Abstract
Objectives:
The aim of this study was to review the literature about the effect of whole body vibration exercise in the BMD in patients with postmenopausal osteoporosis without medications.
Methods:
A systematic review was performed.
Results:
The frequency of the mechanical vibration used in the protocols has varied from 12 to 90 Hz. The time used in the protocols varied from 2 up to 22 months. Techniques with X-rays were used in nine of the twelve publications analyzed, the Dual energy X-ray absorptiometry (DEXA) in eight studies and the High resolution peripheral quantitative computed tomography (HR-pQCT) in one publication. The concentration of some biomarkers was determined, as the sclerostin, the bone alkaline phosphatase, N-telopeptide X and 25-hydroxyvitamin D. Among the twelve articles analyzed, seven of them have shown an improvement of the BMD of some bone of postmenopausal women exposed to whole body vibration exercises not associated to medications; as well as modifications in biomarkers.
Keywords: Postmenopausal Women, Osteoporosis, Bone Mineral Density, Whole Body Vibration Exercise
Introduction
Menopause is one of the most important events in the life of a woman that brings several permanent physiological changes with health consequences. Menopause occurs in the climacteric cycle, which is the transition from reproductive to non-reproductive period. Menopause is a clinical condition defined retrospectively. It is the time of the final menstrual period, followed by amenorrhea for 12 months. Postmenopausal (PM) describes the period following the final menstruation[1].
With the increase in the proportion of elderly populations in the world, and in consequence the number of postmenopausal woman (PMW), sarcopenia (the loss in skeletal muscle mass and muscle strength) and osteoporosis (low bone mass and structural deterioration of bone tissue) are important public health issues[2,3]. Between the ages of 40 and 80 years, muscle mass decreases by 30% to 50%[2], leading to physical frailty, increased risk of falls, impaired mobility, and a possible contribution to several age related chronic disorders (e.g., osteoporosis, type 2 diabetes, insulin resistance, obesity and cardiovascular diseases)[4]. Dalal and Agarwal, 2015[5] consider that the major consequences of menopause are related primarily to estrogen deficiency and that is very difficult to distinguish the consequences of this deficiency from those of aging, since aging and menopause are strongly related. It is considered that the estrogen deficiency exacerbates bone demineralization processes resulting in bone abnormal microarchitecture[6].
Osteoporosis (OP) is a systemic skeletal disease that affects millions of people around the world[7] and it is mostly observed in female population, where a significant increase in incidence is recorded after MP[4]. The World Health Organization (WHO) describes OP as a disease characterized by low bone mass and structural deterioration of bone tissue, leading to bone fragility and increased susceptibility to fractures of the hip, spine, and wrist[4]. OP has been defined by the WHO on the basis of BMD assessment, as a BMD that lies 2.5 standard deviations or more below the average value for young healthy women (a T-score of <-2.5 SD)[8].
Weber-Rajek et al., 2015[9] pointed out that postmenopausal osteoporosis (PMO) treatment has evolved focusing on prevention, screening, diagnosis, and early and specified therapy. However, treatment of OP is a long-term process, may be harmful, and may not be 100% preventive of its development and consequences[10]. This is particularly true for regimens involving drugs with potential adverse effects, or that need adherence of over 90% of time for to be effective[10]. It also occurs in those who cannot afford certain medication options or are in conflict with taking any medications for prolonged periods[11].
Nonpharmacologic approaches have been suggested for the management of women with OP, and physical activities have been considered important for maintaining bone health[11].
The effects of exercise on the prevention of the postmenopausal symptoms have been discussed and accepted[12]. Authors have reported that postmenopausal symptoms can be prevented significantly by encouraging women over middle age to exercise regularly[11-13]. Moreover, authors have pointed out that in the treatment of osteoporosis, physical therapy could improve the quality of life of patients and to reduce the risk of falls, that are strictly related to fractures[12]. Although exercises are a widely used strategy to enhance muscle strength, flexibility and prevent bone loss in MP, there are conflicting results among the different types of exercises[14,15]. Although the multi-component exercises may have shown significant results in the maintenance of bone mass during aging process[11,15], the popular walking and jogging modalities do not demonstrate uniform results, and are potentially harmful for people with impaired balance and flexibility[14].
The safety of the physical activity is an important consideration in the management of the patients with osteoporosis, due to the increased risk of falls[16,17]. Mechanical vibrations produced in oscillating/vibratory platforms (OVP) can be transmitted to the body of the patient generating whole body vibration (WBV) exercises. These WBV exercises (WBVE), in appropriate parameters, such as frequency (F), amplitude (A), peak-to-peak distance (D), and peak acceleration (apeak), are considered a safe form of physical activity[18,19]. Following the piezoelectric theory, the interaction of mechanical vibration with the structures of the body would induce the process of bone formation[9]. Moreover, WBV may affect the levels of growth hormone, parathyroid hormone and testosterone in serum[20-21], which may prevent sarcopenia and osteoporosis[23,24].
WBV exercises increase the muscular strength and power that could lead to an increased and better quality neuromuscular function. As cited in the review of Totosy de Zepetnek (2009)[25] there are intrinsic several mechanisms of actions of the mechanical vibrations may be responsible for the prevention of decline, increase or maintenance of bone mass[25]. It has been demonstrated that the fluid flow in canaliculi and bone lacunae may be increased due to the loading frequency. The mechanotransduction may be influenced by the mechanical loading adequate to increase fluid flow in bone and by the effects of forces from muscle contraction applied to bone during physical activity[21,25].
In the case of patients with OP, these findings may help to reduce the risk of falls with a consequent decrease of the bone fractures[20]. WBVE is also one of the suggestions of the Innovative Comprehensive Active Rehabilitation of Osteoporosis (ICARO) strategy for the treatment of osteoporosis, cited by Weber-Rajek et al, with the proposal of increasing bone mass to delay the disease progress and limit its sequelae[9].
Several biomarkers are used in clinical studies to evaluate bone metabolism and relation with exercises effects, such as osteocalcin, parathyroid hormone, bone alkaline phosphatase, calciferol, N-terminal propeptide of type I procollagen (P1NP) and C-terminal cross-linking telopeptide of type I collagen[26].
Recently it has been highlighted the influence of osteocytes in bone and muscle activation stimuli (Yavropoulou, 2014) through their expression of sclerostin[27]. Being a SOST gene product, sclerostin inhibits osteoblastic bone formation[27]. Bone disorders that downregulate sclerostin like esclerosteosis and van Buchem disease results in high bone mineral density and low risk of fractures (Sharif, 2015)[28]. Exercise and experimental loading through mechanical stimulation of the skeleton may induce bone formation, while immobilization increases the number of sclerostin positive osteocytes. Osteocytes are able to detect mechanical strain and respond to it leading to bone formation (Cidem 2014)[29]. Measurements of serum sclerostin levels appear useful for understanding the mechanisms by which osteocytes respond to hormonal, physical (like mechanical vibration) and pharmacological stimuli, but other issues regarding gender, genetics and physiological differences must be cleared[27].
Despite the already demonstrated effectiveness of the WBVE to manage OP, the protocols are not consensual, due the variation of the parameters of mechanical vibrations and other physical parameters[18]. Moreover, in some studies, the participants are also taking medications, besides the utilization of the WBVE[30]. The first published meta-analysis on the effects of WBV in OP was in 2010, by Slatkovska et al. In their analysis, it was excluded studies in which patients were taking medications, or that protocols lasted less than 6 months[31]. Amongst other conclusions, the authors highlighted the importance of the positioning of patients in the platforms, since the effects of mechanical vibrations may vary according to postural and anatomical differences[31].
The aim of this study was to review recent literature and highlight novel findings on the effect of whole body vibration exercise on the BMD in women with PMO without medications.
Material and methods
Search strategy used to find the publications
Three reviewers independently accessed bibliographical databases in the Universidade do Estado do Rio de Janeiro on November 1st 2015; two searches were performed.
In the first, the keyword “osteoporosis” was searched in the PubMed database to verify the number of publications (NP) in ten years (1995-2015). All the publications were considered in this study. In the second, the keywords “whole body vibration” and “postmenopausal women” were searched together in the PubMed and PEDro databases. All these publications were screened following the exclusion and inclusion criteria. About the databases used, briefly, PubMed comprises more than 24 million citations for biomedical literature from MEDLINE, life science journals and online book (http://www.ncbi.nlm.nih.gov/pubmed) and PEDro is the Physiotherapy Evidence Database and it is a free database of over 29 thousand randomized trials, systematic reviews and clinical practice guidelines in physiotherapy (http://www.pedro.org.au).
Exclusion criteria to select the publications
Exclusion criteria allowed the elimination of unnecessary publications identified in the second search. Papers were excluded if they were: (i) published in a language different of the English; (ii) review articles; (iii) with combined treatments, (iv) case reports and findings not related to the bone.
Inclusion criteria to select the publications
In the search with the keywords “whole body vibration” and “postmenopausal women”, all the publications found in the databases (PubMed and PEDro) were preliminarily considered to be included in this current review. Papers from personal files of the authors were selected first.
To be included in this review, all studies investigating the effects of WBV on the bone of PMW needed to fulfill the following criteria: be a randomized controlled trial (RCT); be a single group experimental study (crossover designs) in the absence of RCT’s; and published in the English language. Studies were included if the PMW were taking supplementation of vitamin D and/or performed static or dynamic exercises on an OVP.
As the three reviewers carried out the searches for publications independently, they then decided which publications should be excluded from the search results. Full papers were included for this literature review if they met the search criteria and described a study using WBV generated by an OVP used for the evaluation or treatment of PMW independently on the year of the publication. Three of the authors independently ed data and disagreements were resolved by consensus.
A flowchart (Figure 1), based in the PRISMA analysis, was done to show the steps in the selection of the full papers analyzed in this review[32].
Figure 1.
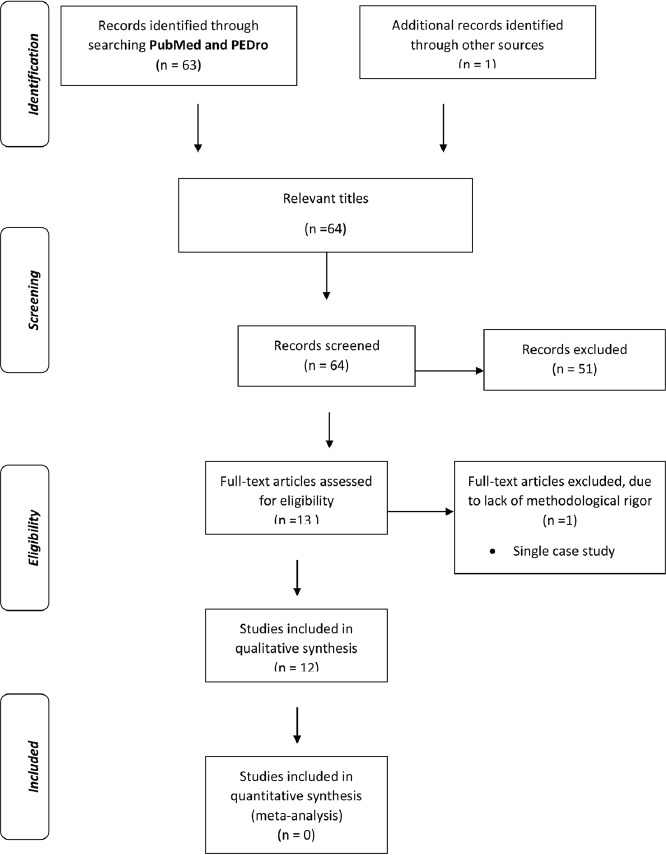
Flowchart indicating the steps to select the full papers analyzed in this review.
Levels of evidence (LE) of the selected papers
Included studies were classified according to the National Health and Medical Research Council Hierarchy of evidence (NHMRC, 2003-2007)[33] (Figure 2).
Figure 2.
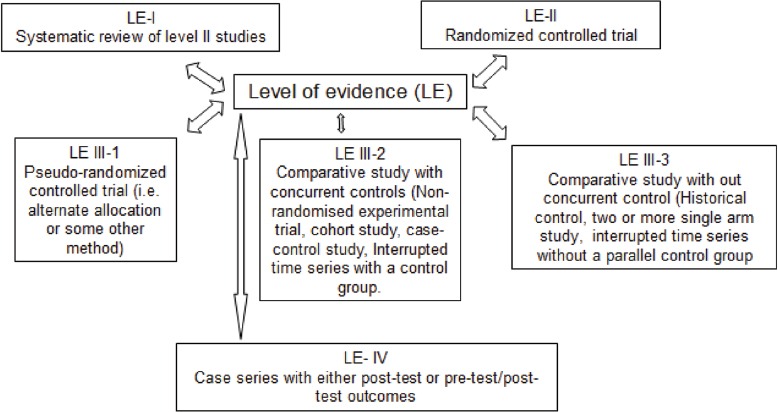
Designation of levels of evidence according to the intervention research question
Each article was assigned to one reviewer, crosschecked by a second reviewer and when there was disagreement, a third researcher was consulted and the issue discussed until consensus was reached.
Data analysis
Data was not comparable and therefore statistical pooling was not appropriate. The results of the findings of this review were summarized in a narrative form.
Results
In the [Table 1] is shown the number of publications searched of with the keyword “osteoporosis” in the PubMed (November 1st 2015) in ten years. It is possible to verify that the NP has increased in the various years as well as the awareness strategy, possibly demonstrating popularity, due to the burden of the disease[31].
Table 1.
Number of publications containing the keyword “osteoporosis” in PubMed from 1995 to 2015.
| Year | Number of publications |
|---|---|
| 1995 | 1181 |
| 2000 | 1988 |
| 2007 | 2884 |
| 2008 | 2951 |
| 2009 | 3047 |
| 2010 | 3147 |
| 2011 | 3369 |
| 2012 | 3472 |
| 2013 | 3584 |
| 2014 | 3775 |
| 2015 | 3502 |
In the [Table 2] is shown the total number of publications searched with the keywords “whole body vibration” and “postmenopausal women” in the databases PubMed and PEDro, it was found more publications with this keyword in PubMed.
Table 2.
Total number of publications with the keywords “whole body vibration” and “postmenopausal women” in two databases from 1995 to 2015.
| Database | Number of publications |
|---|---|
| PubMed | 39 |
| PEDro | 24 |
Figure 1 shows the flowchart[32] indicating the steps to select the full papers analyzed in this review using the keywords “whole body vibration” and “postmenopausal women”. Of the 64 papers screened, only 12 have reached the inclusion and exclusion criteria. Some of the publications were in duplicate, or were reviews, were in a language different of the English or were cases reports. Moreover, the publications that did not evaluate bone metabolism were excluded.
Of the twelve included studies, seven were in the Level II (RCT) and five Level III-2 according to the NHMRC[33]. The main objective of the all the selected papers were to verify whether WBVE were effective for the management of PMO considering the parameters related to the bone improvement.
The number of participants was 1,002 and this ranged from 22 (Liphardt et al, 2015)[34] to 202 (Slatkovska et al, 2014)[35]. The ages varied from 46 (Lai et al, 2013)[36] up to 85.6 (Verschueren et al, 2011)[22] years old.
[Table 3] shows the descriptions of the level of evidence of the publications, the aims of the studies, characteristics of the participants and the protocols used to the management of PMW with WBVE. The parameters for evaluation of WBVE effects recommended by the International Society of Musculoskeletal and Neuronal Interactions (ISMNI)[18] were calculated if other parameters were provided.
Table 3.
Levels of evidence (LE), aims, applied protocols, tools, outcomes and conclusions of the studies on the effects of WBVE on BMD.
| Reference | LE | Aim of the study | Protocol | A or D / apeak | Footwear [42] | Tools | Outcomes | Conclusions |
|---|---|---|---|---|---|---|---|---|
| Liphardt et al, 2015[34] | III-2 | To verify if WBVT will improve or maintain bone microarchitecture and bone strength in osteopenic postmenopausal women. | 22 women (50-65 yr) received WBVT for 2-3 sessions/wk (22 mos) and were compared with 20 controls. The WBVT group, WBV (3-4 mm) at 20 Hz applied for 10 min per training day (in ten sets of I min with vibration and 1 min break between vibration bouts). | A=4 mm / 1.2 g | Not specified | Bone outcomes were measured by HR-pQCT and finite element estimated bone strength. Balance and jump performance and MVC of knee flexor and extensor muscles were recorded. | Total BMD, cortical area, cortical thickness, and cortical porosity all decreased in both groups; WBVT did not affect the response. All other bone outcomes were not affected by WBVT or time. No difference in measures of balance, jump height, and MVC due to WBVT were detected. | WBVT did not lead to improved bone quality in postmenopausal osteopenic women after 12 mos of training compared to controls, and there were no detected benefits related to balance and muscle strength outcomes. |
| Karamehmetoğlu et al, 2014[37] | III-2 | To assess whether osteocytes have a response on reflex myoelectrical activity during WBV in postmenopausal women. | Participants (56.8 ± 6.5 yr) were classified into 2 groups: the low BMD group (n = 37) and normal BMD group (n = 43). Two sets of WBV (amplitude: 2 mm, frequency: 40 Hz, set duration: 30 s) were used for all participants. There was a rest period of 10 s between sets. | A=2 mm / 6.4 g | bare feet | Hip BMD was measured using DEXA. SE data from the adductor longus muscle were processed to obtain vibration-induced reflex myoelectrical activity. Changes in plasma sclerostin (SOST) levels with WBV were expressed as a standardized vibration-induced SOST index. | The vibration-induced SOST index was 1.03 ± 0.24 in the low BMD group and 0.99 ± 0.33 in the normal BMD group. For plasma SOST levels, no group-by-time interaction was found. The resting myoelectrical activities of adductor muscles increased during WBV in both groups. However, there was no significant difference in the main effects of WBV on resting myoelectrical activity between the groups. | This study suggests that osteocytes serve as mechanoreceptors of reflex electromyography during WBV. The vibration-induced plasma SOST index was found to be a significant independent predictor of the vibration-induced reflex myoelectrical activity of the adductor muscle in both groups. |
| Slatkovska et al, 2014[35] | II | To examine the effect of WBV on calcaneus by QUS measurements; which has rarely been examined. | A single-centre, 12-mos, randomized controlled trial. 202 postmenopausal women (53.5-67.5 yr) with BMD T score between -1.0 and -2.5, not receiving bone medications, were asked to stand on a 0.3 g WBV platform oscillating at either 90- or 30-Hz for 20 consecutive minutes daily, or to serve as controls. Calcium and vitamin D were provided to all participants. | D<50 µm / 0.3 g | bare feet | BUA, speed of sound, and QUS index were obtained as pre-specified secondary endpoints at baseline and 12 mos by using a Sonometer. 12-mos of WBV did not improve QUS parameters in any of analyses. | Most of the analyses showed no statistical differences between the WBV groups and the CG, but the mean calcaneal BUA decreased in the 90 and 30-Hz WBV groups and increased in the control group. Decreases in BUA in the 90 and 30-Hz or combined WBV groups were different from the CG in a few of the analyses including all randomized participants, as well as in analyses excluding participants who had missing QUS measurement and those who initiated hormone therapy or were <80% adherent. | Although there are consistent trends, not all analyses reached significance. 0.3 g WBV at 90 or 30 Hz prescribed for 20 min daily for 12 mos did not improve any QUS parameters, but instead resulted in a decrease in calcaneal BUA in PMW in several analyses. |
| Zaki, 2014[41] | III-2 | To evaluate the impact of two exercise programs, WBV and resistance training on BMD and anthropometry in obese postmenopausal women. | Eighty Egyptian obese postmenopausal women (50-68 yr, with body mass index ranged between 30-36 kg/m2). The exercise prescription consisted of WBV and resistance training (8 mos). In the first session of training, the WBV group performed three sets of 1 min vibration with a frequency of 16 Hz, separated by 1-min resting periods. The training load increased during the following sessions, increasing by one set every session until the 10 sets of WBV that is considered to be the load of this intervention. | Not specified | Not specified | BMD (DEXA) and anthropometrical parameters were measured at the beginning and at the end of the study. Changes from baseline to eight months in BMD and anthropometric parameters were investigated. | BMD at the greater trochanter, at ward’s triangle, and at lumbar spine were significantly higher after physical training, using both WBV and resistive training. Moreover, both exercise programs were effective in BMI and waist to the hip ratio. Simple and multiple regression analyses showed significant associations between physical activity duration and BMD at all sites. The highest values of R (2) were found for the models incorporating WBV plus BMI. | The study suggests that both types of exercise modalities had a similar positive effect on BMD at all sites in obese postmenopausal women. Significant association was noted between physical activity and anthropometric variables and BMD measures at all sites. |
| Lai et al, 2013[36] | II | To investigate the effect of high-frequency and high-magnitude WBV on the BMD of the lumbar spine in postmenopausal women. | Study randomized 28 postmenopausal women (46–69 yr) into either the WBV group or the CG for a 6-mos trial. The WBV group received an intervention of high-frequency (30 Hz) and high-magnitude (3.2 g) WBV in a natural full-standing posture for 5 min, three times per wk. | D<2 mm / 3.2 g | bare feet | DEXA was used to measure the lumbar BMD of the two groups before and after the intervention. | Six months later, the BMD of the WBV group had significantly increased, while that of the control group had decreased. The comparison between the two groups showed that the BMD of the WBV group had increased significantly. | This study found that 6 mos of high-frequency and high-magnitude WBV yielded benefits to the BMD of the lumbar spine in PMW, and could therefore be provided as an alternative exercise. |
| Turner et al, 2011[38] | II | To examine the effects of two doses low-frequency (12 Hz), low-magnitude (0.3 g), WBV on markers of bone formation and resorption in postmenopausal women. | 46 women (59.8 ± 6.2 yr) were randomized into a sham vibration control group, one time per wk vibration group (1×/wk, frequency 12 Hz, 0.5 mm peak-to-peak displacement), or three times per wk vibration group (3×/week). Vibration exposure consisted of 20 min of intermittent vibration for the 1×/wk and 3×/wk groups, and sham vibration (<0.1 g) for the CG for 2 mos. | D=0.5 mm / <1 g | Double-blinded primary outcome measures were urine markers of bone resorption: NTx/Cr and bone formation: bone ALP. | NTx/Cr was reduced in the 3×/wk vibration group but not in the 1×/wk vibration group compared with sham control. No effect of time or group allocation was observed on the bone formation marker ALP. | Low-frequency, low-magnitude vibration 3×/week for 2 mos in PMW results in a significant reduction in NTx/Cr, a marker of bone resorption, when compared with sham vibration exposure. | |
| Beck and Norling, 2010[40] | II | To observe the effect of low- and higher intensity WBV on risk factors for hip fracture in postmenopausal women. | Forty-seven women (71.5 ± 9.0 yrs) completed the 8 mo randomized controlled trial design to examine the influence of twice-weekly low-intensity WBV (15 min, 30 Hz, 0.3 g) or higher intensity WBV (2 × 3 min, 12.5 Hz, 1 g). | D=0.5 mm / 0.3 g x 1 g | Not specified | Anthropometrics, bone (whole body, hip, spine, forearm, and heel), muscle (wall squat and chair rise), and balance (tandem walk and single leg stance) were determined. Physical activity, daily calcium, and compliance were recorded. | There were no between-group differences in any measure at 8 mos, but within-group effects were evident. Controls lost bone at the trochanter and lumbar spine, whereas WBV groups did not. WBV subjects improved wall squat and chair rise performance. | Eight mos of twice-weekly WBV may reduce bone loss at the hip and spine and improve lower limb muscle function. These changes may translate to a decreased risk of falls and hip fracture. |
| Von Stengel et al, 2011a[24] | II | To determine the effect of different WBV devices on BMD and neuromuscular performance. | 108 postmenopausal women (65.8 ± 3.5 yr) were randomly allocated to 1) rotational vibration training (RVT), i.e., 12.5 Hz, 12 mm, 12 mo, three sessions per week, for 15 min, including dynamic squat exercises; 2) vertical vibration training (VVT), i.e., 35 Hz, 1.7 mm, as above; and 3) a wellness CG, i.e., two blocks of 10 low-intensity gymnastics sessions. | D=12 mm / 3.7 g | Not specified | BMD was measured at the hip and lumbar spine at baseline and after 12 months of training using DEXA. Maximum isometric leg extension strength and leg power were determined using force plates. | A BMD gain at the lumbar spine was observed in both vibration VT groups (RVT and VVT), which was significant compared with the CG value for RVT and borderline non significant for VVT. In the neck region, no significant treatment effect occurred. Neck BMD values tended to increase in both VT groups and remained stable in CG. Both VT groups gained maximum leg strength compared with CG, whereas power measurements did not reach the level of significance. | WBV training is effective for reducing the risk for osteoporosis by increasing lumbar BMD and leg strength. |
| Verschueren et al, 2011[22] | III-2 | To study the potential benefit of WBVT given a conventional or a high dose of daily vitamin D supplementation in improving strength, muscle mass, and bone density in postmenopausal women. | In a 2 × 2 factorial-design trial, 113 institutionalized females (73.1-85.6 yr) were randomly assigned either to a WBV or a no-training group (6 mo), receiving either a conventional dose (880 IU/day) or a high dose (1600 IU/day) of vitamin D(3). The frequency of the vibration was 30 to 40 Hz, the acceleration of 1.6 to 2.2 g, the longest duration of vibration loading without rest from 15 to 60 s, and the rest period between exercises loading without rest from 60 up to 5 s. | D<1 mm / 1.6 up to 2.2 g | Not specified | Isometric and dynamic strength, leg muscle mass, and hip BMD (DEXA) were evaluated. Additionally, serum 25(OH)D levels were compared between conventional and high-dose supplementation. | After 6 mos of treatment, dynamic muscle strength, hip BMD, and serum vitamin D levels improved significantly in all groups, whereas isometric strength and muscle mass did not change. When compared with no training, the WBV program did not result in additional improvements. When compared with 880 IU, a high dose of 1600 IU of vitamin D did result in higher serum vitamin D levels but did not result in improvements. | In institutionalized women older than 70 years, the WBV training protocol tested is not more efficient in enhancing muscle mass, strength, and hip BMD compared with vitamin D supplementation. A higher dose of 1600 IU of vitamin D does not provide additional musculoskeletal benefit in this population compared with conventional doses. |
| Bemben et al, 2010[20] | III-2 | To examine the effects of an 8-month program involving WBV plus resistance training on BMD and bone metabolism in older postmenopausal women. | 55 estrogen-deficient postmenopausal women (55-75 yr) were in the resistance training group (R, n=22), a WBV plus resistance training group (WBVR, n=21), or a CG (n=12) for 8 mos. R and WBVR performed upper and lower body resistance exercises 3 days/week at 80% 1 Repetition Maximum (1RM). WBVR received vibration (30-40 Hz, 2-2.8 g) in three different positions preceding the resistance exercises. | D=2 to 4 mm/ 2.16 g up to 2.8 g | Athletic shoes | Daily calcium intake, bone markers Bone ALP; C-terminal telopeptide of Type I collagen (CTX), and BMD of the spine, dual femur, forearm, and total body (DEXA) were measured at baseline and after the intervention. | After 8 mos of R or WBVR, there were no significant group or time effects in Bone ALP, CTX, or total body, spine, left hip or right trochanter BMD. However, right total hip and right femoral neck BMD decreased in all groups. A group x time interaction was detected at radius 33% BMD site, with CON slightly increasing, and WBVR slightly decreasing. R and WBVR increased 1RM strength for all exercises, while CON generally maintained strength. WBVR had greater percent increases in muscular strength than R at 4 mos for lat pull down, seated row, hip abduction and hip adduction and at 8 mos for lat pull down, hip abduction and hip adduction. | Bone metabolism in PMW was not affected by resistance training either with or without WBV. In contrast, the addition of WBV augmented the positive effects of resistance training on muscular strength in these older women. |
| von Stengel et al, 2011b[39] | II | To determine whether the effect of exercise on BMD and falls can be enhanced by WBV. | 151 postmenopausal women (68.5 ± 3.1 yr) were randomly assigned to a: (1) conventional TG; (2) conventional training group including vibration (TGV); and (3) wellness CG. TG conducted an exercise program consisting of 20 min dancing aerobics, 5 min balance training, 20 min functional gymnastics, and 15 min dynamic leg-strength training on vibration plates (without vibration) twice a wk (18 mos). TGV performed an identical exercise regimen with vibration (25-35 Hz) during the leg-strengthening sequence. CG performed a low-intensity wellness program. | A=1.4 mm / 4.2 g up to 8.3 g | Not specified | BMD was measured at the hip and lumbar spine at baseline and follow-up using the DEXA method. Falls were recorded daily via the calendar method. | After 18 mos, an increase in BMD at the lumbar spine was observed in both training groups. The difference between the TG and the CG was significant. At the hip no changes were determined in either group. The fall frequency was significantly lower in TGV compared with CG, whereas the difference between TG and CG was not significant. | A multifunctional training program had a positive impact on lumbar BMD. The application of vibration did not enhance these effects. However, only the training including WBV affected the number of falls significantly. |
| Verschueren et al, 2004[23] | II | In this randomized controlled trial, hip BMD was measured in postmenopausal women after a 24-week WBV training program. | 70 volunteers (age, 58-74 yr) were randomly assigned to a WBVT (n=25), a RES (n=22), or a CG (n=23). The WBV group and the RES group trained three times weekly for 6 mos. The WBV group performed static and dynamic knee-extensor exercises on a vibration platform (35-40 Hz, 2.28-5.09g), which mechanically loaded the bone and evoked reflexive muscle contractions. The RES group trained knee extensors by dynamic leg press and leg extension exercises, increasing from low (20 RM) to high (8 RM) resistance. The CG did not participate in any training. | A=1.7 mm up to 2.5 mm / 2.28 g up to 5.09 g | Gymnastic shoes | Hip BMD was measured using DEXA at baseline and after the 6-month intervention. Isometric and dynamic strength were measured by means of a motor-driven dynamometer. | Vibration training improved isometric and dynamic muscle strength and also increased BMD of the hip. No changes in hip BMD were observed in women participating in resistance training or age-matched controls. Serum markers of bone turnover did not change in any of the groups. | These findings suggest that WBV training may be a feasible and effective way to modify well-recognized risk factors for falls and fractures in older women and support the need for further human studies. |
25(OH)D - 25-hydroxyvitamin D; apeak – peak acceleration; A – acceleration; BMD- Bone mineral density; Bone ALP - Bone alkaline phosphatase; BUA - Calcaneal broadband attenuation; CG - control group; DEXA- dual energy X-ray absorptiometry; HR-pQCT - high-resolution peripheral quantitative CT; min- minute; mos – months; D – peak-to-peak displacement; MVC- maximum voluntary contraction; NTx - N-telopeptide X; NTx/Cr - N-terminal cross-linking telopeptide of type I collagen normalized to creatinine ratio; PMW- postmenopausal women; QUS - calcaneal quantitative ultrasound; RES- resistance training group; SE- Surface electromyography; TG – training group; WBV- Whole body vibration; WBVT - Whole body vibration training; wk- week; year – yr; year – yr.
Not all studies followed the ISMNI recommendations when describing their protocols, nor the studies were comparable since different parameters were used. The frequency of the mechanical vibration used in the protocols has varied from 12 to 90 Hz; the amplitude or peak-to-peak displacement varied from <1 mm to 12 mm, the peak acceleration from <1 to 8.3 g. The duration of the protocols varied from 2 months to 22 months, according to the aim of the study.
The tools for evaluation, the outcomes and the conclusion of the selected articles is also demonstrated in [Table 3]; and through their findings it is possible to observe effects of the use of WBVE in PMO. Techniques with X-rays were used in nine of the twelve publications analyzed, the Dual energy X-ray absorptiometry (DEXA or DXA) in eight studies and High resolution peripheral quantitative computed tomography (HR-pQCT) in one publication[34].
Karamehmetoğlu et al, 2014[37] has performed a study to assess whether osteocytes had an effect on reflex myoelectrical activity through eletromiography study and serum sclerostin levels with a protocol with WBV. Although the activity was diminished in all PMW there was no significant differences among the PMW with or without OP.
Other serum/urinary concentrations of some biomarkers were evaluated, like the bone alkaline phosphatase (Bone ALP) (Turner et al, 2011, Bemben et al, 2010)[20,38], the N-telopeptide X (NTx) as well as the N-terminal cross-linking telopeptide of type I collagen normalized to creatinine ratio (NTx/Cr) (Turner et al, 2011)[38]; and 25-hydroxyvitamin D [25(OH)D] (Verschueren et al, 2011)[22].
Turner et al, 2011 have shown that WBV 3 times/week for a short period (2 months) in PMW with OP resulted in a significant reduction in urinary NTx/Cr ratio, possibly reflecting diminished bone resorption[38]. In the study performed by Verschueren et al (2011) the comparison of two groups with high or low doses of oral vitamin D submitted or not to WBV, demonstrated the positive effects of WBVE on leg strength[22].
Among the twelve articles analyzed, seven of them have shown improvement of the BMD PMW exposed to whole body vibration exercises.
Discussion
Osteoporosis is a skeletal disease that leads to the structural deterioration of bone tissue and has been increasingly studied throughout the years[8]. Several pharmacological therapies are used to treat this disease and consequently adverse events may occur with undesirable results[6,7,10].
The number of articles searched with the keyword “osteoporosis” in the PubMed increased from 1,181 publications in 1995 to 3,502 publications in 2015. This fact can be related to the increase in the proportion of elderly population in the world, and in consequence the number of PMW. Moreover, the undesirable consequences of osteoporosis, in particular the fractures[7,8,11], that demands high costs for surgical treatment and rehabilitation may also have stimulated investigations about OP. Several awareness campaigns on osteoporosis are regularly performed by health services throughout the world[8].
The articles involving only the use of WBV exercises as therapy were selected in PubMed and PEDro databases. Amongst the 12 selected, seven were Level II (RCT) and five were Level III-2 according to the NHMRC; consequently, most of the studies were performed with control groups and demonstrated significant results.
There was a large variation on the number of participants (22 to 202) and ages (from 46 to 85.6 years old). As shown in [Table 3], the biomechanical parameters as frequency (Hz), duration (months), amplitude or peak-to-peak displacement (mm) and peak acceleration (in multiples of g) according to the aims of the study were discrepant.
It is curious that Turner et al, 2011[38] using low frequency for 2 months, with less <1 g of peak acceleration reported a significant reduction of a marker of bone resorption when compared with sham vibration exposure. Liphardt et al, 2015[34], using a protocol with a frequency of 20 Hz (3.2 g) and a duration of 22 months, did not find improvements in the bone quality in postmenopausal women. This contradicts the proposed apeak range of 1 to 5 g in which bone loss is supposed to be decreased by WBVE[25].
Amongst the different tools (Table 3) used to evaluate the effect of the WBV exercises in PMO, it was mostly (eight of the twelve publications) used DEXA, affirming the relevance and accessibility of this technique, when adequately applied, for the screening and evaluation of bone loss. The HR-pQCT may be more accurate technique to evaluate bone microarchitecture, but it is not as accessible for large populations studies. In the one publication that used this technique (Liphardt et al, 2015)[34], no alterations in bone characteristics were found. This technique is more frequently applied for research purposes.
Considering the findings presented in this review, the majority of studies have shown an improvement of the BMD or other parameters regarding muscle, bone and functional outcomes, like reduction of falls[24,39] in postmenopausal women exposed to WBVE[9].
But aside these already known important outcomes, it was also demonstrated effects on biomarkers that are related to muscle/bone activity. These substances may permit the evaluation of the interventions effects on the prevention or treatment of OP[40], because they may elucidate alterations in the physiopathology of bone loss.
In the selected articles of this review bone alkaline phosphatase, 25-hydroxyvitamin D, sclerostin and N-terminal cross-linking telopeptide of type I collagen normalized to creatinine (NTx/Cr) were analyzed[37,38].
Sclerostin is a contemporary target for new medications that are being evaluated to treat OP[28].
In the study by Karamehmetoğlu et al, although there was no significant difference between the low and normal BMD groups in terms of the effects on vibration induced reflex myoelectrical activity through sclerostin detection, it was demonstrated that osteocytes serve as a mechanoreceptor of reflex electromyography (EMG) during WBV[37]. Since osteocytes may be sensitive to mechanical stimulation and help to control the matrix formation process in response to vibration, they are considered to cause changes within bone tissue[27]. Therefore, it was suggested that osteocytes subjected to mechanical stimulation also affect muscle activity and it may be possible to assess in vivo the response of the bone to mechanical loading using electrophysiological methods and its consequences by sclerostin measurements[29,37].
To our knowledge, this is the first review to point out the potential use of serum sclerostin, and its evaluation regarding bone loss and muscle activity concerning WBVE.
More clarification that is accurate is needed to determine the relationships between biochemical factors, individual characteristics regarding genetics and environmental factors, muscle and bone structure, and biomechanical parameters of the therapy with WBVE for PMO. In addition, the evaluation of WBVE effects with associated oral calcium and vitamin D, as well as hormonal replacement therapy, may determine the benefits that may be comparable to traditional pharmacological therapy for OP without the adverse events. The proper and universal use of the ISMNI recommendations may reduce bias and enhance quality of studies until consensus is not achieved.
In conclusion, WBVE are relevant non-pharmacological option, as one of the modalities of exercises recommended for the management of postmenopausal osteoporosis. More studies must be performed to the establishment of the parameters for protocols as well as relevant outcomes.
Author’s Contributions
CD, DSC, EMM, HPV and MBF participated in the conception and design of the study, as well, preparing the manuscript. CD, HPV, DSM, DB, and MBF coordinated the clinical approaches of the study. DSC, CRG, AM, and L did the searches in the databases and aided in the selection of the papers to be discussed in the manuscript. EMM, DSC and CD aided in the corrections of the Tables. MBF have done the final version of the manuscript. MBF conceived the protocol, obtained funding and oversaw the study. All the authors read and approved the final manuscript.
Acknowledgements
The authors thank the Brazilian Government agencies (CNPq, FAPERJ) and UERJ for the support.
Footnotes
The authors have no conflict of interest.
Edited by: F. Rauch
References
- 1.Silva TR, Franz R, Maturana MA, Spritzer PM. Associations between body composition and lifestyle factors with bone mineral density according to time since menopause in women from Southern Brazil: a cross-sectional study. BMC Endocr Disord. 2015;15(1):71. doi: 10.1186/s12902-015-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faulkner JA, Larkin LM, Claflin DR, Brooks SV. Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol. 2007;34(11):1091–6. doi: 10.1111/j.1440-1681.2007.04752.x. [DOI] [PubMed] [Google Scholar]
- 3.Iniguez-Ariza NM, Clarke BL. Bone biology, signaling pathways, and therapeutic targets for osteoporosis. Maturitas. 2015;82(2):245–55. doi: 10.1016/j.maturitas.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Kanis JA, McCloskey EV, Johansson H, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24(1):23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalal PK, Agarwal M. Postmenopausal syndrome. Indian J Psychiatry. 2015;57(Suppl 2):S222–32. doi: 10.4103/0019-5545.161483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McClung MR. Late skeletal effects of early menopause. Menopause. 2015;22(10):1027–9. doi: 10.1097/GME.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 7.Klop C, Gibson-Smith D, Elders PJ, et al. Anti-osteoporosis drug prescribing after hip fracture in the UK: 2000-2010. Osteoporos Int. 2015;26(7):1919–28. doi: 10.1007/s00198-015-3098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kling JM, Clarke BL, Sandhu NP. Osteoporosis prevention, screening, and treatment: a review. J Womens Health (Larchmt) 2014;23(7):563–72. doi: 10.1089/jwh.2013.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber-Rajek M, Mieszkowski J, Niespodziński B, Ciechanowska K. Whole-body vibration exercise in postmenopausal osteoporosis. Prz Menopauzalny. 2015;14(1):41–7. doi: 10.5114/pm.2015.48679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid IR, Black DM, Eastell R, et al. Reduction in the risk of clinical fractures after a single dose of zoledronic Acid 5 milligrams. J Clin Endocrinol Metab. 2013;98(2):557–63. doi: 10.1210/jc.2012-2868. doi: 10.1210/jc.2012-2868. [DOI] [PubMed] [Google Scholar]
- 11.Rizzoli R. Osteoporosis: non-hormonal treatment. Climacteric. 2007;10(Suppl.2):74–8. doi: 10.1080/13697130701600815. [DOI] [PubMed] [Google Scholar]
- 12.Rizzoli R, Reginster JY, Arnal JF, et al. Quality of life in sarcopenia and frailty. Calcif Tissue Int. 2013;93(2):101–20. doi: 10.1007/s00223-013-9758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mithal A, Bonjour JP, Boonen S, et al. Impact of nutrition on muscle mass, strength, and performance in older adults. Osteoporos Int. 2013;24(5):1555–66. doi: 10.1007/s00198-012-2236-y. [DOI] [PubMed] [Google Scholar]
- 14.Gómez-Cabello A, Ara I, González-Agüero A, et al. Effects of training on bone mass in older adults: a systematic review. Sports Med. 2012;42(4):301–25. doi: 10.2165/11597670-000000000-00000. doi: 10.2165/11597670-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Sota T, Matsuo S, Uchida Y, Hagino H, Kawai Y. Effects of lower body positive pressure on cardiovascular responses during walking in elderly women. Physiol Res. 2013;62(6):653–62. doi: 10.33549/physiolres.932459. [DOI] [PubMed] [Google Scholar]
- 16.Gerdhem P. Osteoporosis and fragility fractures: Vertebral fractures. Best Pract Res Clin Rheumatol. 2013;27(6):743–55. doi: 10.1016/j.berh.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Howe TE, Shea B, Dawson LJ. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;7:000333. doi: 10.1002/14651858.CD000333.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Rauch F, Sievanen H, Boonen S, et al. Reporting whole-body vibration intervention studies: recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J Musculoskelet Neuronal Interact. 2010;10(3):193–8. [PubMed] [Google Scholar]
- 19.Sá-Caputo D, Ronikeili-Costa P, Carvalho-Lima RP, et al. Whole body vibration exercises and the improvement of the flexibility in patient with metabolic syndrome. Rehabil Res Pract 2014. 2014:628518. doi: 10.1155/2014/628518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bemben DA, Palmer IJ, Bemben MG, Knehans AW. Effects of combined whole-body vibration and resistance training on muscular strength and bone metabolism in postmenopausal women. Bone. 2010;47(3):650–6. doi: 10.1016/j.bone.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Rauch F. Vibration therapy. Dev Med Child Neurol. 2009;51(Suppl.4):166–8. doi: 10.1111/j.1469-8749.2009.03418.x. [DOI] [PubMed] [Google Scholar]
- 22.Verschueren SM, Bogaerts A, Delecluse C, et al. The effects of whole-body vibration training and vitamin D supplementation on muscle strength, muscle mass, and bone density in institutionalized elderly women: a 6-month randomized, controlled trial. J Bone Miner Res. 2011;26(1):42–9. doi: 10.1002/jbmr.181. [DOI] [PubMed] [Google Scholar]
- 23.Verschueren SM, Roelants M, Delecluse C, et al. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res. 2004;19(3):352–9. doi: 10.1359/JBMR.0301245. [DOI] [PubMed] [Google Scholar]
- 24.Von Stengel S, Kemmler W, Bebenek M, Engelke K, Kalender WA. Effects of whole-body vibration training on different devices on bone mineral density. Med Sci Sports Exerc. 2011;43(6):1071–9. doi: 10.1249/MSS.0b013e318202f3d3. [DOI] [PubMed] [Google Scholar]
- 25.Totosy de Zepetnek JO, Giangregorio LM, Craven BC. Whole-body vibration as potential intervention for people with low bone mineral density and osteoporosis: a review. J Rehabil Res Dev. 2009;46(4):529–42. doi: 10.1682/jrrd.2008.09.0136. Review. [DOI] [PubMed] [Google Scholar]
- 26.Nishizawa Y, Ohta H, Miura M, et al. Guidelines for the use of bone metabolic markers in the diagnosis and treatment of osteoporosis (2012 edition) J Bone Miner Metab. 2013;31(1):1–15. doi: 10.1007/s00774-012-0392-y. [DOI] [PubMed] [Google Scholar]
- 27.Yavropoulou MP, Xygonakis C, Lolou M, et al. The sclerostin story: From human genetics to the development of novel anabolic treatment for osteoporosis. Hormones. 2014;13(4):476–487. doi: 10.14310/horm.2002.1552. [DOI] [PubMed] [Google Scholar]
- 28.Sharifi M, Ereifej L, Lewiecki EM. Sclerostin and skeletal health. Rev Endocr Metab Disord. 2015;16:149–156. doi: 10.1007/s11154-015-9311-6. [DOI] [PubMed] [Google Scholar]
- 29.Çidem M, Karakoç Y, Ekmekçi H, et al. Effects of whole-body vibration on plasma sclerostin level in healthy women. Turk J Med Sci. 2014;44:404–410. doi: 10.3906/sag-1302-88. [DOI] [PubMed] [Google Scholar]
- 30.Iwamoto J, Sato Y, Takeda T, Matsumoto H. Whole body vibration exercise improves body balance and walking velocity in postmenopausal osteoporotic women treated with alendronate: Galileo and Alendronate Intervention Trail (GAIT) J Musculoskelet Neuronal Interact. 2012;12(3):136–43. [PubMed] [Google Scholar]
- 31.Slatkovska L, Alibhai SM, Beyene J, Cheung AM. Effect of whole-body vibration on BMD: a systematic review and meta-analysis. Osteoporos Int. 2010;21(12):1969–80. doi: 10.1007/s00198-010-1228-z. doi: 10.1007/s00198-010-1228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Merlin T, Weston A, Tooher R. Extending an evidence hierarchy to include topics other than treatment: revising the Australian ‘levels of evidence’. BMC Med Res Methodol. 2009;9:9. doi: 10.1186/1471-2288-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liphardt AM, Schipilow J, Hanley DA, Boyd SK. Bone quality in osteopenic postmenopausal women is not improved after 12 months of whole-body vibration training. Osteoporos Int. 2015;26(3):911–20. doi: 10.1007/s00198-014-2995-8. [DOI] [PubMed] [Google Scholar]
- 35.Slatkovska L, Beyene J, Alibhai SM, Wong Q, Sohail QZ, Cheung AM. Effect of whole-body vibration on calcaneal quantitative ultrasound measurements in postmenopausal women: a randomized controlled trial. Calcif Tissue Int. 2014;95(6):547–56. doi: 10.1007/s00223-014-9920-1. [DOI] [PubMed] [Google Scholar]
- 36.Lai CL, Tseng SY, Chen CN, et al. Effect of 6 months of whole body vibration on lumbar spine bone density in postmenopausal women: a randomized controlled trial. Clin Interv Aging. 2013;8:1603–9. doi: 10.2147/CIA.S53591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karamehmetoğlu S, Karacan I, Çidem M, et al. Effects of osteocytes on vibration-induced reflex muscle activity in postmenopausal women. Turk J Med Sci. 2014;44:630–38. [PubMed] [Google Scholar]
- 38.Turner S, Torode M, Climstein M, et al. A randomized controlled trial of whole body vibration exposure on markers of bone turnover in postmenopausal women. J Osteoporos. 2011:710387. doi: 10.4061/2011/710387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Stengel S, Kemmler W, Engelke K, Kalender WA. Effects of whole body vibration on bone mineral density and falls: results of the randomized controlled ELVIS study with postmenopausal women. Osteoporos Int. 2011;22(1):317–25. doi: 10.1007/s00198-010-1215-4. [DOI] [PubMed] [Google Scholar]
- 40.Beck BR, Norling TL. The effect of 8 mos of twice-weekly low- or higher intensity whole body vibration on risk factors for postmenopausal hip fracture. Am J Phys Med Rehabil. 2010;89(12):997–1009. doi: 10.1097/PHM.0b013e3181f71063. [DOI] [PubMed] [Google Scholar]
- 41.Zaki ME. Effects of whole body vibration and resistance training on bone mineral density and anthropometry in obese postmenopausal women. J Osteoporos 2014. 2014:702589. doi: 10.1155/2014/702589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marín PJ, Bunker D, Rhea MR, Ayllón FN. Neuromuscular activity during whole-body vibration of different amplitudes and footwear conditions: implications for prescription of vibratory stimulation. J Strength Cond Res. 2009;23(8):2311–6. doi: 10.1519/JSC.0b013e3181b8d637. [DOI] [PubMed] [Google Scholar]


