Abstract
Fusionless devices are currently designed to treat spinal deformities such as scoliosis by the application of a controlled mechanical loading. Growth modulation by dynamic compression was shown to preserve soft tissues. The objective of this in vivo study was to characterize the effect of static vs. dynamic loading on the bone formed during growth modulation. Controlled compression was applied during 15 days on the 7th caudal vertebra (Cd7) of rats during growth spurt. The load was sustained in the “static” group and sinusoidally oscillating in the “dynamic” group. The effect of surgery and of the device was investigated using control and sham (operated on but no load applied) groups. A high resolution CT-scan of Cd7 was acquired at days 2, 8 and 15 of compression. Growth rates, histomorphometric parameters and mineral density of the newly formed bone were quantified and compared. Static and dynamic loadings significantly reduced the growth rate by 20% compared to the sham group. Dynamic loading preserved newly formed bone histomorphometry and mineral density whereas static loading induced thicker (+31%) and more mineralized (+12%) trabeculae. A significant sham effect was observed. Growth modulation by dynamic compression constitutes a promising way to develop new treatment for skeletal deformities.
Keywords: Mechanical Growth Modulation, Calcification Zone, Endochondral Ossification, Spine Deformity
Introduction
Mechanical loading influences bone growth. Delpech[1] and later Hueter[2] and Volkmann[3] stated that an increased compression on the epiphyseal growth plates slowed longitudinal growth whereas growth was promoted by a release of pressure. Based on this principle, new fusionless devices have been developed for the treatment of progressive deformities such as idiopathic scoliosis[4,5]. By applying a localized loading on the vertebral growth plates, these implants exploit the residual growth potential to guide the growing spine towards realignment at maturity. Recently, anterior vertebral body tethering has proven its efficiency in correcting moderate scoliotic curves (Cobb angle ≈ 40°) for skeletally immature patients[6]. During this surgery, a flexible tether is attached via pedicle screws on the convex side of the spine, increasing compression on that side and releasing compression on the concave side. This device maintains most of the physiological loadings of the spine and preserves disk health[7,8] but the long-term impact of the additional compressive loading on the surrounding tissues, such as the intervertebral disk, growth plate or newly formed bone, is still unknown.
Indeed, in vivo and in vitro studies on animal models have shown that the type (static or dynamic) and the amplitude of mechanical compression affect the response of the intervertebral disk and of the growth plate. With loadings within physiological ranges, static (or sustained) compression leads to intervertebral disk degeneration[9-11], disorganization of the chondrocytes columnar arrangement and modified protein expression in the growth plate[12-16] and loss of chondrocyte viability when the load is sustained for a long time[17]. Conversely, dynamic compression better preserves the integrity of the intervertebral disk[10,11] and the mechanobiology of the growth plate[13,15-17], while achieving the same growth rate reduction. The effect of static and dynamic loadings on remodelling or adaptation processes has already been studied[18]. However, to our knowledge, the resulting impact of static vs. dynamic compression on the quality of the bone formed during the growth modulation period has not been investigated.
During longitudinal growth, bone is formed at the end of the growth plate by a progressive mineralization of the collagen matrix. Mineralization starts in the lower hypertrophic zone of the growth plate, near terminal chondrocytes. Crystals of hydroxyapatite form in matrix vesicles in the longitudinal septa between the columns of chondrocytes and then proliferate into the cartilage matrix. After vascular invasion, transverse septa are removed while osteoblasts deposit new bone on the surface of the calcified longitudinal septa[19,20]. These templates containing bone and mineralized matrix form the newest primary spongiosa, which is the region of interest for our study. This region is then actively remodelled by the combined action of osteoclasts and osteoblasts to generate more mature primary spongiosa, recognizable by larger marrow cavities and increased mineralization of its trabeculae[19-21].
Rat tail models have been extensively used to study growth modulation by mechanical loading[15,22-24]. Indeed, caudal vertebrae are easily accessible and external fixators can be inserted in targeted vertebrae in order to apply various types of forces. In particular, loading apparatus, calibration procedures and levels of force necessary to modulate bone growth by dynamic compression have been well established[13,15].
This in vivo study, which differed from the previously published studies, aimed at assessing the effect of bone growth modulation by static vs. dynamic compression on the quality of the newly formed bone by endochondral ossification. The specific goals were (1) to evaluate longitudinally, using an in vivo approach, the growth rate reduction achieved by static vs. dynamic compressive loadings; and (2) to quantify the impact of the type of compression on the quality (morphology and mineralization) of the newest primary spongiosa formed during mechanical growth modulation.
Material and methods
Animal model and loading conditions
With the approval of the Institutional Animal Care Committee, 24 male Sprague–Dawley rats were received at the age of 21 days old and were randomly divided into four groups of rats: control, sham, static and dynamic (n=6 per group). After one week of acclimatization, the sham, static and dynamic groups were operated on at the age of 28 days old for a growth modulation period of 15 days centered on their pubertal growth spurt[25,26]. A normalized and finely controlled compression was applied during 15 days on the 7th caudal vertebra (Cd7) of rats from the static and dynamic groups using the growth modulation device presented in Figure 1a. The compression was sustained (0.2 MPa) in the static group and sinusoidally oscillating (0.2 MPa ± 30%, frequency = 0.1 Hz) in the dynamic group. This magnitude of compressive loading, considered within normal physiological range[16], was chosen to slow but not arrest growth[15]. The sham group was operated on but no load was applied in order to investigate the effect of surgery and device implantation. The control group did not undergo any surgery. Calibration and daily adjustments of the device were required to apply a controlled and similar loading during the 15 days of experiment. The protocol and the calibration procedure were previously described[13,15].
Figure 1.
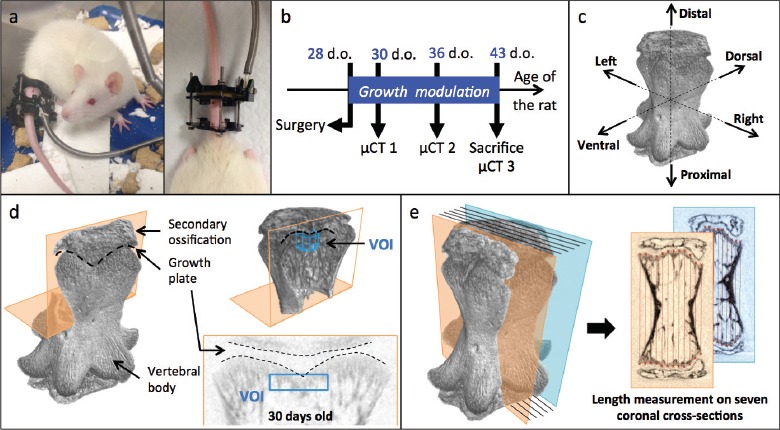
Experimental protocol. a. Growth modulation rat tail device; b. Timeline of the micro-CT acquisitions; c. Reconstructed 7th caudal vertebra; d. Location of the volume of interest (VOI) for histomorphometry analysis; e. Vertebral length measurement.
µCT acquisitions and animal positioning
To evaluate bone histomorphometry, a high-resolution computed tomography (µCT) of Cd7 was performed in vivo at the age of 30 and 36 days old and ex vivo at the age of 43 days old, immediately after euthanasia (Figure 1b). Although growth plate is an area of high metabolic activity, the first scan was performed two days after surgery, for ethical reasons, in order to allow animals enough time to recover from surgery and major anaesthesia. The imaging system was a Skyscan 1176 in vivo µCT (Skyscan, N.V., Belgium) with rotatable X-ray source and detector. The animal was placed in a carbon fiber half-tube bed, which slid into the µCT chamber for imaging. For the control group, the rat tail was placed in polystyrene tubes provided by the manufacturer to position the vertebra at the scanning midline and to prevent any movement of the tail during scanning. For the other groups, the growth modulation device was fixed to a custom-made plastic adapter temporarily fixed to the scanning bed and metallic screws were replaced by plastic screws in the loading device. A resolution of 8.74 µm was chosen to observe in detail the newly formed bone in Cd7, which was completely included in the scanning region. A 1 mm aluminum filter was used to limit the tail exposure to high-energy X-rays. The source voltage was set to 65 kV and the source current to 385 µA as advised by the manufacturer. Using a rotation step of 0.5°, an exposure time of 1.41 s and no frame averaging, the acquisition time for a 180° scan was 12 minutes. During scanning, rats were kept anesthetized with isoflurane. No metal artifacts were observed on the images.
The cross-section reconstruction was performed using a filtered back-projection algorithm (NRecon software, V.1.6.9, Skyscan)[27]. Depending on the vertebral dimensions and orientation, a stack of 600 to 1100 square 8-bit cross-sections was reconstructed for each scan, their width varying between 530 and 880 pixels (4.6 to 7.6 mm). The inter-slice distance was of 1 pixel (8.74 µm), leading to a maximum height of 9.6 mm for the reconstructed volume. Figure 1c shows a typical reconstructed volume for a 43 days-old rat 7th caudal vertebra from the control group.
Histomorphometric analysis and growth rate measurements
The new bone formed by endochondral longitudinal growth was located in the metaphysis of the vertebral body, adjacent to the hypertrophic zone of the growth plate (Figure 1d)[21,28]. The volume of interest (VOI) extracted for histomorphometric analysis was a cylinder oriented along the principal axis of the vertebra and located below the distal growth plate (Figure 1d). The VOI started at the first cross-section in the vertebral body below the growth plate and ended 30 cross-sections further inside the vertebral body (260 µm in thickness); its diameter was 120 pixels (1.05 mm). Using vertebral anatomical landmarks, reconstructed vertebrae were carefully repositioned in 3D in order to align the axis of the VOI with the axis of the vertebra and to ensure reproducibility of VOI selection. Centering the VOI on the vertebral axis also limited the potential bias due to an asymmetric loading that might occur from this loading paradigm. The VOI contained essentially new primary spongiosa, still in the process of calcification, and a small region of more mature primary spongiosa, with trabeculae emerging parallel to the vertebral axis.
The dimensions of the VOI were chosen from scans of 30-days-old rats to include mainly the newest primary spongiosa. Since this zone of provisional calcification was irregular, the VOI also included more mature primary spongiosa, which had already been remodelled, especially at the ages of 36 and 43 days old.
For calculation of the structural parameters, images were segmented using a uniform threshold algorithm. The threshold was adapted in function of the age of the rats in order to select a good representation of the actual bone structure for each age, following the guidelines in following the guidelines from Bouxsein et al.[29]: gray level thresholds of 44, 46 and 48, corresponding to equivalent densities of 0.266 g/cm3, 0.280 g/cm3 and 0.295 g/cm3 of calcium hydroxyapatite (CaHA), were respectively set for 30, 36 and 43 days-old rats. These values were chosen to include most of the trabeculae in the selection and to prevent the selection of non-bone regions. The following parameters were calculated over each VOI using 3D morphometric analysis in CT Analyzer software[27,29]: bone volume fraction (BV/TV), direct trabecular thickness (Tb.Th), direct trabecular separation (Tb.Sp), trabecular number (Tb.N), structure model index (SMI), degree of anisotropy (DA) and trabecular bone pattern factor (Tb.Pf). BV/TV, representing the proportion of bone in the VOI, was calculated using the marching cubes method[30]. Computed using the local sphere-fitting method, Tb.Th measures the average thickness of trabeculae and Tb.Sp the average diameter of the cavities[27,31]. Tb.N is the linear density of trabeculae in the VOI and was calculated from BV/TV and Tb.Th using the following equation: Tb.N = (BV/TV) / Tb.Th. SMI indicates if the structure is more plate-like (SMI ≈ 0) or rod-like (SMI ≈ 3)[31]. DA quantifies the anisotropy of the VOI, ranging from 0 for total isotropy to 1 for total anisotropy. Finally Tb.Pf is a 3D index to characterize connectivity; a low value of Tb.Pf corresponds to a highly connected structure whereas a high value of Tb.Pf means a more disconnected tissue.
Bone mineral density (BMD) and tissue mineral density (TMD) were also calculated for each VOI. BMD is the density of CaHA in the complete VOI, including bone and marrow, and is typically measured for trabecular bone regions. TMD is the density of CaHA inside the bone-segmented regions of the VOI and assesses the material density of the bone itself. Calibrations were necessary to establish a relationship between known CaHA densities and X-ray attenuation coefficients. After every scanning session (generally grouping three samples), a pair of 4 mm rods with known mass concentrations of CaHA was scanned in the same conditions as the samples. The rods were embedded in a wet tissue and placed in an Eppendorf tube to mimic tissues surrounding the caudal vertebra. After reconstruction of the cross-sections, the X-ray attenuation coefficients were measured for all acquisitions. The average values of the obtained attenuation coefficients were used to calibrate the BMD and TMD for all samples.
The length of the vertebral body was assessed, before segmentation, for all samples using a custom-made Matlab software (see Figure 1e)[31] on seven equidistant coronal cross-sections distributed over the vertebra. To do so, the extremities of the vertebral body were first manually identified and modeled as splines. The length of 12 segments parallel to the axis of the vertebra was then automatically calculated for each cross-section. The length of the vertebral body was defined as the mean value of these 84 measurements (7 cross-sections x 12 segments/cross-section) and was assessed for every animal at 30, 36 and 43 days old. To obtain the mean growth rate for a selected period (i.e. between 30 and 36 days old or between 36 and 43 days old), the difference in vertebral length was divided by the number of days between the two corresponding µCT scans (i.e. six or seven days). Thus, the measured mean growth rate comprised the effects of both distal and proximal vertebral growth plates.
Statistical analysis
Statistical analyses were conducted for weight, growth rate and histomorphometric measurements in order (1) to compare the sham group to the control group at each time point to assess the impact of instrumentation only and (2) to detect any difference between the three instrumented groups at each time point and characterize the specific effect of each type of mechanical loading. To that purpose, two-way Repeated Measures ANOVAs were performed over the evaluated parameters, first, from the sham and control groups and second, from the sham, static and dynamic groups. Then, for both statistical analyses, Holm-Sidak’s multiple comparisons test was performed to characterize, at each time point, the difference between each group included in the analysis[32]. A p-value less than 0.05 was considered statistically significant. The analyses were performed with Graphpad Prism (v 6.0).
Results
The mean and standard deviation of the evaluated parameters are presented in [Table 1] for each group and each time point.
Table 1.
Overview of the different parameters (mean ± SD) for each group and each time point. Comparisons between groups for each time point. cp<0.05, compared to the control group; shp<0.05, compared to the sham group; stp<0.05 compared to the static group and dp<0.05 compared to the dynamic group.
| Age (days old) | Control (n=6) | Sham (n=6) | Static (n=6) | Dynamic (n=6) | |
|---|---|---|---|---|---|
| Weight (g) | 30 | 119 ± 20 | 107 ± 10 | 108 ± 14 | 104 ± 10 |
| 36 | 180 ± 28 | 161 ± 20 | 167 ± 23 | 157 ± 18 | |
| 43 | 253 ± 35 | 222 ± 37 | 233 ± 40 | 216 ± 25 | |
| Growth rate (µm/day) | 30 → 36 | 158 ± 25 sh | 131 ± 10 c,st,d | 103 ± 13 sh | 102 ± 10 sh |
| 36 → 43 | 120 ± 17 sh | 83 ± 14 c,d | 70 ± 8 | 67 ± 5 sh | |
| BMD (g.cm-3) | 30 | 279 ± 28 | 268 ± 44 | 274 ± 40 | 291 ± 55 |
| 36 | 148 ± 27 | 127 ± 23 | 147 ± 33 | 149 ± 20 | |
| 43 | 171 ± 28 sh | 89 ± 19 c | 107 ± 36 | 101 ± 28 | |
| TMD (g.cm-3) | 30 | 441 ± 22 | 449 ± 23 | 459 ± 25 | 426 ± 44 |
| 36 | 458 ± 29 | 441 ± 21 | 469 ± 12 | 437 ± 31 | |
| 43 | 505 ± 18 sh | 446 ± 37 c | 500 ± 36 | 461 ± 21 | |
| BV/TV (%) | 30 | 34.5 ± 5.7 | 33.8 ± 9.8 | 36.0 ± 9.7 | 39.4 ± 13.0 |
| 36 | 10.7 ± 3.4 | 5.9 ± 8.1 | 9.4 ± 5.3 | 10.5 ± 4.3 | |
| 43 | 13.1 ± 3.8 sh | 3.1 ± 2.6 c | 5.5 ± 5.6 | 4.5 ± 3.7 | |
| Tb.Th (µm) | 30 | 49 ± 1 | 55 ± 8 | 58 ± 9 | 52 ± 6 |
| 36 | 40 ± 6 | 42 ± 7 | 47 ± 6 | 43 ± 6 | |
| 43 | 56 ± 14 sh | 42 ± 13 c,st | 55 ± 14 sh,d | 40 ± 7 st | |
| Tb.Sp (µm) | 30 | 90 ± 26 | 113 ± 21 | 123 ± 19 | 97 ± 37 |
| 36 | 199 ± 27 sh | 232 ± 16 c | 212 ± 28 | 204 ± 26 | |
| 43 | 200 ± 26 sh | 257 ± 10 c | 251 ± 18 | 245 ± 19 | |
| Tb.N (1/mm) | 30 | 7.3 ± 1.6 | 6.1 ± 1.2 | 6.2 ± 1.3 | 7.5 ± 2.1 |
| 36 | 2.7 ± 1.0 | 1.4 ± 0.6 | 2.0 ± 1.0 | 2.4 ± 0.8 | |
| 43 | 2.4 ± 0.9 sh | 0.7 ± 0.5 c | 0.9 ± 0.7 | 1.0 ± 0.8 | |
| SMI | 30 | 1.1 ± 0.4 | 0.8 ± 0.6 | 0.3 ± 0.8 | 0.4 ± 0.9 |
| 36 | 1.7 ± 0.3 | 2.1 ± 0.2 | 2.1 ± 0.5 | 1.8 ± 0.4 | |
| 43 | 1.9 ± 0.4 | 2.2 ± 0.5 | 2.2 ± 0.9 | 2.1 ± 0.6 | |
| DA | 30 | 0.29 ± 0.11 | 0.37 ± 0.07 | 0.40 ± 0.08 | 0.33 ± 0.06 |
| 36 | 0.55 ± 0.09 | 0.66 ± 0.18 | 0.55 ± 0.11 | 0.66 ± 0.08 | |
| 43 | 0.63 ± 0.09 | 0.77 ± 0.10 | 0.66 ± 0.14 | 0.68 ± 0.12 | |
| Tb.Pf (1/mm) | 30 | -9 ± 7 | -10 ± 11 | -19 ± 16 | -20 ± 18 |
| 36 | 11 ± 11 | 20 ± 10 | 22 ± 13 | 12 ± 14 | |
| 43 | 8 ± 10 sh | 36 ± 27 c | 21 ± 14 | 28 ± 31 |
Weight
The four groups presented similar weight at each time point (Table 1).
Growth rate reduction
Device implantation and mechanical loading affected the longitudinal growth rate (Table 1, Figure 2).
Figure 2.
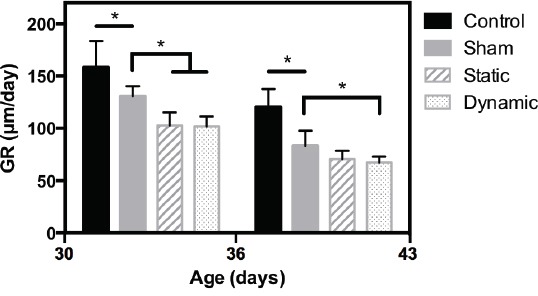
Growth rate (GR) measurements. *p<0.05.
Compared to the control group, the mean growth rate in the sham group was reduced by 17% between 30 and 36 days old and by 31% between 36 and 43 days old. In comparison with the sham group, static and dynamic loadings resulted in a similar decrease in growth rate: -21% for the static group and -22% for the dynamic group between 30 and 36 days old; -15% for the static group and -19% for the dynamic group between 36 and 43 days old. All the observations were statistically significant except the difference between static and sham groups in the mean growth rate measured between 36 and 43 days old.
Morphological evolution
Figure 3 shows the evolution of the microstructure in the VOI for the different groups while the corresponding evolution of the histomorphometric parameters is presented in Figure 4.
Figure 3.
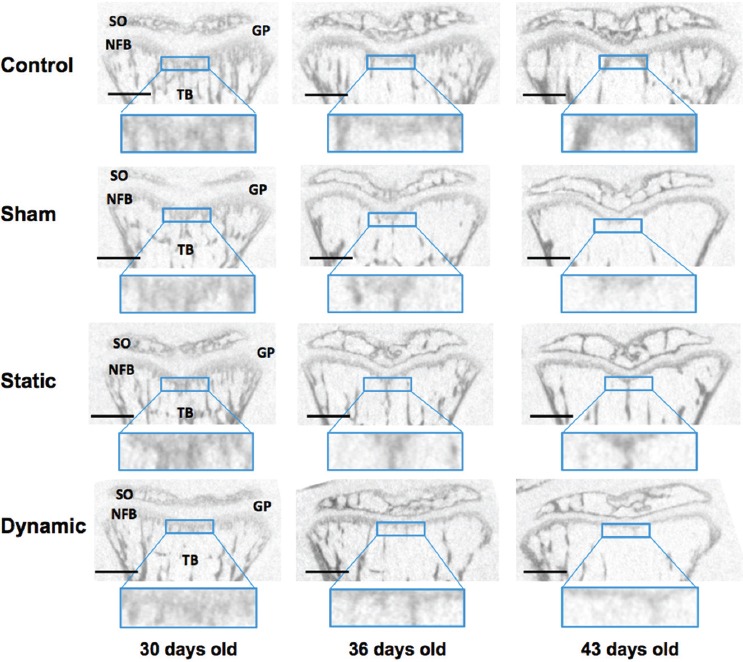
Typical evolution of bone microstructure on sagittal cross-sections. SO: secondary ossification; GP: growth plate; NFB: newly formed bone; TB: trabecular bone. Scale bar: 1 mm.
Figure 4.
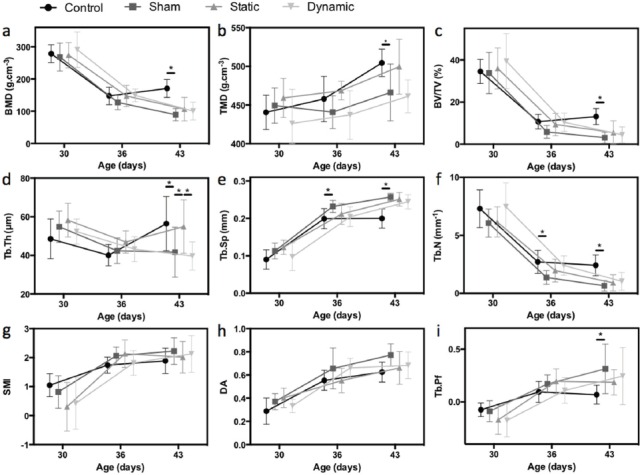
Histomorphometric analysis. a. Bone mineral density (BMD); b. Tissue mineral density (TMD); c. Bone volume fraction (BV/TV); d. Direct trabecular thickness (Tb.Th); e. Direct trabecular separation (Tb.Sp); f. Trabecular number (Tb.N); g. Structure model index (SMI); h. Degree of anisotropy (DA); i. Trabecular bone pattern factor (Tb.Pf); * p<0.05.
Normal evolution of the microstructure with growth
Between 30 and 43 days old, the microstructure inside the VOI evolved for all groups (see Figure 3). The modifications observed for the control group (first line in Figure 3 and black circles in Figure 4) illustrate the normal evolution with growth. At 30 days old, the VOI was mostly formed of new primary spongiosa. It contained about 35% of bone tissue (Figure 4c), which was highly connected (low value of Tb.Pf in Figure 4i, high trabecular number in Figure 4f) and poorly mineralized (low value of TMD in Figure 4b). BMD was high (Figure 4a) because of the elevated percentage of bone tissue in the VOI. No principal orientation of the trabeculae could be distinguished and the microstructure looked rather isotropic (low value of DA in Figure 4h). At 36 days old, the region of new primary spongiosa was reduced while small and slim trabeculae emerged from this region towards the vertebral body. This evolution was highlighted by the decrease of BV/TV, BMD and Tb.Th (Figure 4c, a and d) and by the increase of Tb.Sp (Figure 4e). Tb.N also declined (Figure 4f) and became representative of the number of trabeculae emerging from the primary spongiosa. Tb.Pf increased (Figure 4i), thus illustrating the apparition of the more disconnected trabeculae. Between 36 and 43 days old, the region of new primary spongiosa preserved its shape and thickness while emerging trabeculae were strengthened (becoming thicker - Figure 4d - and more mineralized - Figure 4b). The continuous increase of TMD between 30 and 43 days old (Figure 4b) pointed out the progressive mineralization of the bone tissue during growth. The evolution of the parameters SMI (Figure 4g) and DA (Figure 4h) illustrated the transition from a quasi-isotropic plate-like structure at 30 days old to a more anisotropic rod-like structure with well-defined trabeculae at the end of growth.
Sham effect
Except for SMI and DA, all histomorphometric parameters evaluated in the sham group were significantly different from the parameters of the control group at 43 days old (Figure 4, Table 1). Significant differences appeared from 36 days old for Tb.Sp and Tb.N. Therefore, compared to the controls, at 43 days old, the newly formed bone region in the sham group contained less bone tissue which was less calcified. Even if the general shape of the microstructure was similar (comparable SMI and DA), the VOI contained less trabeculae, which were thinner and more spaced than in the control group. The thickness of the new primary spongiosa region also seemed reduced in the µCT cross-sections from the sham group (Figure 3).
Impact of static vs dynamic compressive loadings
The evolution of trabecular thickness differed according to the type of mechanical loading (Table 1, Figure 4d). At 43 days old, compared to the sham group, Tb.Th was significantly higher in the static group, whereas no difference was observed with the dynamic group. A similar trend was observed for TMD (Table 1, Figure 4b) but the difference between the sham and static groups did not reach statistical significance.
When comparing static and dynamic groups at 43 days old, a significant difference was observed for Tb.Th. A different trend was also observed for TMD between static and dynamic groups but the comparison between groups at 43 days old did not reach the threshold for statistical significance.
As shown in Figure 3, animals from the sham and dynamic groups presented similar microstructure at the end of the growth modulation period whereas animals from the static group exhibited a thicker zone of primary spongiosa, larger trabeculae and more calcified bone tissue.
Discussion
This study investigated the impact of bone growth modulation by static and dynamic compressions on the quality of the newly formed bone by endochondral ossification. Growth rate measurements attested the efficiency of the growth modulation procedure, whereas histomorphometric analyses of the calcification zone pointed out different bone quality according to the type of applied mechanical loading.
Growth reduction by static and dynamic compressions
Dynamic and static compressions induced similar decreases in growth rates (about 20% compared with the sham group) after 8 and 15 days of loading. This result is consistent with previous studies using similar animal models and loading devices, conducted after 7 days of static loading[23] or after 15 days of static or dynamic loadings[12,13,15,33]. In the present study, the methodology for growth rate measurement was different from traditional bone labeling techniques and took into account the growth from both proximal and distal growth plates. To calculate the resulting growth rate for one side of the vertebra (i.e. one growth plate), as classically reported, the values of this study should be divided by two. Thus, after division, the obtained growth rate values are close to the literature[12,13,15,23,33], in particular for the three instrumented groups. This methodology, compared to the ones using fluorochromes as intravital bone marker, presents the advantage of being performed longitudinally in vivo without animal sacrifice. It could however induce some inaccuracy because of the irregular shape of the vertebra and the limited resolution of the µCT. Thus, larger animal groups may be needed to achieve the same level of statistical significance as in previous studies, in particular when the growth rate is lower (i.e. between 36 and 43 days old).
Static loading promotes ossification of the newly formed bone
The impact of static compression over the newly formed bone was assessed by comparison with the sham group. Static loading modified the newly formed bone: after 15 days of sustained compression, the newest primary spongiosa and the trabeculae were thicker and more calcified for the static group. Hence, ossification was promoted by static compression as suggested by Reich et al.[14,34] on a chick model. In their study, chicks were carrying sand bags to increase the compressive stress on their hind limb growth plates. Reich et al. suggested that increased compression on the growth plate accelerated ossification through overexpression of OPN, MMPs and increased vascularization, thus contributing to the reduction of the growth plate height and finally of the growth rate[14]. Their observations were though conducted at the level of gene and protein expressions. They observed an upregulation of osteopontin (OPN) and a deregulation of proteins (Alkaline phosphatase, Gla protein) involved in cartilage and bone mineralization at the chondro-osseous junction in comparison with controls[14,34]. Matrix metalloproteinases, and particularly MMP3, MMP9 and MMP13 are essential players in bone development and remodeling due to their role as extracellular matrix degrading enzymes[35], promoters of angiogenesis[20,36] and of hypertrophic chondrocytes apoptosis[37]. Reich et al.[14] observed an increase in the gene expression of MMP9 and MMP13 associated with enhanced vascularization of the lower growth plate. On the other hand, Cancel et al.[12] also observed an increase of MMP3 but no significant change for MMP13 expression in rat vertebral growth plate subjected to static loading. In vitro studies from our group have however demonstrated an increase in MMP13 expression concomitant with type II collagen decrease on ulnar growth plate explants under static loading using similar parameters[38]. All these modifications in regulatory mechanisms are consistent with an early ossification of bones subjected to growth modulation by static compression.
Dynamic compression preserves newly formed bone histomorphometry
As exhibited in bone histomorphometry analyses, the evolution of the ossification zone was very similar for the sham and dynamic groups, suggesting that dynamic compression does not alter endochondral ossification when load is applied within physiological ranges. To our knowledge, this is the first analysis of the newly formed bone histomorphometry after growth modulation by dynamic compression. Our observations are in agreement with data obtained on the growth plate by Valteau et al.[15] and Tang and Mao[39]. Using similar loading apparatus and animal model as our study, Valteau et al.[15] reported a more preserved histomorphometry of the growth plate when growth modulation is based on dynamic rather than static compression. Tang and Mao[39] did not detect changes in the gene expression of calcification markers, such as OPN or osteocalcin (OCN), and in matrix molecules of the cranial base growth plate subjected to a cyclic loading except for decorin proteoglycan, which was more specifically expressed near the perichondrium. Taken together, these results suggest that the regulation of the growth rate induced by dynamic compression is less detrimental for the growth plate and does not affect the quality of the newly formed bone. Hence, dynamic loading would be more respectful of the physiology of the tissue and should be privileged in the development of fusionless treatment of deformities such as scoliosis.
Sham effect observed on growth rate and histomorphometric parameters
The insertion of the apparatus without applying any mechanical loading had an impact on the growth rate, as also found, but to a lesser extent in other studies[12,13,15,23,40]. The statistical significance observed in our study may be related to the high inter-individual variability of the longitudinal growth rate. To reduce the impact of inter-individual variability, we could use internal controls for growth rate measurements and normalize the growth rate measured on Cd7 with the growth rate measured on an adjacent vertebra not affected by the device (e.g. Cd5). Indeed, the growth rates observed for the control group in our study were higher than growth rates classically reported in the literature and the normalization of the growth rate with internal controls might bring closer the sham and control groups.
Wearing the growth modulation device was also detrimental to endochondral ossification. Indeed, at 43 days old, all histomorphometric parameters except DA and SMI were impaired in the sham group. At the end of the experiment, the first zone of newest primary spongiosa was very thin and the second region of more mature primary spongiosa presented scant trabeculae, which were thinner and less calcified than in the control group. This could be explained by stress-shielding induced in Cd7 by the rigidity of the loading device. Indeed, rats moved freely in their cage but physiological stresses related to daily activities transferred likely through the device between the sixth and eighth caudal vertebrae instead of via Cd7. Observations regarding TMD and BMD parameters are consistent with data from Morey-Holton and Globus[41] who reported that unloading hind limbs of rats induced weight loss and increased the amount of immature bone in the unloaded bones. Conversely, Menard et al.[33] obtained comparable Alizarine red semi-quantifications of calcification in the early calcification zone in shams using a similar growth modulation device and controls. These differences may be due to the observation scale. Indeed, the VOI of our study corresponded to the “radiological calcification zone” reported by Tsai et al.[21], which is intermediate between the “early calcification zone” studied by Menard et al.[33] and the observations on the complete bone reported by Morey-Holton and Globus[41]. Thus, our observations included cartilage matrix resorption followed by generation and probably early remodeling of the new bone whereas the observations of Menard et al. were related to mineralization initiation, in the lower hypertrophic zone and at the beginning of the longitudinal septae at the vicinity of the growth plate.
Some precautions should prevail in the interpretation of the results because of this sham effect. Indeed, the level of stress achieved in the static group (0.2 MPa) was sufficiently high to promote early ossification in the chondro-osseous junction and to form trabeculae with similar characteristics (thickness and tissue mineral density) as those of the controls. However, this stress level is far below stresses related to daily life activities[23] so its impact may be reduced if this sustained compression is added to normal physiological loadings. We also showed that dynamic loading had little impact on the calcification process, even on poorly loaded vertebrae. We expect thus that dynamic loading would not affect the quality of the bone after growth modulation and this, including vertebrae subjected to physiological loadings.
However, the sham effect should be reduced in future studies in order to better characterize the impact of growth modulation on a physiologically loaded bone. Ohashi et al.[42] reported that even short periods (10 min/day) of cyclic compression reduced growth. In this sense, the device may be adapted to reduce the time of growth modulation and preserve periods for daily life solicitations of the vertebra outside modulation.
Limitations
Regarding histomorphometry analysis, the thickness of the VOI was limited to 260 µm in order to focus on the newest primary spongiosa, also called the zone of provisional calcification by Tsai et al.[21]. According to Tsai et al.[21], the size of the zone of provisional calcification is about 100 µm when referred to in the pathology literature whereas it is about 450 µm when referred to in the imaging literature (where the typical resolution is 50 µm). In our study, we chose an intermediate thickness of 260 µm because:
-
1)
it was consistent with the µCT resolution (8.5 µm isotropic);
-
2)
it corresponded to the thickness of the newest primary spongiosa for 30 d.o. rats.
This limited thickness of the VOI could affect the values obtained for different parameters and especially Tb.Sp, which was probably underestimated for the sham group at 43 days old. However, very little bias was expected on Tb.Th since several trabeculae were present in every VOI.
Moreover, three different thresholds were chosen to segment bone for 30, 36 and 43 day old rats in order to provide a good representation of the actual bone structure for each age. Using different thresholds could have impacts in a longitudinal evaluation of the histomorphometric parameters, where a unique threshold should be selected to compare quantitatively two different time points. However, in our study, the comparisons between groups were made for each time point separately. Hence, using three different thresholds for three different time points did not alter the statistical analyses and the conclusions of this study.
Although our study focused on bone formation, the VOI also included more mature primary spongiosa, which had been remodelled, especially at the ages of 36 and 43 days old. Thus, our observations concerned both the endochondral ossification and the early stages of bone remodelling. The region of interest was not adapted to characterize the effect of the type of loading on bone remodelling and on the quality of mature trabecular bone since it was not the subject of our study.
Regarding mechanical loading, the device applied uniaxial compression along one vertebra. This loading paradigm cannot simulate accurately any asymmetrical compression or distraction force or any bending moment that would occur in a human spine deformity involving multiple segments. Also, the static and dynamic compression were only tested at a certain controlled magnitude and frequency and the result might be different if other settings were used. Finally, this work was conducted immediately after growth modulation at a period corresponding to the end of adolescence. It would be interesting to investigate the long-term impact of growth modulation by static and dynamic compression on the quality of bone after a period without any growth modulation. The resumption of growth and the remodeling processes may indeed differ according to the applied treatment and affect the quality of bone after ceasing the growth modulation treatment.
Conclusion
This study showed that bone growth could be reduced by static and dynamic compressions applied in physiological ranges. The histomorphometry of the newly formed bone was preserved under growth modulation by dynamic compression, where a compressive loading of 0.2 MPa ± 30% was applied at a frequency of 0.1 Hz, whereas trabeculae were thicker and more mineralized when growth modulation was applied by static compression. Thus, endochondral ossification was disrupted by static loading whereas the impact of dynamic loading was negligible. Growth modulation by dynamic compression constitutes a promising way to develop new fusionless treatment for deformities affecting children and adolescent such as scoliosis. Future studies should focus on long-term quality of bone in order to observe if bone formation is altered even after ceasing the growth modulation treatment.
Acknowledgement
The authors acknowledge helpful contributions and technical skills of laboratory team members N. Aimene and S. Rhalmi, as well as Ste-Justine hospital’s animal care technicians. This work has been carried out thanks to the support of the Research Group in Biomedical Sciences and Technologies (Fonds de recherche du Québec – Santé), of the MEDITIS program (Natural Sciences and Engineering Research Council of Canada) and of the A*MIDEX project (n° ANR-11-IDEX-0001-02) funded by the «Investissements d’Avenir» French Government program, managed by the French National Research Agency (ANR).
Footnotes
The authors have no conflict of interest.
Edited by: F. Rauch
References
- 1.Delpech J. De l’Orthomorphie. Paris: 1828. [Google Scholar]
- 2.Hueter C. Anatomische studien an den extremitatengelenken neugeborener und erwachsener. Virchows Arch Pathol Anat Physiol. 1862;25:572–99. [Google Scholar]
- 3.Volkmann R. Chirurgische erfahrungen über knochenverbiegung une knochenwachstum. Virchows Archiv. 1862;24:512–40. [Google Scholar]
- 4.Courvoisier A, Eid A, Bourgeois E, Griffet J. Growth tethering devices for idiopathic scoliosis. Expert Rev Med Devices. 2015;12:449–56. doi: 10.1586/17434440.2015.1052745. [DOI] [PubMed] [Google Scholar]
- 5.Cunin V. Early-onset scoliosis - Current treatment. Orthop Traumatol Surg Res. 2015;101:S109–18. doi: 10.1016/j.otsr.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 6.Samdani AF, Ames RJ, Kimball JS, et al. Anterior vertebral body tethering for immature adolescent idiopathic scoliosis: one-year results on the first 32 patients. Eur Spine J. 2015;24:1533–9. doi: 10.1007/s00586-014-3706-z. [DOI] [PubMed] [Google Scholar]
- 7.Braun JT, Ogilvie JW, Akyuz E, Brodke DS, Bachus KN. Creation of an experimental idiopathic-type scoliosis in an immature goat model using a flexible posterior asymmetric tether. Spine. 2006;31:1410–4. doi: 10.1097/01.brs.0000219869.01599.6b. [DOI] [PubMed] [Google Scholar]
- 8.Newton PO, Farnsworth CL, Upasani VV, Chambers RC, Varley E, Tsutsui S. Effects of intraoperative tensioning of an anterolateral spinal tether on spinal growth modulation in a porcine model. Spine. 2011;36:109–17. doi: 10.1097/BRS.0b013e3181cc8fce. [DOI] [PubMed] [Google Scholar]
- 9.Setton LA, Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg AM. 2006;88A:52–7. doi: 10.2106/JBJS.F.00001. [DOI] [PubMed] [Google Scholar]
- 10.Wang D-L, Jiang S-D, Dai L-Y. Biologic response of the intervertebral disc to static and dynamic compression in vitro. Spine. 2007;32:2521–8. doi: 10.1097/BRS.0b013e318158cb61. [DOI] [PubMed] [Google Scholar]
- 11.Ménard AL, Grimard G, Massol E, et al. Static and dynamic compression application and removal on the intervertebral discs of growing rats. J Orthopaed Res. 2015;34:290–8. doi: 10.1002/jor.22991. [DOI] [PubMed] [Google Scholar]
- 12.Cancel M, Grimard G, Thuillard-Crisinel D, Moldovan F, Villemure I. Effects of in vivo static compressive loading on aggrecan and type II and X collagens in the rat growth plate extracellular matrix. Bone. 2009;44:306–15. doi: 10.1016/j.bone.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Menard A-L, Grimard G, Valteau B, Londono I, Moldovan F, Villemure I. In vivo dynamic loading reduces bone growth without histomorphometric changes of the growth plate. J Orthopaed Res. 2014;32:1129–36. doi: 10.1002/jor.22664. [DOI] [PubMed] [Google Scholar]
- 14.Reich A, Jaffe N, Tong A, et al. Weight loading young chicks inhibits bone elongation and promotes growth plate ossification and vascularization. J Appl Physiol. 2005;98:2381–9. doi: 10.1152/japplphysiol.01073.2004. [DOI] [PubMed] [Google Scholar]
- 15.Valteau B, Grimard G, Londono I, Moldovan F, Villemure I. In vivo dynamic bone growth modulation is less detrimental but as effective as static growth modulation. Bone. 2011;49:996–1004. doi: 10.1016/j.bone.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Villemure I, Stokes IAF. Growth plate mechanics and mechanobiology. A survey of present understanding. J Biomech. 2009;42:1793–803. doi: 10.1016/j.jbiomech.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaviani R, Londono I, Parent S, Moldovan F, Villemure I. Compressive mechanical modulation alters the viability of growth plate chondrocytes in vitro. J Orthop Res. 2015;33:1587–93. doi: 10.1002/jor.22951. [DOI] [PubMed] [Google Scholar]
- 18.Meakin LB, Price JS, Lanyon LE. The contribution of experimental in vivo models to understanding the mechanisms of adaptation to mechanical loading in bone. Front Endocrinol. 2014;5:5. doi: 10.3389/fendo.2014.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burdan F, Szumilo J, Korobowicz A, et al. Morphology and physiology of the epiphyseal growth plate. Folia Histochem Cyto. 2009;47:5–16. doi: 10.2478/v10042-009-0007-1. [DOI] [PubMed] [Google Scholar]
- 20.Anderson HC, Shapiro IM. The epiphyseal growth plate. In: Bronner F, Farach-Carson MC, Roach HI, editors. Bone and development. Springer; 2010. pp. 39–64. [Google Scholar]
- 21.Tsai A, McDonald AG, Rosenberg AE, Stamoulis C, Kleinman PK. Discordant radiologic and histological dimensions of the zone of provisional calcification in fetal piglets. Pediatr Radiol. 2013;43:1606–14. doi: 10.1007/s00247-013-2740-z. [DOI] [PubMed] [Google Scholar]
- 22.Akyuz E, Braun JT, Brown NAT, Bachus KN. Static versus dynamic loading in the mechanical modulation of vertebral growth. Spine. 2006;31:E952–8. doi: 10.1097/01.brs.0000248810.77151.22. [DOI] [PubMed] [Google Scholar]
- 23.Stokes IAF, Aronsson DD, Dimock AN, Cortright V, Beck S. Endochondral growth in growth plates of three species at two anatomical locations modulated by mechanical compression and tension. J Orthopaed Res. 2006;24:1327–34. doi: 10.1002/jor.20189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmid EC, Aubin C-E, Moreau A, Sarwark J, Parent S. A novel fusionless vertebral physeal device inducing spinal growth modulation for the correction of spinal deformities. Eur Spine J. 2008;17:1329–35. doi: 10.1007/s00586-008-0723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunziker EB, Schenk RK. Physiological mechanisms adopted by chondrocytes in regulating longitudinal bone-growth in rats. J Physiol-London. 1989;414:55–71. doi: 10.1113/jphysiol.1989.sp017676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez J, Balbin M, Santos F, Fernandez M, Ferrando S, Lopez JM. Different bone growth rates are associated with changes in the expression pattern of types II and X collagens and collagenase 3 in proximal growth plates of the rat tibia. J Bone Miner Res. 2000;15:82–94. doi: 10.1359/jbmr.2000.15.1.82. [DOI] [PubMed] [Google Scholar]
- 27.Perilli E, Le V, Ma B, Salmon P, Reynolds K, Fazzalari NL. Detecting early bone changes using in vivo micro-CT in ovariectomized, zoledronic acid-treated, and sham-operated rats. Osteoporosis Int. 2010;21:1371–82. doi: 10.1007/s00198-009-1082-z. [DOI] [PubMed] [Google Scholar]
- 28.Mc Lean FC, Bloom W. Calcification and ossification. Calcification in normal growing bone. Anat Rec. 1940;78:333–59. [Google Scholar]
- 29.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Mueller R. Guidelines for Assessment of Bone Microstructure in Rodents Using Micro-Computed Tomography. J Bone Miner Res. 2010;25:1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 30.Lorensen WE, Cline HE. Marching cubes: a high resolution 3D surface construction algorithm. Comput Graphics. 1987;21:163–9. [Google Scholar]
- 31.Hildebrand T, Ruegsegger P. A new method for the model-independent assessment of thickness in three-dimensional images. J Microsc-Oxford. 1997;185:67–75. [Google Scholar]
- 32.Glantz SA. Primer of Biostatistics. 6ed. McGraw-Hill Medical; 2005. [Google Scholar]
- 33.Ménard A-L, Grimard G, Londono I, et al. Bone growth resumption following in vivo static and dynamic compression removal on rats. Bone. 2015;81:662–8. doi: 10.1016/j.bone.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Reich A, Sharir A, Zelzer E, Hacker L, Monsonego-Ornan E, Shahar R. The effect of weight loading and subsequent release from loading on the postnatal skeleton. Bone. 2008;43:766–74. doi: 10.1016/j.bone.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasky-Negev M, Simsa S, Tong A, Genina O, Ornan EM. Expression of matrix metalloproteinases during vascularization and ossification of normal and impaired avian growth plate. J Anim Sci. 2008;86:1306–15. doi: 10.2527/jas.2007-0738. [DOI] [PubMed] [Google Scholar]
- 37.Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–22. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sergerie K, Parent S, Beauchemin P-F, Londono I, Moldovan F, Villemure I. Growth Plate Explants Respond Differently to In Vitro Static and Dynamic Loadings. J Orthopaed Res. 2011;29:473–80. doi: 10.1002/jor.21282. [DOI] [PubMed] [Google Scholar]
- 39.Tang MH, Mao JJ. Matrix and gene expression in the rat cranial base growth plate. Cell Tissue Res. 2006;324:467–74. doi: 10.1007/s00441-005-0143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stokes IA, Gwadera J, Dimock A, Farnum CE, Aronsson DD. Modulation of vertebral and tibial growth by compression loading: diurnal versus full-time loading. J Orthopaed Res. 2005;23:188–95. doi: 10.1016/j.orthres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Morey-Holton ER, Globus RK. Hindlimb unloading of growing rats: A model for predicting skeletal changes during space flight. Bone. 1998;22:83S–88S. doi: 10.1016/s8756-3282(98)00019-2. [DOI] [PubMed] [Google Scholar]
- 42.Ohashi N, Robling AG, Burr DB, Turner CH. The effects of dynamic axial loading on the rat growth plate. J Bone Miner Res. 2002;17:284–92. doi: 10.1359/jbmr.2002.17.2.284. [DOI] [PubMed] [Google Scholar]


