Abstract
The skeleton has the ability to perfectly adapt to external forces of the operating environment, by altering its morphology and metabolism in order to meet different needs. This unique adaptive capacity of the skeleton creates an interesting range of biological questions concerning the perception of mechanical or other kinds of signals, the type of receptor, and the molecular pathways involved in this adaptation. Studies of the characteristics of the cellular engineering provide a host of new information that confers to osteocytes the role of the protagonist in the perception and regulation of mechanical effects on the skeleton. The identity of mechanoreceptors is manifold and concerns ion channels, integrins, cell membrane, the cytoskeleton, and other systems. A similar multiplicity characterizes the intracellular signaling. This review describes recent data concerning the outward force reception systems and intracellular transduction pathways of information transfer leading to the continuous adaptation of bone tissue. Increased appreciation of the importance of the mechanical environment in regulating and determining the effectiveness of structural adjustment of the skeleton defines new horizons for the discovery of novel therapeutic approaches to diseases associated with bone loss.
Keywords: Bone Mechanotransduction, Fluid Shear Stress, Mechanical Loading, Osteocytes
Introduction
In 1892, Julius Wolff postulated that bone is a dynamic tissue that adapts to meet the physical demands of its external environment[1]. Reduced mechanical loading from spaceflight, immobilization, aging, and injury all lead to a decreased bone density and strength. Prolonged microgravity associated with spaceflight can cause the loss of bone density at a rate of 1-2% per month[2]. Mechanical disuse causes osteoporosis and, on the other hand, physical activity is known to increase bone mass and density. Up to now the strategies addressing the issue of skeletal fragility fail to take advantage of the intrinsic ability of bone tissue to adapt to external forces, which relies on the close orchestration of both formation and resorption in those specific sites of the skeleton that are subject to specific loads. It is well known that skeletal response to greater physical stresses results in larger and stronger bones[3], a response achieved by site-specific adaptations rather than an overall skeletal change[4]. Conversely, reduced physical demands, such as those associated with chronic immobilization or the consequences of aging, will accelerate losses in bone quantity and quality, and the following osteopenia can lead to increased risk of fracture, and eventually to a loss of independence. Mechanotransduction, the process by which cells convert mechanical energy, including electromagnetic energy, gravity, tension, compression, and shear, into electrical and/or biochemical signals, is important to maintain adult bone health and homeostasis. Theoretically, all eukaryotic cells are probably mechanosensitive and physical forces influence growth and remodeling in all living tissues at the cellular level. Understanding the molecular pathways governing the ability of bone to respond to functional demands should lead to novel therapeutic strategies for musculoskeletal disorders, from optimized exercise regimens to drugs that exploit key signaling molecules involved in mechanosensitivity. Although it is not possible to encompass the entire field of mechanosensing and transduction, our purpose is to provide a perspective towards the multiplicity, and complexity, of signaling systems that respond to mechanical input. The first part of this review we will consider the fundamentals of bone remodeling and mechanotransduction in bone and in the latter parts we will review the current data regarding the sensing, transduction and intracellular processing in skeletal cells, as well as the knowledge gap in the mechanisms involved, which are now enlisted in the new discipline of “mechanomics”.
| Abbreviations | |
|---|---|
| Akt | protein kinase B |
| MAPK | mitogen activated protein kinase |
| FAK | focal adhesion kinase |
| GTPase | guanine triphosphatase |
| MSC | mesenchymal stem cell |
| RANKL | receptor activator of NF kappa B ligand |
| GSK3β | glycogen synthase kinase 3 beta |
| mTOR | mammalian target of rapamycin |
| PGE2 | prostaglandin E2 |
| COX-2 | yclooxygenase 2 |
| LCS | lacuna-canalicular system |
| ECM | extracellular matrix |
| GEF | guanine exchange factor |
| ERK1/2 | extracellular signal-regulated kinases 1/2 |
| PC1 | polycystin 1 |
| PC2 | polycystin 2 |
| STAT6 | signal transducer and activator of transcription 6 |
| PKA | protein kinase A |
| PI3K | phosphoinositide 3-kinase |
| Cx43 | onnexin 43 |
| VSCC | voltage sensitive calcium channel |
| JNK | -Jun N-terminal kinases |
| SH2 | Src homology 2 domain |
| c-Src | hicken sarcoma gene |
| Grb2 | growth factor receptor-bound protein 2 |
| Ras | rat sarcoma gene |
| RhoA | ras homolog gene family member A |
| ER | estrogen receptor |
| Sost | sclerostin gene |
Basic aspects of bone architecture
The human skeleton is composed of cortical and trabecular bone. Cortical bone is found primarily in the cortex of long bones, whereas trabecular bone is prevalent in the ends of long bones surrounded by a cortical bone shell. Cortical and trabecular bone differ in their porosity where the former ranges between 5% to 10%, and the latter between 50 to 90%. As such the mechanical properties of these two types of bone vary significantly. Cortical bone is comprised of repeating structural units known as osteons, which are made of concentric interconnected rings of osteocytes, the most abundant cell type in bone. Osteocytes are buried in the bone matrix in the lacunae, while their long cell processes connected in a three-dimentional pattern to other osteocytes via gap junctions, to bone surface lining cells and cells within the bone marrow through narrow channels called canaliculi[5,6]. The resulting network is known as the lacuno-canalicular system. In fact there are three kinds of porosities in cortical bone: a) vascular porosity containing blood vessels, nerves and interstitial fluid in osteonal lumens and Haversian canals; b) lacunar-canalicular porosity and c) collagen-apatite porosity of the bone extracellular matrix (Figure 1).
Figure 1.
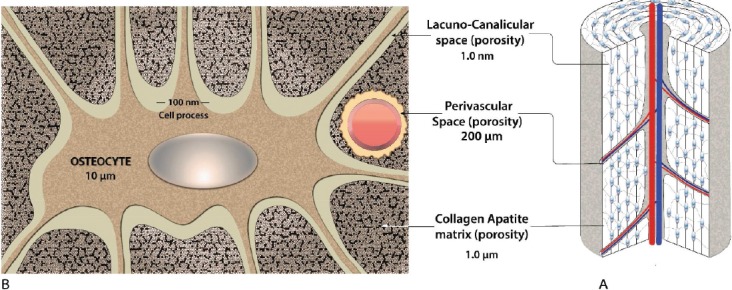
Bone tissue anatomy and cellular architecture. (A) Macroscopic bone tissue anatomy: osteon, osteocyte, and cytoplasmic processes. (B) Schematic representation of an osteocyte. Osteocytes are embedded into a mineralized bone matrix surrounded by three different and communicating porosities.
The space surrounding the osteocytes is filled with an interstitial fluid that moves upon mechanical loading of bone. In contrast, the collagen-apatite porosity is not interconnected and the water found in the pores is tightly bound[7]. In the absence of external applied forces, diffusion-based molecular transport of only small molecules takes place from vascular capillaries to the lacunar-canalicular porosity[7]. Trabecular bone, besides inter-trabecular space also has collagen-apatite and lacunar-canalicular porosities.
The bone porosity with the largest, but quite variable, characteristic linear dimension (up to 1 mm) is associated with cancellous bone and is referred as inter-trabecular porosity of cancellous bone. It is the porosity external to, and surrounding, the trabeculae. This porosity is connected to the medullary cavity and may contain marrow, fat and blood vessels. The characteristic linear dimension of this space varies with anatomical location. It is smaller near the load-bearing surfaces and increases to its greatest magnitude near the medullary canal. This porosity contains also fluids or gels. The viscosity of these fluids is not uniform, but generally is two orders of magnitude larger than the viscosity of fluid in other types of porosity. More recent data have shown that the overall bone permeability is strongly affected by the pore fluid viscosity, which, in case of polarized fluids, is strongly increased due to the presence of electrically charged pore walls[8].
Mechanical loading in bone
Bone cells are tightly coupled to their extracellular environment in a complex dynamic fashion to biophysical stimuli that includes gravitational force, strain, stress, shear, pressure, fluid flow, streaming potentials, acceleration and damage. While it may not be possible to separate specific effects of each of these factors, it is clear that several of these parameters independently have the ability to regulate cellular responses and influence remodeling events within bone. Furthermore, components of these specific factors — such as magnitude, frequency, strain rate — also affect the cellular response. In order to characterize the overall mechanism by which bone mass is adjusting to physical forces by means of longitudinal growth, modeling or remodeling Harold Frost[9] coined the term “mechanostat”.
Frost suggested the existence of a homeostatic regulatory mechanism in bone responsible for sensing changes in the mechanical demands placed on bone and subsequently altering the mass and conformation of bone to better meet these new mechanical demands. Specifically, Frost postulated that several mechanical thresholds control whether bone is added or taken away from the skeleton. He theorized that below a certain threshold of mechanical use, bone is resorbed, and is therefore rid of excess mass. Above another threshold, in which bone is exposed to greater than typical peak mechanical loads, bone formation occurs on the existing structure to increase bone strength[9].
According to this mechanostat hypothesis non-physical factors (e.g. cell-cell interactions, hormones, blood pH, minerals, disease, etc.) are needed to control bone adaptation mechanisms, while physical and mainly mechanical factors are needed to temporally and spatially guide these processes. Furthermore, while non-mechanical factors can promote or hinder the guidance of bone adaptation processes, they cannot replace the role of mechanical factors, for example by maintaining bone structure and strength in immobilized patients.
To determine the effect of mechanical forces on bone remodeling, it is important to understand how bone cells experience these forces in their native environment. Since Piekarski and Munro’s original proposal[10], there has been increasing evidence from mathematical and experimental models suggesting that mechanical forces on bone induce fluid flow in the lacunar-canalicular porosity[11,12]. Structural models of osteocytic processes and the pericellular matrix suggested that fluid flows in the lacunar-canalicular system in an oscillatory pattern, where changes in matrix deformation during repeated loading and unloading causes oscillatory fluid displacement generating direct cell strain. Zhang et al.[13] estimated that the fluid component could transfer as much as 12% of the imposed mechanical load and produce peak pressures of 2-3 MPa. In one study using fluorescence recovery after photobleaching (FRAP) in combination with computational modeling, fluid flow in the lacunar-canalicular system of mechanically loaded bone has been measured in real-time and predicted the peak canalicular fluid velocity to be 60 µm/s in loaded bone, with a peak shear stress at the osteocyte process of 5 Pa[12]. Mechanical forces on bone can also impose hydraulic pressure on osteocytes in the lacunar-canalicular system. Normal walking, modeled as oscillatory hydraulic compression loading of 0-18 MPa at 1 Hz, was translated to be 0.27 MPa of cyclic hydraulic pressure (CHP) in the lacunar-canalicular system[13].
Strain amplification in osteocytes
Locomotion induces tissue-level strains that are typically below 0.2% (2000 microstrain). Since in vitro findings indicate that cellular-level strains greater than 0.5%, which would normally cause bone tissue damage, are required for intracellular signaling, it has been proposed that osteocytes possess the machinery for strain amplification through the ultrastructural features in their cellular processes, where proteoglycan-based tethering elements attach the cell processes to the canalicular wall (Figure 2). Fluid flow due to bone deformation would impose drag forces on the tethering elements, thereby producing a hoop strain on the process membrane and underlying central actin filament bundle. Although, the cell body is more mechanoresponsive as it is less stiff, fluid shear stress in vivo is at least two orders of magnitude higher on processes than the cell body since the pericellular space in lacunae is much larger than in canaliculi[14]. Yet less is known about the mechanosensory organs of osteocytes as cells in culture lack their native extracellular environment and tethering elements necessary for the strain amplification model; hence in vitro results from fluid shear stress experiments must be interpreted with caution. In addition to their cell bodies and processes, osteocytes are able to sense mechanical stimuli through actin to extracellular matrix attachments by means of integrins and CD44 receptors on the cell membrane[15]. Integrins have also been suggested to be associated with stretch-activated ion channels and hemichannels[16] to convey fluid shear stress to the cytoskeleton[17]. It has been shown that the formation of strong integrin attachments requires the presence of the glycocalyx of the osteocyte dendritic process[16]. Wang et al. applying a theoretical model predicted that tensile forces acting on the integrins are <15 pN providing stable attachment for the range of physiological loadings[18]. Their model also predicted that axial strains caused by the sliding of actin microfilaments in the fixed integrin attachments are an order of magnitude larger than the radial strains and two orders of magnitude greater than whole-tissue strains and thus are able to open stretch-activated cation channels[18].
Figure 2.
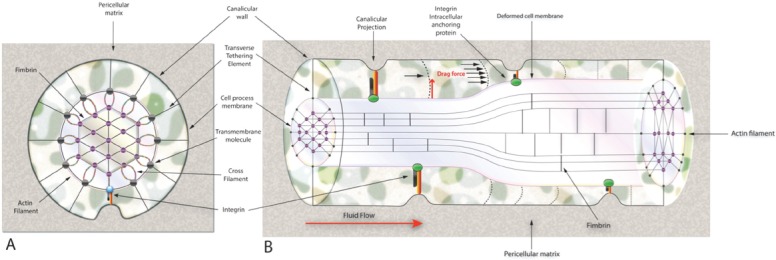
(A) Transverse cross-section of a structural model for a cell process in a canaliculus attached to a focal attachment complex and tethered by the pericellular matrix. (B) Longitudinal cross-section of the deformed transverse tethering elements and sliding actin filaments.
Osteocytes also seem to sense loads through primary cilia, which are solitary immotile microtubule-based cellular projections that deflect in the presence of fluid flow and are necessary for bone homeostasis[19]. The location of the primary cilium, i.e. on the osteocyte cell body, makes it difficult to have a role as a flow sensor for osteocytes in vivo, because loading-induced fluid flow will primarily occur around the osteocyte cell processes. Bell et al., suggested that cells sense hydraulic pressure by using the primary cilium as a sensor of hydrostatic pressure, but the exact mechanisms have not yet been completely verified[20].
Mechanoregulatory function of osteocytes
Mechanical stimulation has been demonstrated to be necessary for maintaining osteocyte viability, whereas the absence of mechanical loading causes osteocyte apoptosis[21-23]. In fact, mechanical strain has been shown to elicit a biphasic relationship with osteocyte viability in vivo, where apoptosis was reduced by 40% at strains of 0.003 or 0.004 but increased eight-fold at microdamage-inducing (i.e., fatigue loading) strains of 0.008[21]. The percentage of bone resorption surfaces has also been found to be directly correlated to strain-induced osteocyte apoptosis in vivo, suggesting that apoptosis may be a targeting mechanism for inducing a bone resorption response[21,24-26]. Moreover, osteocyte-ablated mice did not develop osteoporosis in the absence of mechanical loading[27], suggesting that osteocytes are responsible for unloading-related bone loss. While it is not clear why osteocyte apoptosis is regulated by mechanical loading, an analytical model has suggested that regions of osteocyte apoptosis in fatigue-loaded bone correspond to reduced fluid flow[28]. It is well known that fluid flow is a critical solute transport mechanism in bone, and that diffusion alone is not sufficient for molecular (and, hence, nutrient) transport to osteocytes in the lacunar-canalicular system[10].
Models examining the direction of the flow showed that the load-induced fluid flow is directed radially from the cement line toward the osteonal central canal, and that the relaxation time for this flow correlated with the decay of streaming potentials when the molecular sieve for the matrix is about the size of albumin molecules (7 nm)[29]. This theoretical model was confirmed by Wang et al.[7] who showed the bone’s interstitial fluid pathway in vivo using tracers of various sizes. These elegant studies confirmed the importance of mechanically induced flow for the transport of metabolites to and from osteocytes in an osteon, to ensure osteocyte viability. Also, tracer studies show that the size of the molecular sieve is ~6 nm[30,31], and easily allows the passage of microperoxidase (~2 nm), and that a small tracer, such as procion red (~1 nm), is confined within the boundaries of the LCS[32].
Mechanosensors
A mechanosensor may be defined as any cellular product or structure capable of detecting alterations in a variety of external or internal forces. Mechanosensors exist in nearly every cell type and are responsible for many essential functions. The ability of cells to perceive the mechanical signals from the environment requires either direct contact of the mechanosensor with the extracellular space, or its ability to detect changes in an interfering medium as e.g. pressure, gravitational field or fluid shear on the plasma membrane. These sensors constitute a group of specific receptors that respond to external force with conformational change and can be proteins, primary cilium or composite cellular structures that interact with cellular proteins, alter the composition of membrane lipids or interact with components of the extracellular matrix or cytoskeleton. Although in many cellular models it is suggested that a single mechanosensor is responsible for specific cellular responses, it is more likely that many of these receptors participate to the final response of the cell to its environment. In this part, we consider current knowledge about cellular candidates of mechanosensing.
Integrins and ECM proteins (Figure 3)
Figure 3.
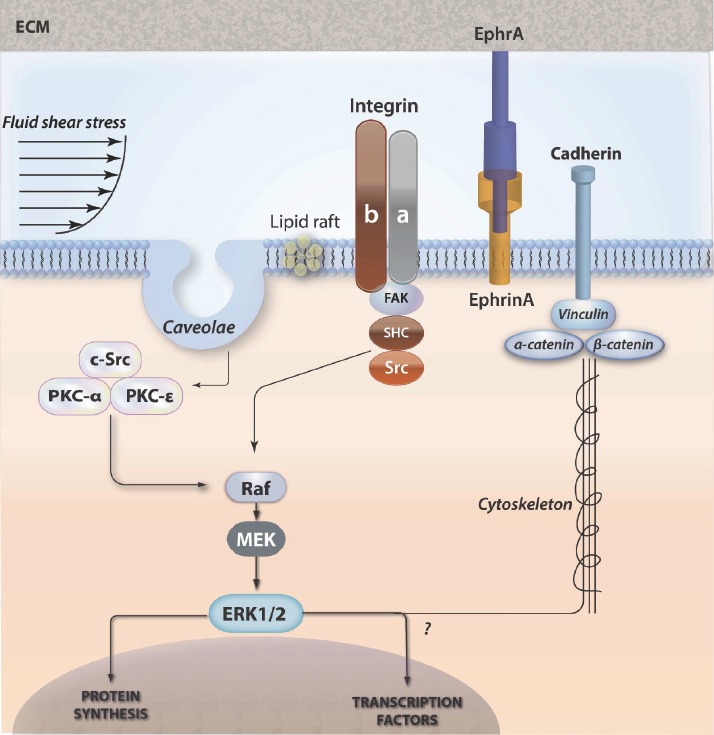
Schematic of co-ordidated actions and responses of integrins, lipid rafts and cadherins involved in osteocyte mechanobiology. (See text for details).
Integrins are heterodimeric protein complexes that connect the cell to the pericellular environment by spanning the plasma membrane and forming adhesions with the adjacent tissues or cells. The binding of ligands to the extracellular domain of integrins may transmit signals activating intracellular signaling, while modification of intracellular domains also regulates the binding affinity of extracellular molecules. The integrin dimers are composed from α and β subunits, both of which carry intracellular domains. Activation of this heterodimer causes a conformational change in the β-subunit. β1 integrin plays important roles in both osteoblasts and osteocytes. Osteoblasts exposed to fluid flow shear stress up-regulated β1 integrin expression and activated αvβ3, which co-localized with shc[33]. Integrins can connect with other adhesion-associated tethering proteins to form adhesions capable to mediate mechanotransduction signaling cascades. The ECM proteins include talin[34], p130Cas[35], paxillin and focal adhesion kinase (FAK). Talin and paxillin enchain to the focal adhesion binding sequence of FAK and talin associates with the cytoplasmic tail of β integrins[36]. Paxillin binds to the cytoplasmic side of the focal adhesion initiating signals since it is a substrate for FAK and src kinase[37]. The p130Cas, promotes association of src family kinases during cell stretching[35]. The FAK tyrosine kinase congregates in areas close to focal adhesions[38] and its activation induces integrin concentration[39]. These focal adhesion adapter proteins probably modulate external signaling in cooperation with integrins. Newer studies have shown the spatial location of integrins in the osteocyte LCS[40] and their ability to modulate fluid shear stress-mediated gene expression.
The tethering elements (Figure 2)
Tethering elements are transverse and elongated proteoglycan molecules that extend across the pericellular space of the osteocyte, connecting the mineralized matrix to the membrane of the cell and its processes[41]. The transverse tethering elements seem to be involved in sensing fluid movement[42]. Current research has demonstrated that the heparan sulfate proteoglycan perlecan is present along the cell membrane of the osteocyte. Also, in a recent study, the number of transverse tethering elements was significantly reduced in mice that were deficient in perlecan[43]. It is possible that perlecan cross the pericellular space of the osteocyte canaliculi tethering the cell to the ECM. Perlecan possibly regulates the size of the pericellular space of the osteocyte canaliculi, as the canalicular space was significantly reduced in the perlecan-deficient mice. The presence of numerous regulatory regions in the protein core of the perlecan molecule provide to this molecule with the ability to interact with several membrane receptors that mediate the adjustment of osteocytes to dynamic flow shear stress[43].
Cytoskeleton and focal adhesions
Cytosceletal proteins support the mechanical strength of the cell and when extracellular forces are applied, signals are directly transmitted on the cytoskeleton which in turn regulates the perception and adaptation of the cell to mechanical or gravitational force. Fluid shear stress across osteoblasts induces reorganization of actin filaments into contractile stress fibers[44] while disruption of the actin cytoskeleton modifies the response of bone cells to fluid shear stress[45]. Furthermore, enhancement of actin polymerization induces osteogenic differentiation[46]. Focal adhesions on the other hand are macromolecular proteins that connect the cytoskeleton and the pericellular space, mediating regulatory effects on cell behavior[47]. These adhesion sites contain transmembrane integrins as well as, a collection of associated adhesion proteins forming a macromolecular mechanotransducer complex. The connection of focal adhesions to the actin fibrils transmit the exerted force throughout the cell, activating simultaneously several signaling pathways. It has been shown that cells respond to mechanical challenge by forming focal adhesions, which provide a cytoskeletal pathway for signal enhancement through the β-catenin cascade. Repeated mechanical challenge induces cytoskeletal adaptation with formation of new focal adhesions/mechanotransducer complexes that generate an amplified signal. It appears that focal adhesions contribute to precursor recruitment through their attachment with cytoskeletal structure[48].
Plasma membrane structures
Various types of lipids, tethering and transmembrane protein molecules, lipid rafts and caveolar formations has been shown to form flexible adjustable signaling facilities within the plasma membrane.
Lipid rafts and membrane proteins (Figure 3)
Lipid rafts are highly organized and dynamic assemblies of glycosphingolipids and cholesterol forming intra-membrane domains which are able to coordinate the association of signaling molecules such as GTP-binding proteins, kinases, and integrins. Recruitment of regulatory molecules in one location gives lipid rafts the ability to create the appropriate concentration of signaling effectors whereby crosstalk and directionality of signals can efficiently occur[49]. It has been shown that cholesterol depletion of lipid raft micro-domains prevents activation of the small GTPase H-Ras required for the anti-osteoclastic effects of mechanical strain[50]. Furthermore, lipid rafts are substantial for hydrostatic pressure and fluid shear stress-induced activation of ERK1/2 and c-fos expression in osteoblasts[51]. Lipid rafts can be categorized as caveolar or non-caveolar. The presence of caveolin proteins produces the characteristic caveolar invaginations, and through binding sites, allows close associations of proteins into signaling complexes[52]. One possible explanation for the regulation of caveolae-induced intracellular signaling is that ECM-associated molecules are clustered with signaling effectors. Indeed, caveolae are important for β1 integrin-mediated mechanical activation of the Src-like kinase Csk[53]. Previous studies also demonstrated that fluid flow stimulated nitric oxide (NO) production from caveolae-associated endothelial nitric oxide synthase (eNOS)[54]. Furthermore, a recent study identified caveolin-enriched membrane fractions as important structures necessary for proper growth factor-mediated signaling in osteoblasts[55]. It has been shown that cholesterol depletion attenuates integrin-dependent caveolin-1 phosphorylation, Src activation and Csk association with β1-integrin, supporting the concept that β1-integrin mediated mechanotransduction is mediated by caveolar domains. Caveolin-1 regulates the mechanical properties of bone in vivo as well: caveolin-1 knockout mice display increased bone formation rate at trabecular and cortical sites. This appears to involve the ability of caveolin-1 to chronically restrict osteoprogenitor recruitment[56], perhaps through limiting the availability of β-catenin[57]. It has also been shown that lipid rafts may orchestrate nongenomic estrogenic effects not limited to membrane ER. In fact, recent findings indicate that under certain stimulation, raft-like microdomains located on mitochondria, may contribute to apoptosis-associated signaling[58]. ERb has been detected in mitochondria where probably could mediate certain estrogenic actions such as Ca2+ influx, ATP production, apoptosis, and free radical species generation[59,60].
Cadherins and other cell-cell connections (Figure 3)
Cadherins are a family of integral membrane glycoproteins composed of a long extracellular domain, a single-pass transmembrane domain, and a small, intracellular c-terminal tail. The intracellular domain anchors the cadherin to the cytoskeleton by associating with multiprotein complexes that include vinculin, α- and β-catenin[61]. These transmembrane anchoring systems are important in numerous processes including differentiation, cell polarity, immune response, cell division and apoptosis.
Ephrins (Figure 3)
Eph receptors belong to a subfamily of receptor tyrosine kinases activated by ligands called ephrins. Generally, EphA receptors interact with ephrinA and EphB receptors interact with ephrinB ligands. Eph receptors interact with ephrins at the cell surface, triggering bidirectional signaling: forward through Eph receptors and reverse through ephrins. Forward Eph signaling depends on both Eph kinase activity and kinase-independent signals, while reverse ephrin signaling depends on Src family kinases and other effector molecules. In communication between other cell types, activation of ephrin/Eph-mediated bidirectional signaling alters cell adhesion, migration and proliferation. Ephrin-A1 has been shown to regulate cell morphology and motility through the activation of EphA receptors, which signal to the PI3K pathway to induce cell retraction. Ephrin-A1 also serves as an inhibitory substrate for cell spreading and migration. Moreover, Ephrin-A1 treatment results in the dephosphorylation of paxillin and induces the reorganization of phosphopaxillin-containing focal adhesions. The Ephrin-A1 regulated paxillin dephosphorylation is phosphatase dependent, but p85b independent. Bone cells such as chondrocytes, osteoblasts, osteocytes and osteoclasts express ephrin ligands and Eph receptors. Cells of the human osteoblast lineage express most ephrinAs, ephrinBs, EphA2 and EphB2 throughout differentiation[62]. Treatment of cultured osteoblasts with a specific EphrinB2/EphB4 inhibitor increased RANKL expression[63]. This suggests that the dependence of osteoclast formation on EphrinB2 may relate to EphrinB2/EphB4 interactions specifically within the osteoblast lineage. This finding is in concern with the low osteoclast numbers in EphB4-overexpressing mice[64]. Although little is known about function of ephrins and Ephs in osteocytes, these abundant bone cells do express ephrins/Ephs such as ephrinB1, ephrinB2 and EphB4 and blockade of ephrinB2/EphB4 interaction results in decreased expression of sclerostin, a potent inhibitor of osteoblastogenesis. Therefore, osteocytes may communicate bi-directionally with osteoclasts or osteoblasts in response to various stimuli through ephrins/Ephs. Ephrins contribute to osteoblast function, and appear to be necessary for differentiation and possibly communication with the osteoclasts during remodeling[63]. The restriction of ephrin clustering within the plasma membrane leads to altered signaling, suggesting that the distribution of ephrin receptors modulates the response to extracellular or trans-cellular signals. By this way the continuously changing cellular environment of signals can be adjusted, via mechanical tuning through physical connections, cytoskeleton and substrate.
Primary cilia (Figure 4)
Figure 4.
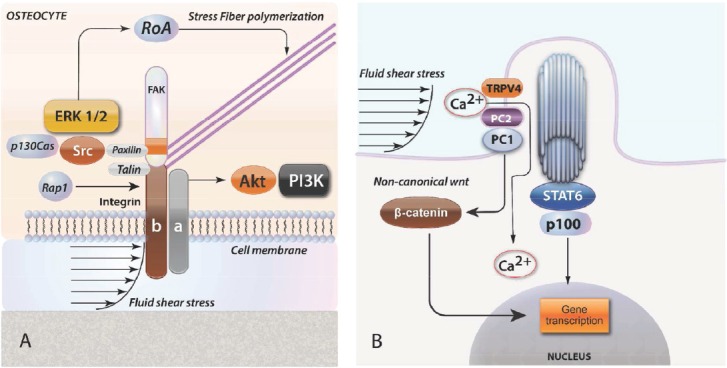
Mechanotransduction through integrins and primary cilia. (A) Cell cytoskeleton senses loading at the membrane through integrins that transmit force through ERK, Src and RoA to stress fiber polymerization. (B) Primary cilia may sense flow shear strain, activating ion flux through PC1 and TRPV4, which can activate Stat signals. Cilia also modulate Wnt signaling via non-canonical pathway that leads to β-catenin degradation.
Primary cilia consists of a central axis composed of nine microtubules surrounded by a specialized membrane which connected with the cell membrane, but has different characteristics. Primary cilia derive from the basal body, which is a modified structure of the mother centriole. In a recent study deletion of the kinesin family member 3A (Kif3A), a gene that is essential for primary cilia formation lead to a significant decrease of bone formation in response to mechanical load[65]. Espinha et al[66], showed an increase in microtubules around primary cilia both with time and shear rate in response to oscillatory fluid flow stimulation. They also showed that the primary cilium is required for this loading-induced cellular response providing a new insight into how cilia may regulate its mechanics and thus the cells mechanosensitivity[66].
There is a primary cilium in each cell. The primary cilia are adaptive mechanosensors and possess a specific mechanism that can regulate cellular mechanosensitivity. It has been shown that cilium stiffness changes in response to mechanical and chemical stimuli through an acetylation-mediated mechanism by which the cell can regulate ciliary stiffness and in turn, regulate cellular responsiveness. This sensory adaptation occurs on a very short time, making mechanosensors therapeutic targets for disorders involving impaired mechanosensitivity[67]. It has also been shown that bending of the cilium causes calcium movement intracellularly in kidney cells[68]. Another interesting study showed that the primary cilium forms a Ca(2+) microdomain dependent on Ca(2+) entry through TRPV4 [69].
The molecular mechanism of this transport is based on polycystines, which are proteins that concentrate at the base of the cilium. More specifically, polycystine 2 (PC2) is a cationic channel that may be involved in this transfer. Recent data show that they are involved in mechanotransduction[19], possibly through the release of PGE2 which seems to be independent of intracellular Ca2+ influx and appears to be cilia dependent[19]. Furthermore, MSCs exposed to conditioned media from mechanically stimulated osteocytic cells showed induction of osteogenic genes, an effect that was abolished when primary cilia formation was inhibited in the osteocyte[70]. Primary cilia has recognized to modulate Wnt signaling, inducing β-catenin degradation via non-canonical pathway[71]. Studies have shown that osteocyte primary cilia are exquisitely sensitive to deflection[19] and tiny, motions may indeed be sufficient to elicit a response. Such motions may be the result of flow on the cilium, flow on the cell body, or relative displacement of the cell body within the lacuna if the distal aspect of the cilium is attached to the mineralized matrix (Figure 4).
Gap-junctions and Ion channels
Gap junctions are created by connexins. Connexins are membrane spanning protein hexamer complexes, the connexons. Connexons form pores within the plasma membrane of cells. Alignment of connexons with their counterpart on an adjacent cell creates functional connections called gap junctions. The gap junction pore allows for intracellular communication isolated from the extracellular environment, and can pass small molecules (<1 kDA) including calcium, inositol phosphates, ATP, and cAMP[72]. Independent of cell-cell gap junctions, connexin hemichannels serve as a portal through which prostaglandins are released from the osteocyte in response to fluid shear stress. Gap junctions found at the ends of cell protuberances of osteocyte and between osteocytes and osteoblasts[73]. Functional coupling through the existence of a syncytium signaling has been demonstrated in bone cells in vivo and in vitro. Intercellular communication via gap junctions show that adjusts the bone remodeling signals from osteocytes to the surface osteoblasts. Indeed, the response of bone to biochemical[74] and electrical stimulation seems to be required functional gap junctions. Further, intracellular calcium signaling due to mechanical stimulation appear to operate with the same mechanism[75,76]. Recent data suggest that the ability of osteocytes networks to develop calcium oscillations required gap junctions[76,77]. Phosphorylation and functioning gap junction seems to be regulated by mechanical stimulation[78] and has been associated with the expression of bone matrix genes. It has to be noted that, autocrine/paracrine activation of cAMP/PKA and PI3K/Akt pathways leads to inactivation of GSK3β leading to increased nuclear translocation of β-catenin, which regulates connexin 43 (Cx43) transcription[79]. Data from Cx43 knockout mice have shown delayed expression of genes coding for bone matrix proteins such as osteocalcin and osteopontin[80]. The increased expression of connexins in vitro and in vivo in response to mechanical stimulation suggests that cells create enhanced connections with surrounding tissue to tranfer the mechanical information through the osteocyte network, enabling a cellular “syncytium” operating in a manner similar to neural networks[81]. While bone has generally been considered a non-excitable tissue, there is an increasing body of evidence suggesting that membrane excitability may subject to mechanical force induced regulation. It is known that membrane depolarization through ion channels is responsible for maintenance of proper electrochemical gradients. Bone cells express several different ion channels involved in force - actinated pathways. Bone cells express several different ion channels involved in mechanosensitive pathways. These include the gadolinium-sensitive stretch-activated cation channels, transient receptor potential (TRP) channels[82], and the multimeric voltage sensitive calcium channels (VSCC)[83,84]. Shear stress-activated cation channels alter membrane potential in response to membrane strecing, causing local depolarizations enabling the activation of VSCCs. Furthermore, VSCCs can independently regulate membrane stretch and shear-induced mechanosensitive events in osteoblasts initiating anabolic signals in response to mechanical stimulation[85]. While osteoblasts predominantly express the L-type VSCC variant, recent studies demonstrate that the T-type VSCC is the functional subunit found in osteocytes[84]. Interestingly, the T-type channel in osteocytes was shown to associate with the membrane-anchored extracellular α2δ1 subunit and this association seem to be important for the regulation of stress induced ATP release[86].
Signal transduction pathways
Kinase signaling
Extracellular forces can activate mitogen activated protein kinase (MAPK) pathways in many cell types. The MAPKs are serine/threonine protein kinases with a fundamental role in the differentiation, proliferation and cell survival. In endothelial cells physical forces can also activate extracellular signal regulated kinases (ERK1/2), p38 families, BMK-1 and c-Jun N-terminal kinases (JNK)[87]. Studies have shown that when ERK1/2 is activated by external forces in bone cells[88] causes RANKL down-regulation, increased eNOS protein and MMP-13 expression, leading eventually in decreased osteoclastic activity and increased bone formation[89]. It has also been shown that activation of ERK in bone cells under shear stress requires ATP dependent activation of calcium channels[90]. Another serine/threonine kinase with multiple cellular activities, Akt kinase, is also activated by fluid shear stress in bone cells and alters the differentiation process of MSCs leading to increased osteoblast commitment and thereby bone mass. FAK is also connected to other signaling proteins such as the family of Src kinases, the phosphatidylinositol 3-kinases (PIK3) kinases and paxillin[91]. With these connections, FAK forms networks responding to activation of integrins and transfer signals to other intracellular pathways such as MAPK[92], ERK, and Akt/mTor/p70S6K pathway enhancing proliferation of osteoblasts. Similarly FAK increases expression of osteopontin, osteocalcin and COX2 in mature osteoblasts, while it is also involved in osteoblastic differentiation by promoting the expression of the transcription factors Runt-related transcription factor 2 (Runx2) and Osterix (Osx), which are considered the key regulators of osteoblastic differentiation and osteogenesis[93].
Calcium Signaling (Figure 5)
Figure 5.
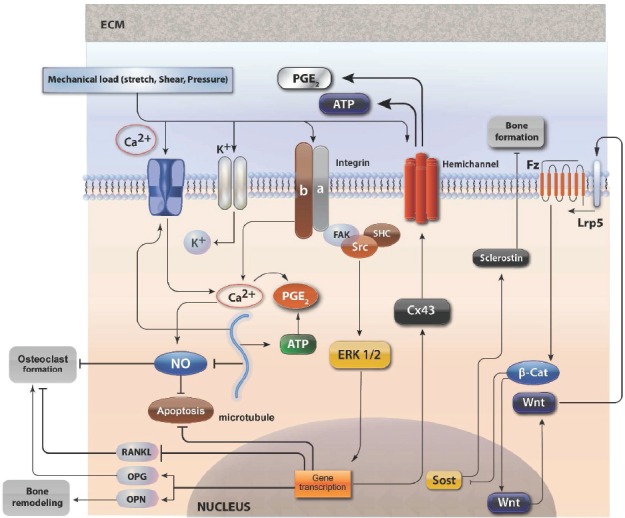
Schematic of sensors, signaling pathways, and responses involved in osteocyte mechanobiology. Fluid shear stress activates a mechanosensitive calcium channel in the plasma membrane, leading to Ca2+ influx through the voltage sensitive complex. Acting as a second messenger, Ca2+ promotes PGE2 synthesis via ATP and also inhibits NO generation. ERK1/2 induses transcription of Cx43 to form connexin hemichanels for PGE2 and ATP release. The action of canonical wnt on inhibition of SOST gene is also depicted. (see text for details).
Intracellular calcium levels are maintained low by mechanisms such as extracellular movement, and transfer to the endoplasmic reticulum. Changes in intracellular calcium levels affect the proliferation, differentiation and mobility in a variety of cell systems. A rapid increase in intracellular levels is evident after mechanical stimulation of bone cells[94] and calcium channels play an important role in these changes. The signals that cause these changes relate to all forms of mechanical action, e.g stretch of cell membrane, fluid flow or osmotic changes. It is notable that the frequency of changes are more important than the amplitude, since the rest period increases the response of osteoblastic cells to the calcium[95]. Calcium mobilization activates various routes such as IP3[96], ATP and nitric oxide[97]. The intracellular movement of calcium can also activate protein kinase PKA[98], MAPK and c-Fos. Recent studies have also shown that the release of PGE2 is also dependent on intracellular calcium movement through L-type channels. Based on current knowledge, it is clear that intracellular calcium levels serve in many ways the intracellular activation that occurs as a result of mechanical action.
G-Protein-Mediated signaling
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins involved in transmitting signals from the exterior compartment into the cell and belong to the larger group of enzymes called GTPases. Mechanical signals has been shown to activate heterotrimeric GTPases via G-protein coupled receptors, stimulating rises in intracellular calcium, cAMP and cGMP. A G-protein-coupled receptor includes a transmembrane receptor and an intracellular G-protein, which is activated through a conformational alteration that occurs with ligand binding or mechanical stimulation. Osteocytes exposed to fluid shear stress showed a transient decrease in cAMP production[99]. cAMP is catalyzed from ATP via adenylate cyclase (AC), an enzyme that is activated by GTPases. AC isoform 6 has shown to be localized on the primary cilium and the decreases in cAMP levels in response to fluid shear stress were dependent on AC6 activity. Primary cilium-mediated AC6 activation in osteocytes cells regulated COX-2 gene expression and PGE2 signaling. Also, fluid flow activates phospholipase C and initiates IP3 signaling through a G-protein-mediated mechanism leading to COX2 expression. Furthermore, mechanical force can activate RhoA GTPases in MSCs. RhoA has an important role in actin cytoskeletal organization regulating stress fiber formation in response to mechanical strain[100]. RhoA activity is a further prominent example of mechanically regulated signaling systems that affect MSC lineage commitment and final phenotype underscoring the role of MSCs as a mechanical target determining bone morphology.
Wnt/β-catenin (Figure 5)
The wnt signal pathway is particularly important in bone biology[101]. Activating mutations of the co-receptor LRP-5 leads to increased bone density, whereas inactivating mutations reduce bone mass favoring fractures[102]. The downstream effector of the system, β-catenin is the main regulatory element in bone remodeling both in osteoblasts and osteocytes. Studies have shown that β-catenin may be a direct target of mechanotransduction regardless of the classical activation pathway through LRP-5 receptor[103]. This alternative pathway probably involves the inhibition of the activity of GSK3β through the mTORPs2 activation by mechanical shear stretch. In osteoblasts, β-catenin associates with cadherins on the inner leaflet of the plasma membrane. Fluid shear stress decreases the amount of β-catenin bound to N-cadherin thus increasing the cytoplasmic pool of β-catenin[104]. The increase in unbound β-catenin coupled with activation of GSK3β and Akt that occurs after fluid shear stress has been proposed as a potential upstream regulator of β-catenin nuclear translocation. Thus, cadherins may serve as launching platforms for β-catenin in response to mechanical stimulation[105]. Sclerostin, which is constitutively expressed by osteocytes, is a negative regulator of bone formation that acts by deactivating the Wnt receptor. Upon loading, sclerostin expression is down-regulated, effectively increasing osteoblast activity[106]. Osteocytes may also stimulate local bone remodeling as a consequence of undergoing apoptosis[22]. Recently it has been shown that fatigue damage in the absence of osteocyte apoptosis is insufficient to induce bone remodeling[26]. It is now considered that dying osteocytes, act as the signal to target remodeling to regions of cell death[107].
Prostaglandins (Figure 5)
Prostaglandins and prostacyclin are eicosanoids derived from phospholipids, and play a significant role in the skeletal function. Their levels increase in various load conditions of the skeleton[108], including the substrate strain, fluid flow, magnetic fields, and hyper-gravity[109-111] and their exogenous administration stimulates bone formation[112]. Biochemical inhibition of their actions by the administration of indomethacin reduce the adaptive capacity of bone to mechanical loading[113]. The exogenous PGE2 increases the sensitivity of bone to external loads, demonstrating its role as intercellular messenger for mechanical loading information both in vitro and in vivo. Similar is also the effects of prostacyclin[114]. It should be pointed out that the release is parallel to that of NO which makes possible their cooperation in mechanical loading conditions[115]. Increased gap junctional communication is mediated by PGE2, and mechanically stimulated PGE2 release is reduced in gap-junction-deficient cells, suggesting cross talk between those two systems through connexin hemichannels. The response in osteocytes and osteoblasts appears to occur through different mechanisms and is higher in osteocytes than in other bone cell types. Interestingly, mechanical vibration leads to a reduction in PGE2 release. PGE2 release due to mechanical stimulation has been reported to require a competent cytoskeleton[116] and is increased with the formation of focal adhesions[117], although conflicting findings exist[45]. Expression of the inducible form of the enzyme responsible for PGE2 synthesis (COX-2) is also increased with mechanical stimulation, as well as by estrogen and PTH.
Nitric oxide
Nitric oxide (ΝΟ) is a highly reactive intracellular messenger in many organ systems. It is produced by NO synthase in three isoforms, neuronal (nNOS), endothelial (eNOS) and induced (iNOS). All forms exist in bone cells, but with different representation. The iNOS is present in osteoblasts, but is absent from osteoclasts, while the other two forms exist in all skeletal cells. NO signaling has been implicated in the adaptation of bone to mechanical loading. It has been shown that eNOS knockout mice exhibit reduced bone mass during development due to decreased osteoblastic function. Mechanical stress increases iNOS and eNOS activity in vivo, and inhibition of this activity suppresses loading-induced bone formation. In vitro, release of NO from bone cells in response to mechanical stimulation has been reported[118] and has been implicated in MAPK signaling, cytoskeletal adaptation, and possibly PGE2 signaling[119]. It is noteworthy that the polycystins of the primary cilium have recently been shown to be involved in NO signaling[120].
Other factors
Stromal cell–derived factor-1 (SDF-1)
SDF-1 is a protein with 89 amino acids that binds to the membrane receptor CXCR4 activating multiple signaling pathways involving cell adhesion and migration. These paths include FAK, PI3K, MEK and Jak/Tyk. The presence of SDF-1 is necessary for differentiation and recruitment of mesenchymal cells under the influence of BMP-2[121] and display significant increases in response to treatment with PTH[122]. The mechanical load increases the expression of the SDF-1 in osteocytes and periosteal osteoblasts and its inhibition slows load-induced bone formation.
Nucleotide signaling
ATP can function as a cell-cell signaling molecule when released into the extracellular space. ATP binds purinergic receptors which are ligand-gated ion channels that mediate extracellular calcium influx. Purinergic receptors were shown to mediate the propagation of mechanically induced intracellular calcium mobilization from cell to cell in the presence of ATP/UTP[123]. ATP/UTP has been implicated in mechanically induced activation of MAPK signaling, upregulation of the transcription factor RUNX2, and cellular proliferation. Inhibition of purinergic receptors decreases loading-induced changes in gene expression. There is evidence showing that ATP release, similar to PGE2 release, occurs as a result of the activity of connexin hemichannels[72].
Estrogens
Estrogens are important regulators of mechanically induced osteogenesis controlling the adaptation of osteoblasts and osteocytes to mechanical loads[124]. The mechanisms of action of estrogens can be both genomic, and non-genomic. The non-genomic actions are associated with activation of TGF1 receptors and activation induced by genes, such as COX2[125]. Another mechanism is also associated with the expression of the sclerostin gene[126].
This acute down-regulation of the sclerostin seems to be mediated by estrogen Receptor β[127]. ERa stimulates gene transcription via two activation functions (AFs), AF-1 in the N-terminal and AF-2 in the ligand -binding domain. ERa is required for the osteogenic response to mechanical loading in a ligand-independent manner involving AF-1 but not AF-2[128]. Recently studies have shown that functional ERα enhances the net-osteogenic response to loading in cortical but not cancellous bone in female mice but reduces it in males. ERβ decreases the response to loading in cortical bone of males and females but has no effect in cancellous bone. Bone loss due to disuse in cortical bone is unaffected by ER status, but in cancellous bone, functional ERα contributes to greater disuse-related bone loss[129].
Conclusions
The musculoskeletal system is constantly under an ever changing environment of mechanical, hydraulic or electromagnetic external or internal loading of varying intensities. This constant need of the body to adapt is carried out by a variety of mechano-sensors and cellular signaling pathways. The diversity of signals received by systems mounted on the cell membrane, such as integrins, the cadherins, the ion channels and cilia, as well as other variations of sensors that shape or stretch the cell membrane. These signals are transferred intracellularly from varied signaling systems of, such as MAPK, β-catenin, the GTPases and affecting the intracellular metabolism, recruitment, integration and proliferation of bone cells determining the way, time and locations of bone reconstruction or repair various damage from fatigue or rupture. It is now clear that little is known about the mechanical side of this system than the biological. The evolving discipline of mechanomics target to the conclusive understanding of the cellular and the pericellular mechanics of bone and other tissues. It is now clear that as little is known in this area of research to yield critical new insights. Recent technological advances have shown impressive progress in bone biology. Micro-fabricated devices have been developed to enable applied, as well as, basic research concerning the biology of the skeleton. As a new technology, microfluidics created an increasing interest in the biological and medical research for requiring less time, reduced sample quantities and low cost. A distinct advantage of microfluidics technology is the precise control and manipulation of fluids that are spatially constrained to a sub-millimeter scale. Thus, microfluidics technology is an ideal tool to study the effects of fluid flow on cells. Finally, the research on the epigenetic mechanisms offers a new approach to the study of mechanobiology. With these new developments we hope that this knowledge will create fascinating ways for development of effective treatments for the global epidemic of osteoporosis and other bone diseases.
Footnotes
The authors have no conflict of interest.
Edited by: F. Rauch
References
- 1.Wolff J, Maquet PGJ, Furlong R. The law of bone remodelling. Berlin; London: Springer-Verlag; 1986. [Google Scholar]
- 2.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–12. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 3.Karlsson MK, Johnell O, Obrant KJ. Bone mineral density in weight lifters. Calcif Tissue Int. 1993;52:212–5. doi: 10.1007/BF00298721. [DOI] [PubMed] [Google Scholar]
- 4.Kannus P, Haapasalo H, Sievanen H, Oja P, Vuori I. The site-specific effects of long-term unilateral activity on bone mineral density and content. Bone. 1994;15:279–84. doi: 10.1016/8756-3282(94)90289-5. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro F. Variable conformation of GAP junctions linking bone cells: a transmission electron microscopic study of linear, stacked linear, curvilinear, oval, and annular junctions. Calcif Tissue Int. 1997;61:285–93. doi: 10.1007/s002239900337. [DOI] [PubMed] [Google Scholar]
- 6.Kamioka H, Honjo T, Takano-Yamamoto T. A three-dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopy. Bone. 2001;28:145–9. doi: 10.1016/s8756-3282(00)00421-x. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Ciani C, Doty SB, Fritton SP. Delineating bone’s interstitial fluid pathway in vivo. Bone. 2004;34:499–509. doi: 10.1016/j.bone.2003.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdalrahman T, Scheiner S, Hellmich C. Is trabecular bone permeability governed by molecular ordering-induced fluid viscosity gain? Arguments from re-evaluation of experimental data in the framework of homogenization theory. J Theor Biol. 2015;365:433–44. doi: 10.1016/j.jtbi.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 10.Piekarski K, Munro M. Transport mechanism operating between blood supply and osteocytes in long bones. Nature. 1977;269:80–2. doi: 10.1038/269080a0. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Wang Y, Han Y, et al. In situ measurement of solute transport in the bone lacunar-canalicular system. Proc Natl Acad Sci U S A. 2005;102:11911–6. doi: 10.1073/pnas.0505193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price C, Zhou X, Li W, Wang L. Real-time measurement of solute transport within the lacunar-canalicular system of mechanically loaded bone: direct evidence for load-induced fluid flow. J Bone Miner Res. 2011;26:277–85. doi: 10.1002/jbmr.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang D, Weinbaum S, Cowin SC. Estimates of the peak pressures in bone pore water. J Biomech Eng. 1998;120:697–703. doi: 10.1115/1.2834881. [DOI] [PubMed] [Google Scholar]
- 14.Anderson EJ, Kaliyamoorthy S, Iwan J, Alexander D, Knothe Tate ML. Nano-microscale models of periosteocytic flow show differences in stresses imparted to cell body and processes. Ann Biomed Eng. 2005;33:52–62. doi: 10.1007/s10439-005-8962-y. [DOI] [PubMed] [Google Scholar]
- 15.Aarden EM, Nijweide PJ, van der Plas A, et al. Adhesive properties of isolated chick osteocytes in vitro. Bone. 1996;18:305–13. doi: 10.1016/8756-3282(96)00010-5. [DOI] [PubMed] [Google Scholar]
- 16.Burra S, Nicolella DP, Francis WL, et al. Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc Natl Acad Sci U S A. 2010;107:13648–53. doi: 10.1073/pnas.1009382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reilly GC, Haut TR, Yellowley CE, Donahue HJ, Jacobs CR. Fluid flow induced PGE2 release by bone cells is reduced by glycocalyx degradation whereas calcium signals are not. Biorheology. 2003;40:591–603. [PubMed] [Google Scholar]
- 18.Wang Y, McNamara LM, Schaffler MB, Weinbaum S. A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proc Natl Acad Sci U S A. 2007;104:15941–6. doi: 10.1073/pnas.0707246104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malone AM, Anderson CT, Tummala P, et al. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104:13325–30. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell A. The pipe and the pinwheel: is pressure an effective stimulus for the 9+0 primary cilium? Cell Biol Int. 2008;32:462–8. doi: 10.1016/j.cellbi.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Noble BS, Peet N, Stevens HY, et al. Mechanical loading: biphasic osteocyte survival and targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol. 2003;284:C934–43. doi: 10.1152/ajpcell.00234.2002. [DOI] [PubMed] [Google Scholar]
- 22.Plotkin LI, Mathov I, Aguirre JI, Parfitt AM, Manolagas SC, Bellido T. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am J Physiol Cell Physiol. 2005;289:C633–43. doi: 10.1152/ajpcell.00278.2004. [DOI] [PubMed] [Google Scholar]
- 23.Bonewald LF. Mechanosensation and Transduction in Osteocytes. Bonekey Osteovision. 2006;3:7–15. doi: 10.1138/20060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verborgt O, Gibson GJ, Schaffler MB. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res. 2000;15:60–7. doi: 10.1359/jbmr.2000.15.1.60. [DOI] [PubMed] [Google Scholar]
- 25.Clark WD, Smith EL, Linn KA, Paul-Murphy JR, Muir P, Cook ME. Osteocyte apoptosis and osteoclast presence in chicken radii 0-4 days following osteotomy. Calcif Tissue Int. 2005;77:327–36. doi: 10.1007/s00223-005-0074-z. [DOI] [PubMed] [Google Scholar]
- 26.Cardoso L, Herman BC, Verborgt O, Laudier D, Majeska RJ, Schaffler MB. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res. 2009;24:597–605. doi: 10.1359/JBMR.081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tatsumi S, Ishii K, Amizuka N, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–75. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Tami AE, Nasser P, Verborgt O, Schaffler MB, Knothe Tate ML. The role of interstitial fluid flow in the remodeling response to fatigue loading. J Bone Miner Res. 2002;17:2030–7. doi: 10.1359/jbmr.2002.17.11.2030. [DOI] [PubMed] [Google Scholar]
- 29.Zeng Y, Cowin SC, Weinbaum S. A fiber matrix model for fluid flow and streaming potentials in the canaliculi of an osteon. Ann Biomed Eng. 1994;22:280–92. doi: 10.1007/BF02368235. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka T, Sakano A. Differences in permeability of microperoxidase and horseradish peroxidase into the alveolar bone of developing rats. J Dent Res. 1985;64:870–6. doi: 10.1177/00220345850640060201. [DOI] [PubMed] [Google Scholar]
- 31.Fritton SP, Weinbaum S. Fluid and Solute Transport in Bone: Flow-Induced Mechanotransduction. Annu Rev Fluid Mech. 2009;41:347–74. doi: 10.1146/annurev.fluid.010908.165136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knothe Tate ML, Niederer P, Knothe U. In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone. 1998;22:107–17. doi: 10.1016/s8756-3282(97)00234-2. [DOI] [PubMed] [Google Scholar]
- 33.Weyts FA, Li YS, van Leeuwen J, Weinans H, Chien S. ERK activation and alpha v beta 3 integrin signaling through Shc recruitment in response to mechanical stimulation in human osteoblasts. J Cell Biochem. 2002;87:85–92. doi: 10.1002/jcb.10278. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010;2:a005066. doi: 10.1101/cshperspect.a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawada Y, Tamada M, Dubin-Thaler BJ, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–26. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horwitz A, Duggan K, Buck C, Beckerle MC, Burridge K. Interaction of plasma membrane fibronectin receptor with talin - a transmembrane linkage. Nature. 1986;320:531–3. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- 37.Subauste MC, Pertz O, Adamson ED, Turner CE, Junger S, Hahn KM. Vinculin modulation of paxillin-FAK interactions regulates ERK to control survival and motility. J Cell Biol. 2004;165:371–81. doi: 10.1083/jcb.200308011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–78. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 39.Schaller MD. The focal adhesion kinase. J Endocrinol. 1996;150:1–7. doi: 10.1677/joe.0.1500001. [DOI] [PubMed] [Google Scholar]
- 40.McNamara LM, Majeska RJ, Weinbaum S, et al. Attachment of osteocyte cell processes to the bone matrix. Anat Rec (Hoboken) 2009;292:355–63. doi: 10.1002/ar.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.You LD, Weinbaum S, Cowin SC, Schaffler MB. Ultrastructure of the osteocyte process and its pericellular matrix. Anat Rec A Discov Mol Cell Evol Biol. 2004;278:505–13. doi: 10.1002/ar.a.20050. [DOI] [PubMed] [Google Scholar]
- 42.You L, Cowin SC, Schaffler MB, Weinbaum S. A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J Biomech. 2001;34:1375–86. doi: 10.1016/s0021-9290(01)00107-5. [DOI] [PubMed] [Google Scholar]
- 43.Thompson WR, Modla S, Grindel BJ, et al. Perlecan/Hspg2 deficiency alters the pericellular space of the lacunocanalicular system surrounding osteocytic processes in cortical bone. J Bone Miner Res. 2011;26:618–29. doi: 10.1002/jbmr.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pavalko FM, Chen NX, Turner CH, et al. Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeleton-integrin interactions. Am J Physiol. 1998;275:C1591–601. [PubMed] [Google Scholar]
- 45.Malone AM, Batra NN, Shivaram G, et al. The role of actin cytoskeleton in oscillatory fluid flow-induced signaling in MC3T3-E1 osteoblasts. Am J Physiol Cell Physiol. 2007;292:C1830–6. doi: 10.1152/ajpcell.00352.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR. Mechanically induced osteogenic differentiation--the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci. 2009;122:546–53. doi: 10.1242/jcs.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen CS, Alonso JL, Ostuni E, Whitesides GM, Ingber DE. Cell shape provides global control of focal adhesion assembly. Biochem Biophys Res Commun. 2003;307:355–61. doi: 10.1016/s0006-291x(03)01165-3. [DOI] [PubMed] [Google Scholar]
- 48.McBeath R, Pirone DM, Nelson CM, et al. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–95. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 49.Lajoie P, Goetz JG, Dennis JW, Nabi IR. Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J Cell Biol. 2009;185:381–5. doi: 10.1083/jcb.200811059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubin J, Murphy TC, Rahnert J, et al. Mechanical inhibition of RANKL expression is regulated by H-Ras-GTPase. J Biol Chem. 2006;281:1412–8. doi: 10.1074/jbc.M508639200. [DOI] [PubMed] [Google Scholar]
- 51.Ferraro JT, Daneshmand M, Bizios R, Rizzo V. Depletion of plasma membrane cholesterol dampens hydrostatic pressure and shear stress-induced mechanotransduction pathways in osteoblast cultures. Am J Physiol Cell Physiol. 2004;286:C831–9. doi: 10.1152/ajpcell.00224.2003. [DOI] [PubMed] [Google Scholar]
- 52.Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 53.Radel C, Carlile-Klusacek M, Rizzo V. Participation of caveolae in beta1 integrin-mediated mechanotransduction. Biochem Biophys Res Commun. 2007;358:626–31. doi: 10.1016/j.bbrc.2007.04.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rizzo V, McIntosh DP, Oh P, Schnitzer JE. In situ flow activates endothelial nitric oxide synthase in luminal caveolae of endothelium with rapid caveolin dissociation and calmodulin association. J Biol Chem. 1998;273:34724–9. doi: 10.1074/jbc.273.52.34724. [DOI] [PubMed] [Google Scholar]
- 55.Solomon KR, Danciu TE, Adolphson LD, Hecht LE, Hauschka PV. Caveolin-enriched membrane signaling complexes in human and murine osteoblasts. J Bone Miner Res. 2000;15:2380–90. doi: 10.1359/jbmr.2000.15.12.2380. [DOI] [PubMed] [Google Scholar]
- 56.Rubin J, Schwartz Z, Boyan BD, et al. Caveolin-1 knockout mice have increased bone size and stiffness. J Bone Miner Res. 2007;22:1408–18. doi: 10.1359/jbmr.070601. [DOI] [PubMed] [Google Scholar]
- 57.Case N, Ma M, Sen B, Xie Z, Gross TS, Rubin J. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008;283:29196–205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sorice M, Manganelli V, Matarrese P, et al. Cardiolipin-enriched raft-like microdomains are essential activating platforms for apoptotic signals on mitochondria. FEBS Lett. 2009;583:2447–50. doi: 10.1016/j.febslet.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 59.Yang SH, Liu R, Perez EJ, et al. Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci U S A. 2004;101:4130–5. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maselli A, Pierdominici M, Vitale C, Ortona E. Membrane lipid rafts and estrogenic signalling: a functional role in the modulation of cell homeostasis. Apoptosis. 2015;20:671–8. doi: 10.1007/s10495-015-1093-5. [DOI] [PubMed] [Google Scholar]
- 61.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–7. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arthur A, Zannettino A, Panagopoulos R, et al. EphB/ephrin-B interactions mediate human MSC attachment, migration and osteochondral differentiation. Bone. 2011;48:533–42. doi: 10.1016/j.bone.2010.10.180. [DOI] [PubMed] [Google Scholar]
- 63.Martin TJ, Allan EH, Ho PW, et al. Communication between ephrinB2 and EphB4 within the osteoblast lineage. Adv Exp Med Biol. 2010;658:51–60. doi: 10.1007/978-1-4419-1050-9_6. [DOI] [PubMed] [Google Scholar]
- 64.Zhao C, Irie N, Takada Y, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–21. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 65.Chen JC, Hoey DA, Chua M, Bellon R, Jacobs CR. Mechanical signals promote osteogenic fate through a primary cilia-mediated mechanism. FASEB J. 2016;30:1504–11. doi: 10.1096/fj.15-276402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Espinha LC, Hoey DA, Fernandes PR, Rodrigues HC, Jacobs CR. Oscillatory fluid flow influences primary cilia and microtubule mechanics. Cytoskeleton (Hoboken) 2014;71:435–45. doi: 10.1002/cm.21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen AM, Young YN, Jacobs CR. The primary cilium is a self-adaptable, integrating nexus for mechanical stimuli and cellular signaling. Biol Open. 2015;4:1733–8. doi: 10.1242/bio.014787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol. 2003;191:69–76. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- 69.Lee KL, Guevarra MD, Nguyen AM, Chua MC, Wang Y, Jacobs CR. The primary cilium functions as a mechanical and calcium signaling nexus. Cilia. 2015;4:4. doi: 10.1186/s13630-015-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoey DA, Kelly DJ, Jacobs CR. A role for the primary cilium in paracrine signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochem Biophys Res Commun. 2011;412:182–7. doi: 10.1016/j.bbrc.2011.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009;19:R526–35. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212:207–14. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yellowley CE, Li Z, Zhou Z, Jacobs CR, Donahue HJ. Functional gap junctions between osteocytic and osteoblastic cells. J Bone Miner Res. 2000;15:209–17. doi: 10.1359/jbmr.2000.15.2.209. [DOI] [PubMed] [Google Scholar]
- 74.Vander Molen MA, Rubin CT, McLeod KJ, McCauley LK, Donahue HJ. Gap junctional intercellular communication contributes to hormonal responsiveness in osteoblastic networks. J Biol Chem. 1996;271:12165–71. doi: 10.1074/jbc.271.21.12165. [DOI] [PubMed] [Google Scholar]
- 75.Guo XE, Takai E, Jiang X, et al. Intracellular calcium waves in bone cell networks under single cell nanoindentation. Mol Cell Biomech. 2006;3:95–107. [PubMed] [Google Scholar]
- 76.Huo B, Lu XL, Hung CT, et al. Fluid Flow Induced Calcium Response in Bone Cell Network. Cell Mol Bioeng. 2008;1:58–66. doi: 10.1007/s12195-008-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saunders MM, You J, Trosko JE, et al. Gap junctions and fluid flow response in MC3T3-E1 cells. Am J Physiol Cell Physiol. 2001;281:C1917–25. doi: 10.1152/ajpcell.2001.281.6.C1917. [DOI] [PubMed] [Google Scholar]
- 78.Alford AI, Jacobs CR, Donahue HJ. Oscillating fluid flow regulates gap junction communication in osteocytic MLO-Y4 cells by an ERK1/2 MAP kinase-dependent mechanism. Bone. 2003;33:64–70. doi: 10.1016/s8756-3282(03)00167-4. [DOI] [PubMed] [Google Scholar]
- 79.Xia X, Batra N, Shi Q, Bonewald LF, Sprague E, Jiang JX. Prostaglandin promotion of osteocyte gap junction function through transcriptional regulation of connexin 43 by glycogen synthase kinase 3/beta-catenin signaling. Mol Cell Biol. 2010;30:206–19. doi: 10.1128/MCB.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chaible LM, Sanches DS, Cogliati B, Mennecier G, Dagli ML. Delayed osteoblastic differentiation and bone development in Cx43 knockout mice. Toxicol Pathol. 2011;39:1046–55. doi: 10.1177/0192623311422075. [DOI] [PubMed] [Google Scholar]
- 81.Turner CH, Robling AG, Duncan RL, Burr DB. Do bone cells behave like a neuronal network? Calcif Tissue Int. 2002;70:435–42. doi: 10.1007/s00223-001-1024-z. [DOI] [PubMed] [Google Scholar]
- 82.Abed E, Labelle D, Martineau C, Loghin A, Moreau R. Expression of transient receptor potential (TRP) channels in human and murine osteoblast-like cells. Mol Membr Biol. 2009;26:146–58. doi: 10.1080/09687680802612721. [DOI] [PubMed] [Google Scholar]
- 83.Li J, Duncan RL, Burr DB, Turner CH. L-type calcium channels mediate mechanically induced bone formation in vivo. J Bone Miner Res. 2002;17:1795–800. doi: 10.1359/jbmr.2002.17.10.1795. [DOI] [PubMed] [Google Scholar]
- 84.Shao Y, Alicknavitch M, Farach-Carson MC. Expression of voltage sensitive calcium channel (VSCC) L-type Cav1.2 (alpha1C) and T-type Cav3.2 (alpha1H) subunits during mouse bone development. Dev Dyn. 2005;234:54–62. doi: 10.1002/dvdy.20517. [DOI] [PubMed] [Google Scholar]
- 85.Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res. 2005;20:41–9. doi: 10.1359/JBMR.041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thompson WR, Majid AS, Czymmek KJ, et al. Association of the alpha(2)delta(1) subunit with Ca(v)3.2 enhances membrane expression and regulates mechanically induced ATP release in MLO-Y4 osteocytes. J Bone Miner Res. 2011;26:2125–39. doi: 10.1002/jbmr.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yan C, Takahashi M, Okuda M, Lee JD, Berk BC. Fluid shear stress stimulates big mitogen-activated protein kinase 1 (BMK1) activity in endothelial cells. Dependence on tyrosine kinases and intracellular calcium. J Biol Chem. 1999;274:143–50. doi: 10.1074/jbc.274.1.143. [DOI] [PubMed] [Google Scholar]
- 88.Rubin J, Murphy TC, Fan X, Goldschmidt M, Taylor WR. Activation of extracellular signal-regulated kinase is involved in mechanical strain inhibition of RANKL expression in bone stromal cells. J Bone Miner Res. 2002;17:1452–60. doi: 10.1359/jbmr.2002.17.8.1452. [DOI] [PubMed] [Google Scholar]
- 89.Rubin J, Murphy TC, Zhu L, Roy E, Nanes MS, Fan X. Mechanical strain differentially regulates endothelial nitric-oxide synthase and receptor activator of nuclear kappa B ligand expression via ERK1/2 MAPK. J Biol Chem. 2003;278:34018–25. doi: 10.1074/jbc.M302822200. [DOI] [PubMed] [Google Scholar]
- 90.Liu D, Genetos DC, Shao Y, et al. Activation of extracellular-signal regulated kinase (ERK1/2) by fluid shear is Ca(2+)- and ATP-dependent in MC3T3-E1 osteoblasts. Bone. 2008;42:644–52. doi: 10.1016/j.bone.2007.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hehlgans S, Haase M, Cordes N. Signalling via integrins: implications for cell survival and anticancer strategies. Biochim Biophys Acta. 2007;1775:163–80. doi: 10.1016/j.bbcan.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 92.Chatzizacharias NA, Kouraklis GP, Theocharis SE. Disruption of FAK signaling: a side mechanism in cytotoxicity. Toxicology. 2008;245:1–10. doi: 10.1016/j.tox.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 93.Wang B, Du T, Wang Y, Yang C, Zhang S, Cao X. Focal adhesion kinase signaling pathway is involved in mechanotransduction in MG-63 cells. Biochem Biophys Res Commun. 2011;410:671–6. doi: 10.1016/j.bbrc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 94.Hung CT, Pollack SR, Reilly TM, Brighton CT. Real-time calcium response of cultured bone cells to fluid flow. Clin Orthop Relat Res. 1995:256–69. [PubMed] [Google Scholar]
- 95.Donahue SW, Donahue HJ, Jacobs CR. Osteoblastic cells have refractory periods for fluid-flow-induced intracellular calcium oscillations for short bouts of flow and display multiple low-magnitude oscillations during long-term flow. J Biomech. 2003;36:35–43. doi: 10.1016/s0021-9290(02)00318-4. [DOI] [PubMed] [Google Scholar]
- 96.Chen NX, Ryder KD, Pavalko FM, et al. Ca(2+) regulates fluid shear-induced cytoskeletal reorganization and gene expression in osteoblasts. Am J Physiol Cell Physiol. 2000;278:C989–97. doi: 10.1152/ajpcell.2000.278.5.C989. [DOI] [PubMed] [Google Scholar]
- 97.McAllister TN, Frangos JA. Steady and transient fluid shear stress stimulate NO release in osteoblasts through distinct biochemical pathways. J Bone Miner Res. 1999;14:930–6. doi: 10.1359/jbmr.1999.14.6.930. [DOI] [PubMed] [Google Scholar]
- 98.Ryder KD, Duncan RL. Parathyroid hormone enhances fluid shear-induced [Ca2+]i signaling in osteoblastic cells through activation of mechanosensitive and voltage-sensitive Ca2+ channels. J Bone Miner Res. 2001;16:240–8. doi: 10.1359/jbmr.2001.16.2.240. [DOI] [PubMed] [Google Scholar]
- 99.Kwon RY, Temiyasathit S, Tummala P, Quah CC, Jacobs CR. Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic AMP in bone cells. FASEB J. 2010;24:2859–68. doi: 10.1096/fj.09-148007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–15. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Case N, Rubin J. Beta-catenin--a supporting role in the skeleton. J Cell Biochem. 2010;110:545–53. doi: 10.1002/jcb.22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boyden LM, Mao J, Belsky J, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–21. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 103.Case N, Sen B, Thomas JA, et al. Steady and oscillatory fluid flows produce a similar osteogenic phenotype. Calcif Tissue Int. 2011;88:189–97. doi: 10.1007/s00223-010-9448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Norvell SM, Alvarez M, Bidwell JP, Pavalko FM. Fluid shear stress induces beta-catenin signaling in osteoblasts. Calcif Tissue Int. 2004;75:396–404. doi: 10.1007/s00223-004-0213-y. [DOI] [PubMed] [Google Scholar]
- 105.Bidwell JP, Pavalko FM. The Load-Bearing Mechanosome Revisited. Clin Rev Bone Miner Metab. 2010;8:213–23. doi: 10.1007/s12018-010-9075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Robling AG, Niziolek PJ, Baldridge LA, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–75. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 107.Kogianni G, Mann V, Noble BS. Apoptotic bodies convey activity capable of initiating osteoclastogenesis and localized bone destruction. J Bone Miner Res. 2008;23:915–27. doi: 10.1359/jbmr.080207. [DOI] [PubMed] [Google Scholar]
- 108.Thorsen K, Kristoffersson AO, Lerner UH, Lorentzon RP. In situ microdialysis in bone tissue. Stimulation of prostaglandin E2 release by weight-bearing mechanical loading. J Clin Invest. 1996;98:2446–9. doi: 10.1172/JCI119061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Donahue TL, Haut TR, Yellowley CE, Donahue HJ, Jacobs CR. Mechanosensitivity of bone cells to oscillating fluid flow induced shear stress may be modulated by chemotransport. J Biomech. 2003;36:1363–71. doi: 10.1016/s0021-9290(03)00118-0. [DOI] [PubMed] [Google Scholar]
- 110.Batra NN, Li YJ, Yellowley CE, et al. Effects of short-term recovery periods on fluid-induced signaling in osteoblastic cells. J Biomech. 2005;38:1909–17. doi: 10.1016/j.jbiomech.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 111.Searby ND, Steele CR, Globus RK. Influence of increased mechanical loading by hypergravity on the microtubule cytoskeleton and prostaglandin E2 release in primary osteoblasts. Am J Physiol Cell Physiol. 2005;289:C148–58. doi: 10.1152/ajpcell.00524.2003. [DOI] [PubMed] [Google Scholar]
- 112.Jee WS, Ke HZ, Li XJ. Long-term anabolic effects of prostaglandin-E2 on tibial diaphyseal bone in male rats. Bone Miner. 1991;15:33–55. doi: 10.1016/0169-6009(91)90109-d. [DOI] [PubMed] [Google Scholar]
- 113.Chow JW, Chambers TJ. Indomethacin has distinct early and late actions on bone formation induced by mechanical stimulation. Am J Physiol. 1994;267:E287–92. doi: 10.1152/ajpendo.1994.267.2.E287. [DOI] [PubMed] [Google Scholar]
- 114.Rawlinson SC, Mohan S, Baylink DJ, Lanyon LE. Exogenous prostacyclin, but not prostaglandin E2, produces similar responses in both G6PD activity and RNA production as mechanical loading, and increases IGF-II release, in adult cancellous bone in culture. Calcif Tissue Int. 1993;53:324–9. doi: 10.1007/BF01351837. [DOI] [PubMed] [Google Scholar]
- 115.Pitsillides AA, Rawlinson SC, Suswillo RF, Bourrin S, Zaman G, Lanyon LE. Mechanical strain-induced NO production by bone cells: a possible role in adaptive bone (re)modeling? FASEB J. 1995;9:1614–22. doi: 10.1096/fasebj.9.15.8529841. [DOI] [PubMed] [Google Scholar]
- 116.Ajubi NE, Klein-Nulend J, Alblas MJ, Burger EH, Nijweide PJ. Signal transduction pathways involved in fluid flow-induced PGE2 production by cultured osteocytes. Am J Physiol. 1999;276:E171–8. doi: 10.1152/ajpendo.1999.276.1.E171. [DOI] [PubMed] [Google Scholar]
- 117.McGarry JG, Klein-Nulend J, Prendergast PJ. The effect of cytoskeletal disruption on pulsatile fluid flow-induced nitric oxide and prostaglandin E2 release in osteocytes and osteoblasts. Biochem Biophys Res Commun. 2005;330:341–8. doi: 10.1016/j.bbrc.2005.02.175. [DOI] [PubMed] [Google Scholar]
- 118.Klein-Nulend J, Semeins CM, Ajubi NE, Nijweide PJ, Burger EH. Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts - correlation with prostaglandin upregulation. Biochem Biophys Res Commun. 1995;217:640–8. doi: 10.1006/bbrc.1995.2822. [DOI] [PubMed] [Google Scholar]
- 119.Bakker AD, Joldersma M, Klein-Nulend J, Burger EH. Interactive effects of PTH and mechanical stress on nitric oxide and PGE2 production by primary mouse osteoblastic cells. Am J Physiol Endocrinol Metab. 2003;285:E608–13. doi: 10.1152/ajpendo.00501.2002. [DOI] [PubMed] [Google Scholar]
- 120.Abou Alaiwi WA, Lo ST, Nauli SM. Primary cilia: highly sophisticated biological sensors. Sensors (Basel) 2009;9:7003–20. doi: 10.3390/s90907003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhu W, Boachie-Adjei O, Rawlins BA, et al. A novel regulatory role for stromal-derived factor-1 signaling in bone morphogenic protein-2 osteogenic differentiation of mesenchymal C2C12 cells. J Biol Chem. 2007;282:18676–85. doi: 10.1074/jbc.M610232200. [DOI] [PubMed] [Google Scholar]
- 122.Jung Y, Wang J, Schneider A, et al. Regulation of SDF-1 (CXCL12) production by osteoblasts;a possible mechanism for stem cell homing. Bone. 2006;38:497–508. doi: 10.1016/j.bone.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 123.You J, Jacobs CR, Steinberg TH, Donahue HJ. P2Y purinoceptors are responsible for oscillatory fluid flow-induced intracellular calcium mobilization in osteoblastic cells. J Biol Chem. 2002;277:48724–9. doi: 10.1074/jbc.M209245200. [DOI] [PubMed] [Google Scholar]
- 124.Callewaert F, Bakker A, Schrooten J, et al. Androgen receptor disruption increases the osteogenic response to mechanical loading in male mice. J Bone Miner Res. 2010;25:124–31. doi: 10.1359/jbmr.091001. [DOI] [PubMed] [Google Scholar]
- 125.Liedert A, Wagner L, Seefried L, Ebert R, Jakob F, Ignatius A. Estrogen receptor and Wnt signaling interact to regulate early gene expression in response to mechanical strain in osteoblastic cells. Biochem Biophys Res Commun. 2010;394:755–9. doi: 10.1016/j.bbrc.2010.03.065. [DOI] [PubMed] [Google Scholar]
- 126.Zaman G, Saxon LK, Sunters A, et al. Loading-related regulation of gene expression in bone in the contexts of estrogen deficiency, lack of estrogen receptor alpha and disuse. Bone. 2010;46:628–42. doi: 10.1016/j.bone.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Galea GL, Meakin LB, Sugiyama T, et al. Estrogen receptor alpha mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist Sost is mediated by estrogen receptor beta. J Biol Chem. 2013;288:9035–48. doi: 10.1074/jbc.M112.405456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Windahl SH, Saxon L, Borjesson AE, et al. Estrogen receptor-alpha is required for the osteogenic response to mechanical loading in a ligand-independent manner involving its activation function 1 but not 2. J Bone Miner Res. 2013;28:291–301. doi: 10.1002/jbmr.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Saxon LK, Galea G, Meakin L, Price J, Lanyon LE. Estrogen receptors alpha and beta have different gender-dependent effects on the adaptive responses to load bearing in cancellous and cortical bone. Endocrinology. 2012;153:2254–66. doi: 10.1210/en.2011-1977. [DOI] [PubMed] [Google Scholar]


