Abstract
Objectives:
To evaluate the effects of whole-body vibration (WBV) on skeletal muscle activity and power performance of the upper body during decline bench press exercise at different loads.
Methods:
Forty-seven healthy young and active male students volunteered. Each performed dynamic decline bench press repetitions with and without WBV (50 Hz, 2.2 mm) applied through a hamstring bridge exercise at three different loads of their 1-repetition maximum (1RM): 30%, 50%, and 70% 1RM. Muscle activity of the triceps brachii (TB), biceps brachii (BB), pectoralis major (PM), and biceps femoris (BF) was measured with surface electromyography electrodes and kinetic parameters of the repetitions were measured with a rotary encoder.
Results:
WBV increased peak power (PP) output during the 70% 1RM condition (p<0.01). Muscle activity was increased with WBV in the TB and BF muscles at all loads (p<0.05). There were no effects of WBV on BB or PM muscles.
Conclusion:
WBV applied through a hamstring bridge exercise increases TB muscle activity during a decline bench press and this augmentation contributes to an increased peak power at higher loads and increased peak acceleration at lower loads.
Keywords: Vibration Exercise, Kinematics, Power, Acceleration, Muscle Activity
Introduction
Whole body vibration (WBV) as a mode of exercise training has been increasingly more popular in health, physical therapy, rehabilitation, professional sports, and wellness applications because of its effects on the neuromusculoskeletal system[1]. The main benefits effects of the WBV training are increased maximal power output[2,3], strength gains[4], and muscle activity evaluated with surface electromyography (EMG). The majority of the previous studies have focused on the effects of WBV in muscle groups near the vibration stimulus.
While most WBV studies apply vibration in a semi-squat position, it has been postulated based on lower-body examinations that WBV has is greatest effects on muscles most proximal to the generation of the WBV stimulus[5]. Recently the application of WBV to an isometric push-up position (hands directly on platform) acutely increased both growth hormone and testosterone concentrations as well as likely inducing central fatigue[6]. The push-up was chosen as this basic exercise may represent a potent physiological system stressor due to its large muscle volume (trunk + upper arm + lower arm) involvement[7] and the trunk muscles are more strongly activated in the horizontal position compared to the typical standing (half squat) positions during WBV[8]. Other research has demonstrated EMG activity of the biceps brachii and triceps brachii muscles can maximize agonist activation and antagonist activation of these muscles during static vibration exercise[9]. On the other hand, athletes who trained at loads which maximized mechanical power achieved the greatest enhancement in dynamic athletic performance[10] suggesting training with a load that maximizes power output is the best stimulus for further improvements in power[11-13]. Thus, the search for optimal loads for maximizing power is of particular interest for strength and conditioning coaches. Therefore, the purpose of the present investigation was to examine the effects of WBV on skeletal muscle activity and power performance of the upper body during decline bench press exercise at different loads. We hypothesized that WBV would induce an additional stimulus for neuromuscular system of the upper body and that WBV would evoke greater effects during the higher load conditions.
Materials and methods
Subjects
Forty-seven healthy young and active male students (21.6±1.6 y; 176.0±5.7 cm; 71.9±8.6 kg; mean±SD) volunteered for the study. The participants were recreationally active (with some experience in strength training), but none were involved in a systematic training program at the time of data collection or for at least 2 months prior to the study. Exclusion criteria included were diabetes, epilepsy, gallstones, kidney stones, cardiovascular diseases, joint implants, recent thrombosis, and musculoskeletal problems. Prior to data collection all participants were informed of the requirements associated with participation and provided written informed consent. The research project was conducted according to the Declaration of Helsinki and was approved by the University Review Board for use of Human Subjects.
Experimental design
The present study investigated whether a WBV stimulus applied through a hamstring bridge exercise (Figure 1) would benefit muscle performance compared to a control (CTRL) condition during a decline bench press exercise (using a Smith Machine) and whether different loads would induce differences on neuromuscular and power performance. Dynamic decline bench press repetitions were performed under six conditions: 1) low load, 30% 1 repetition maximum (RM) without WBV (CTRL-30); 2) low load, 30% 1RM with WBV (WBV-30); 3) moderate load, 50% 1RM without WBV (CTRL-50); 4) moderate load, 50% 1RM with WBV (WBV-50); 5) high load, 70% 1RM without WBV (CTRL-70); 6) high load, 70% 1RM with WBV (WBV-70).
Figure 1.
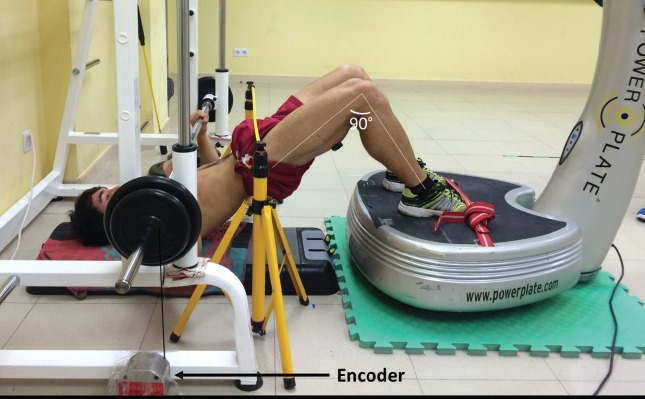
Experimental setup.
Familiarization session
One week before the testing sessions, participants attended two familiarization sessions, where one was used to acclimatize participants with the study protocol and the second determined their 1 repetition maximum (RM) using a Smith Machine (Telju, Toledo, Spain). In both sessions, participants performed a standardized warm-up consisting of a 2 min slow jog, 5 dynamic warm-up exercises (10 reps of pull-backs, squats, lateral lunges, hands-to-feet walking, and 2 sets of 10 repetitions of declined bench press with 20 kg load). Following the warm-up, participants adopted the experimental position (hamstring bridge with feet flat on the platform and hands on the barbell; Figure 1). For the purposes of this study, 1-RM is defined as the maximum weight a participant can lift with good form through the full range of motion (lowered the bar until the chest was touched lightly, approximately 3 cm superior to the xiphoid process). Hand spacing was adjusted individually to no more than 1.5 biacromial width and feet remained in contact with the WBV platform in the hamstring bridge during each lift (Figure 1). All 1-RM lifts were administered according to the NSCA guideline[14]. Briefly, participants performed a warm-up with low resistance (50% of anticipated 1-RM) for 8-10 repetitions followed by 3 min rest. Testing was initiated at 70% of anticipated 1-RM, and weight was increased by 5-10 kg until 1-RM was achieved. Each repetition was separated by 3 min of rest and 1-RM was achieved within 5 attempts or less to prevent fatigue.
Experimental session
Participants were fitted with EMG electrodes (detailed below) before completing a standardized warm-up (identical to familiarization session). Following this warm-up, participants completed a maximal isometric contraction in a press position to determine maximal voluntary contractions (MVC) for all muscles. Participants then adopted the experimental position (Figure 1) to perform the six experimental conditions (WBV-30, Control-30, WBV-50, Control-50, WBV-70, and Control-70), while in a hamstring bridge with feet on the WBV platform. All conditions were performed in random order. Three maximum explosive repetitions were performed where participant’s were instructed to contract as “hard” and “fast” as possible. Repetitions were performed using a Smith machine (Telju, Toledo, Spain), repetition duration was ~1 s each, and five minutes rest was given between each set.
Vibration equipment
The vibration stimulus was applied via of commercial WBV platform (Power Plate® Next Generation pro 5, Power Plate North America, Northbrook, IL, USA) that produced synchronous (uniform) tri-planar oscillations. The WBV stimulus was applied at a frequency of 50 Hz with 2.2 mmp-p (high) amplitude. These WBV stimulus parameters generated a measured acceleration 99.71 m·s-2 via the vector sum of the accelerations was measured using a three-axial accelerometer (Vibration Datalogger DT-178A, Ruby Electronics, Saratoga, USA). During all conditions, subjects wore the same athletic shoes to standardize the damping of the vibration because of the footwear[15].
Kinematic parameters
All repetitions were monitored by linking a rotary encoder (Real Speed, Winlaborat V4.20, Buenos Aires, Argentina) to the barbell (Figure 1). The rotary encoder recorded the position of the load plate within an accuracy of 0.1 mm and time events with an accuracy of 0.001 s. Peak power and peak acceleration, (concentric phase) for each repetition was analyzed.
Surface electromyographic activity (EMG)
Muscle activity of pectoral major (PM), triceps brachii long head (TB), biceps brachii (BB), and biceps femoris (BF) was measured using EMG of the dominant side in writing. Prior to electrode placement, the area was shaved and cleaned with isopropyl alcohol to reduce skin impedance. The electrodes (inter-electrode distance=10 mm) were placed over the mid-belly of the muscle parallel to the direction of the fibres according to recommendations by the SENIAM (Surface ElectroMyoGraphy for the Non-Invasive Assessment of Muscles) project[16].
The double differential technique was used to detect myoelectric raw signals. The surface electrodes were connected to a 16-bit AD converter (TrigoTMWireless System, Delsys Inc., Boston, MA, USA). Raw EMG signals were pre-amplified close to the electrodes (signal bandwidth of 20-450 Hz), sampled at 4000 Hz, and stored on a laptop. EMG data analysis was performed using specific computer software (Delsys EMGworks Analysis 4.0. Delsys Inc. Boston, Massachusetts, USA). The EMG data were averaged by root mean square (rms) in order to obtain averaged amplitude of the EMG signal, and the maximum value of each repetition was selected. This muscle activity was normalized to the EMG signal obtained during the maximal voluntary contraction (MVC).
Statistical analysis
Data were analyzed using PASW/SPSS Statistics 20 (SPSS Inc, Chicago, IL) and significance level was set at P≤0.05. All the measures were normally distributed, as determined by the Kolmogorov-Smirnov test. Sphericity was tested by the Greenhouse-Geisser method. Dependent variables (peak power output, peak acceleration, EMGrms for TB, BB, PM, and BF) were evaluated with a two-way repeated measures analysis of variance (ANOVA) on condition x load. Where significant F-values were achieved, pairwise comparisons were performed using the Bonferroni post hoc procedure. Effect size statistic, η2, was analyzed to determine the magnitude of the effect independent of sample size. From the two familiarization trials, intra-class correlation coefficients were calculated for each dependent variable to determine test-retest reliability, obtaining values always greater than 0.92 (peak power output ICC: 0.94, peak acceleration ICC: 0.94, and EMGrms ICC: 0.93). Values are presented as means±standard deviation (SD).
Results
Kinematic parameters
A load x condition interaction effect (Figure 2) was noted for peak of power (PP) output (p=0.043; η2=0.074). There was a main effect of load indicating higher PP during the 50% vs 30% 1RM loads compared to the 70% 1RM (p<0.001; η2=0.477). There was a main effect for condition on PP (p=0.043; η2=0.074).
Figure 2.
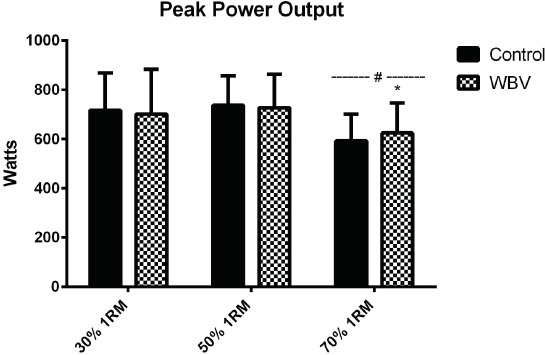
Peak power output during experiment conditions. # Significantly different than 30 % and 50 % 1RM loads (p<0.05).* Significantly different than control condition at the same load (p<0.05).
There was no load x condition interaction (p=0.922; η2=0.002) effect for peak acceleration (PA; Figure 3). A main effect of the condition indicates PA during WBV was increased vs CTRL (p=0.013; η2=0.142). A main effect of load was also observed indicating lower PA during 50% and 70% conditions vs 30% 1RM (p<0.001; η2=0.903).
Figure 3.
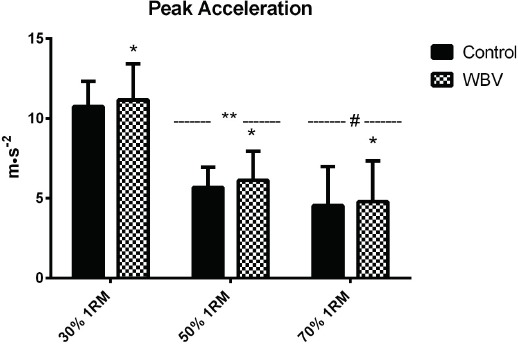
Peak acceleration during experiment conditions. * Significantly different than control condition (p<0.05).** Significantly different than 70 % and 30 % 1RM loads (p <0.05). # Significantly different than 30 % 1RM loads (p<0.05).
Surface electromyographic activity (EMG)
For TB EMGrms (Figure 4), there was no condition x load interaction on TB EMGrms (p=0.904; η2=0.003), though there was a main effect of the condition (p=0.038; η2=0.136) and load (p=0.030; η2=0.174). For condition, EMGrms during WBV was higher vs CTRL and for load the 70% 1RM EMGrms was higher vs 30% 1RM and 50% 1RM (p=0.006). For BB EMGrms, there was no condition x load interaction (p=0.091; η2=0.075) and no main effects for condition (p=0.934; η2<0.001) or load (p=0.399; η2<0.001).
Figure 4.
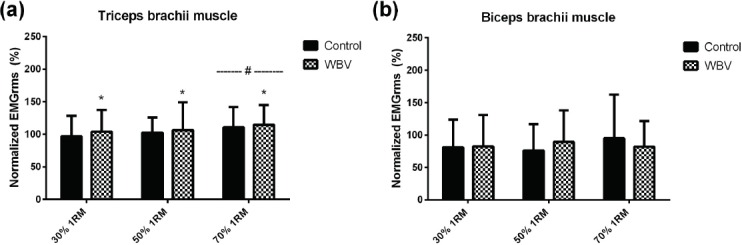
Normalized EMGrms data for triceps brachii muscle (a) and biceps brachii muscle (b). * Significantly different than control condition (p<0.05). # Significantly different than 30 % and 50 % 1RM loads (p<0.01).
For PM EMGrms (Figure 5), there was no condition x load interaction (p=0.121; η2=0.059) and no main effect for condition (p=0.114; η2=0.070). There was a main effect of load where EMGrms during the 70% 1RM condition was increased vs 50% 1RM and 30% 1RM (p<0.001; η2=0.199). For BF EMGrms Figure 5), there was no condition x load interaction (p=0.761; η2=0.008), but there were main effects for condition (p<0.001; η2=0.363) and load (p=0.010; η2=0.122). Within condition, BF EMG was higher during WBV vs CTRL. Within load, the 30%1RM condition was lower vs both 50%1RM (p=0.046) and 70%1RM (p=0.051) conditions.
Figure 5.
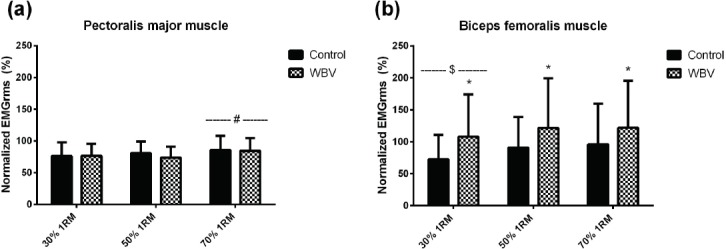
Normalized EMGrms data for pectoral major muscle (a) and biceps femoralis muscle (b). * Significantly different than control condition (p<0.001). # Significantly different than 30 % and 50 % 1RM loads (p<0.01). $ Significantly different than 50 % and 70 % 1RM loads (p<0.01).
Discussion
To the best of our knowledge, this is the first study to assess the effects of WBV applied through the legs on dynamic decline bench press performance. The major finding of the present study was that performing dynamic decline bench press exercise with WBV (applied through a hamstring bridge exercise) and a high load (70% 1RM) produced higher peak power values compared to the same exercise condition without WBV. In addition, while peak acceleration was higher at lower loads as expected, it was increased with WBV exposure. These effects of WBV on peak power and peak acceleration are partly explained by increases in triceps brachii muscle activity.
Exposure to WBV consistently demonstrates increases in lower body skeletal muscle activity while in a standing position on a WBV platform[15,17-20]. While increases in upper body muscle activity are less studied, we have recently demonstrated that exposure through the feet can benefit upper body exercises[21]. The current data extend these effects to WBV augmentation of decline bench press performance with WBV applied through the feet during a hamstring bridge exercise. While there was no effect of WBV during the lower loaded conditions (30% and 50% 1RM), the benefit of WBV during a higher loaded condition such as 70% 1RM is consistent with previous research on loaded WBV exercise 5, which demonstrated no effect of adding a light load (30% of body mass, ~25 kg) load during dynamic squatting. This may suggest the heavy load is increasing the sensitivity of Ia afferents in muscle spindles due to the preceding level of muscle activity[22,23].
Peak acceleration was also affected by WBV exposure. The increased muscle activity is likely contributing to the participants being able to lift the load faster. This increased muscle activity may represent increased muscle fibre activation, which increases the muscles ability to produce force. Other potential factors explaining why WBV exposure would increase peak acceleration include: decreased motor unit recruitment thresholds[24] resulting in an increased activation of the motor unit pool[25], and neural modifications to cortical and spinal areas responsible for excitatory responses during voluntary contractions[26-28]. While an increased motor unit synchronization is attractive possibility, previous research suggests it does not translate to increases in muscle strength[29]. Nevertheless, the level of contribution from the central nervous system remains speculative and requires further investigation.
The increase in triceps brachii muscle activity is in line with the abovementioned increases in peak power and acceleration with WBV exposure. The TB is a prime mover muscle for the decline bench press and increase in its muscle activity would be expected to contribute to increases in dynamic decline bench press performance. Interestingly, the current data also suggest that exposure to the TB muscle through a hamstring bridge with feet on the WBV platform is successful in transmitting the WBV stimuli to the upper body and still derive benefit. Further to the TB data, there was no effect of condition or load on biceps brachii muscle activity. This could be expected, as the BB is not a primary mover for the decline bench press. However, as the tonic vibration reflex stimulated by the WBV stimulus is likely responsible for the increase in TB muscle activity[30,31], this observation of no increase in BB activity suggests there is also no increased co-inhibition of antagonist muscles occurring. This decreased co-inhibition could also contribute to the improved peak power and acceleration.
With the increase in TB muscle activity, it is somewhat surprising that the pectoralis major muscle did not demonstrate an increase in EMG. As the PM muscle is the main primary mover muscle during a decline bench press, the demonstrated improvement in bench press performance would likely be mediated through this muscle. There are several potential reasons for the lack of activation. Perhaps the effect of WBV was inconsistent in this muscle resulting in a variation skewing the results (Type II error). The anatomical arrangement of the PM (fan-shaped) compared to the more longitudinal arrangement of the TB may also play a role in this lack of effect. Further, as the increase in power and acceleration with WBV exposure was not very large, our current data demonstrate the TB muscle response to WBV may be sufficient to mediate this response. It is also possible that the location of the EMG electrode over the mid-belly of the PM muscle could play a role in the lack of activation as a decline bench press would activate the lower portion of the PM.
The use of the hamstring bridge exercise to apply the WBV stimulus during a decline bench press was a novel aspect of the current study. The increases in biceps femoris muscle activity were expected due to its close proximity to the vibration stimulus and that the feet were in direct contact with the platform. Similar to the increase in quadriceps muscle activity during static and dynamic squatting seen in previous WBV research[15,17-19], our data are the first to our knowledge to demonstrate WBV during the hamstring bridge results in increased BF muscle activity. These results suggest the use of the hamstring bridge exercise with WBV applied through the feet as a multi-joint stimulus that increases both lower body (hamstrings) and upper body (triceps brachii) muscle activity and resultant improvements in peak power and acceleration.
Despite the large number of participants and repeated measures design, there were still several limitations of the present study. First, the intensity of the decline bench press was of low- to moderate-intensity (30-70% 1-RM) and future research should consider high-intensity loads (>80% 1-RM). Second, while the location of the electrodes followed standardized procedures, locations of the surface EMG electrodes on the TB and PM muscles may have affected the results and subsequent interpretation.
In summary, this study demonstrates that WBV applied through a hamstring bridge exercise increases triceps brachii muscle activity during a decline bench press and this augmentation contributes to an increased peak power at higher loads and increased peak acceleration at lower loads. This suggests decline bench press exercise benefits from WBV exposure, which could be important in training and athletic environments. To our knowledge, this is the first study to assess the effects of WBV applied through the legs on dynamic decline bench press performance.
Acknowledgements
The authors would like to thank all the participants for their excellent cooperation. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Footnotes
The authors have no conflict of interest.
Edited by: D. Cochrane
References
- 1.Cochrane DJ. Vibration exercise: the potential benefits. Int J Sports Med. 2011;32:75–99. doi: 10.1055/s-0030-1268010. [DOI] [PubMed] [Google Scholar]
- 2.Marin PJ, Rhea MR. Effects of vibration training on muscle power: a meta-analysis. J Strength Cond Res. 2010;24:871–8. doi: 10.1519/JSC.0b013e3181c7c6f0. [DOI] [PubMed] [Google Scholar]
- 3.Cochrane DJ, Stannard SR, Walmsely A, Firth EC. The acute effect of vibration exercise on concentric muscular characteristics. J Sci Med Sport. 2008;11:527–34. doi: 10.1016/j.jsams.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Marin PJ, Rhea MR. Effects of vibration training on muscle strength: a meta-analysis. J Strength Cond Res. 2010;24:548–56. doi: 10.1519/JSC.0b013e3181c09d22. [DOI] [PubMed] [Google Scholar]
- 5.Hazell TJ, Kenno KA, Jakobi JM. Evaluation of muscle activity for loaded and unloaded dynamic squats during vertical whole-body vibration. J Strength Cond Res. 2010;24:1860–5. doi: 10.1519/JSC.0b013e3181ddf6c8. [DOI] [PubMed] [Google Scholar]
- 6.Di Giminiani R, Fabiani L, Baldini G, Cardelli G, Giovannelli A, Tihanyi J. Hormonal and neuromuscular responses to mechanical vibration applied to upper extremity muscles. PLoS One. 2014;9:e111521. doi: 10.1371/journal.pone.0111521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gyulai G, Racz L, Di Giminiani R, Tihanyi J. Effect of whole body vibration applied on upper extremity muscles. Acta Physiol Hung. 2013;100:37–47. doi: 10.1556/APhysiol.99.2012.005. [DOI] [PubMed] [Google Scholar]
- 8.Wirth B, Zurfluh S, Muller R. Acute effects of whole-body vibration on trunk muscles in young healthy adults. J Electromyogr Kinesiol. 2011;21:450–7. doi: 10.1016/j.jelekin.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez Jimenez S, Benitez A, Garcia Gonzalez MA, Moras Feliu G, Maffiuletti NA. Effect of vibration frequency on agonist and antagonist arm muscle activity. Eur J Appl Physiol. 2015;115:1305–12. doi: 10.1007/s00421-015-3108-x. [DOI] [PubMed] [Google Scholar]
- 10.Wilson GJ, Newton RU, Murphy AJ, Humphries BJ. The optimal training load for the development of dynamic athletic performance. Med Sci Sports Exerc. 1993;25:1279–86. [PubMed] [Google Scholar]
- 11.Hoffman JR, Ratamess NA, Cooper JJ, Kang J, Chilakos A, Faigenbaum AD. Comparison of loaded and unloaded jump squat training on strength/power performance in college football players. J Strength Cond Res. 2005;19:810–5. doi: 10.1519/R-16774.1. [DOI] [PubMed] [Google Scholar]
- 12.McBride JM, Triplett-McBride T, Davie A, Newton RU. The effect of heavy- vs. light-load jump squats on the development of strength, power, and speed. J Strength Cond Res. 2002;16:75–82. [PubMed] [Google Scholar]
- 13.Toji H, Kaneko M. Effect of multiple-load training on the force-velocity relationship. J Strength Cond Res. 2004;18:792–5. doi: 10.1519/13933.1. [DOI] [PubMed] [Google Scholar]
- 14.Baechle TR, Earle RW. NCSA’s Essentials of Personal Training. 3rd ed. Champaign: Human Kinetics; 2008. [Google Scholar]
- 15.Marin PJ, Bunker D, Rhea MR, Ayllon FN. Neuromuscular activity during whole-body vibration of different amplitudes and footwear conditions: implications for prescription of vibratory stimulation. J Strength Cond Res. 2009;23:2311–6. doi: 10.1519/JSC.0b013e3181b8d637. [DOI] [PubMed] [Google Scholar]
- 16.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10:361–74. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 17.Abercromby AF, Amonette WE, Layne CS, McFarlin BK, Hinman MR, Paloski WH. Variation in neuromuscular responses during acute whole-body vibration exercise. Med Sci Sports Exerc. 2007;39:1642–50. doi: 10.1249/mss.0b013e318093f551. [DOI] [PubMed] [Google Scholar]
- 18.Cardinale M, Lim J. Electromyography activity of vastus lateralis muscle during whole-body vibrations of different frequencies. J Strength Cond Res. 2003;17:621–4. doi: 10.1519/1533-4287(2003)017<0621:eaovlm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Hazell TJ, Jakobi JM, Kenno KA. The effects of whole-body vibration on upper- and lower-body EMG during static and dynamic contractions. Appl Physiol Nutr Metab. 2007;32:1156–63. doi: 10.1139/H07-116. [DOI] [PubMed] [Google Scholar]
- 20.Marin PJ, Hazell TJ. Effects of whole-body vibration with an unstable surface on muscle activation. J Musculoskelet Neuronal Interact. 2014;14:213–9. [PubMed] [Google Scholar]
- 21.Marin PJ, Herrero AJ, Milton JG, Hazell TJ, Garcia-Lopez D. Whole-body vibration applied during upper body exercise improves performance. J Strength Cond Res. 2013;27:1807–12. doi: 10.1519/JSC.0b013e3182772f00. [DOI] [PubMed] [Google Scholar]
- 22.Burke D, Hagbarth KE, Lofstedt L, Wallin BG. The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol. 1976;261:695–711. doi: 10.1113/jphysiol.1976.sp011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mester J, Spitzenfeil P, Schwarzer J, Seifriz F. Biological reaction to vibration--implications for sport. J Sci Med Sport. 1999;2:211–26. doi: 10.1016/s1440-2440(99)80174-1. [DOI] [PubMed] [Google Scholar]
- 24.Romaiguere P, Vedel JP, Pagni S. Effects of tonic vibration reflex on motor unit recruitment in human wrist extensor muscles. Brain Res. 1993;602:32–40. doi: 10.1016/0006-8993(93)90237-h. [DOI] [PubMed] [Google Scholar]
- 25.Issurin VB TG. Acute and residual effects of vibratory stimulation on explosive strength in elite and amateur athletes. J Sports Sci. 1999;17(3):177–82. doi: 10.1080/026404199366073. [DOI] [PubMed] [Google Scholar]
- 26.Lee M, Carroll TJ. Cross education: possible mechanisms for the contralateral effects of unilateral resistance training. Sports Med. 2007;37:1–14. doi: 10.2165/00007256-200737010-00001. [DOI] [PubMed] [Google Scholar]
- 27.Hendy AM, Spittle M, Kidgell DJ. Cross education and immobilisation: mechanisms and implications for injury rehabilitation. J Sci Med Sport. 2012;15:94–101. doi: 10.1016/j.jsams.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Zhou S. Chronic neural adaptations to unilateral exercise: mechanisms of cross education. Exerc Sport Sci Rev. 2000;28:177–84. [PubMed] [Google Scholar]
- 29.Enoka RM, Fuglevand AJ. Motor unit physiology: some unresolved issues. Muscle Nerve. 2001;24:4–17. doi: 10.1002/1097-4598(200101)24:1<4::aid-mus13>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 30.Pollock RD, Woledge RC, Martin FC, Newham DJ. Effects of whole body vibration on motor unit recruitment and threshold. J Appl Physiol. 2012;112:388–95. doi: 10.1152/japplphysiol.01223.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ritzmann R, Kramer A, Gruber M, Gollhofer A, Taube W. EMG activity during whole body vibration: motion artifacts or stretch reflexes? Eur J Appl Physiol. 2010;110:143–51. doi: 10.1007/s00421-010-1483-x. [DOI] [PubMed] [Google Scholar]


