Abstract
Purpose:
The Achilles tendon (AT) must adapt to meet changes in demands. This study explored AT adaptation by comparing properties within the jump and non-jump legs of jumping athletes. Non-jumping control athletes were included to control limb dominance effects.
Methods:
AT properties were assessed in the preferred (jump) and non-preferred (lead) jumping legs of male collegiate-level long and/or high jump (jumpers; n=10) and cross-country (controls; n=10) athletes. Cross-sectional area (CSA), elongation, and force during isometric contractions were used to estimate the morphological, mechanical and material properties of the ATs bilaterally.
Results:
Jumpers exposed their ATs to more force and stress than controls (all p≤0.03). AT force and stress were also greater in the jump leg of both jumpers and controls than in the lead leg (all p<0.05). Jumpers had 17.8% greater AT stiffness and 24.4% greater Young’s modulus in their jump leg compared to lead leg (all p<0.05). There were no jump versus lead leg differences in AT stiffness or Young’s modulus within controls (all p>0.05).
Conclusion:
ATs chronically exposed to elevated mechanical loading were found to exhibit greater mechanical (stiffness) and material (Young’s modulus) properties.
Keywords: Adaptation, Exercise, Stiffness, Triceps Surae, Ultrasound, Young’s Modulus
Introduction
Tendons enable movement by transmitting muscle-generated forces to the skeleton. In doing so, they also store and release elastic energy to reduce the energy cost of movement1,8. Tendons are exposed to considerable tensile loading in their force transmitting role19 and are subsequently vulnerable to developing load-induced damage2. In order to maintain tissue homeostasis and meet changes in mechanical demands, tendons must possess an ability to respond and adapt to their mechanical environment18. Tendons have indeed been found to be mechanosensitive, with single bouts of loading and long-term habitual loading elevating collagen synthesis levels23,24,29. However, the extent to which the elevated collagen synthesis induces tendon adaptation, as opposed to simply maintaining tissue integrity, remains unclear.
The impact of mechanical loading associated with exercise on tendon properties has previously been assessed by cross-sectionally comparing tendons between cohorts of individuals habitually exposed to different activities. Male runners were shown to have larger and st iffer Achilles tendons (ATs) than non-runners4,26,32, and male athletes who frequently performed weight-bearing exercise (running, jumping) were found to have a larger AT than athletes in non-weight bearing sports (kayakers)20. However, cross-sectional studies comparing tendon properties between cohorts of individuals are typically limited as they do not account for selection bias wherein inherited and other system the change in tendon properties within individuals over time; however, current longitudinal studies have provided contrasting evidence of tendon adaptability to loading. Some longitudinal studies have reported no adaptation17,36, while others have reported loading-induced increases in tendon size28 or mechanical and material properties5,16,21,37, or both3,11,34.
An alternative means of assessing tendon adaptation to load is to compare bilateral tendon properties within individuals who have chronically exposed one lower extremity to elevated loads. This within-subject approach enables the contralateral side to serve as an internal control site for inherited and other systemic factors which contribute to inter-subject variability, and has been successfully used to demonstrate patellar tendon hypertrophy and increased stiffness in the lunging leg of fencing and badminton athletes15. Whether similar side-to-side differences are present in the ATs of athletes who are chronically exposed to unilateral loading has not been established, but would provide novel insight to clarify how the morphological, mechanical and material properties of the AT adapt to withstand elevated mechanical loads. Mechanical properties reflect the features that provide a structure with the ability to withstand load, and are dependent on both the quality (i.e. amount and type) of material present and its arrangement (i.e. structure). In contrast, material properties reflect the ability of the constituent material to withstand load independent of tendon morphology (i.e. structure).
The aim of the current study was to compare AT morphological, mechanical and material properties in the jump and lead (non-jump) legs of collegiate-level jumping (long and/or high jump) athletes. Jumping athletes repetitively and forcibly jump off one leg, with the jump leg being exposed to an active peak vertical ground reaction force during take-off that is more than double that experienced during maximal sprinting14,25,27,30. To confirm exposure of the jump leg to greater loads, we assessed jumping performance in both the jump and contralateral lead legs. A group of non-jumping control athletes were included to ensure that any jump-to-lead leg differences in jumping athletes were not due to elevated habitual unilateral loading associated with simple limb dominance10.
Materials and methods
Participants
Cohorts of male collegiate-level jumping athletes (jumpers; n=10) and cross-country athletes (controls; n=10) aged 18-30 years were recruited. Jumpers were included if they were currently competing or practicing in collegiate-level long and/or high jump and had been continuously participating in competitive jumping for at least 3 years. Participants in both groups were excluded if they had: 1) participated >2 times per month for >6 months within the previous 3 years in an athletic activity (including basketball, triple jump, volleyball) that may preferentially load one lower extremity (except high or long jump in jumpers); 2) a lifetime history of any AT pathology, and; 3) been exposed to lower extremity surgery or lower extremity immobilization for >2 weeks within the past 2 years. The jump leg was defined as the take-off leg in jumpers and preferred hopping leg in controls. The contralateral leg was defined as the non-jumping or lead leg. Jumpers completed a questionnaire to document their participation and best performance in jumping endeavours. Whole-body lean (kg) and fat mass (%) was assessed in all subjects via whole-body dual-energy X-ray absorptiometry (DXA) using the manufacturer’s standard protocols on a Hologic Discovery-W machine equipped with Apex v4.0 software (Hologic, Inc., Waltham, MA, USA). The study was approved by the Institutional Review Board of Indiana University and all participants provided written informed consent.
Jumping performance
Single-leg counter-movement jumps with freely moving arms were used to assess jump height and force for both the jump and lead legs. Subjects were allowed to warm up (e.g., jogging, dynamic stretching), but were requested not to perform any static stretching before testing. During each jump movement, subjects were instructed to take a single step and jump as high as possible off the leg being tested.
Jump height was measured using a Vertec vertical jump meter with moveable vanes every half inch (1.27 cm) (Sports Imports, Columbus, OH). Vertical reach was measured from a flat-footed standing position before subjects performed a single-leg countermovement jump to displace the highest vane possible. Vertical jump height (cm) was measured as the distance difference between standing and jump reach. Subjects were permitted to perform 3 jumps on each leg separated by ≥1 min, with the best jump on each leg recorded as jump height.
Jump force was measured as per jump height, but with subjects performing jump movements on an AMTI force platform (OR6-7-1000 with Gen5 digital amplifiers; Advanced Mechanical Technologies, Inc., Watertown, MA). Body mass force was measured by having subjects stand quietly on the force platform. Subjects subsequently performed single-leg countermovement jumps on each leg during which force within the acceleration phase of the jump was collected at 100 Hz using Vicon Nexus software (version 1.8.5; Vicon, Oxford, UK). Subjects were permitted to perform 3 jumps on each leg separated by ≥1 min, with the highest force on each leg recorded as the jump force. The jump force on each leg was subsequently divided by the force of body mass to convert data into units of body weight (BW).
Tendon morphometry
AT resting length, cross-sectional area (CSA) and internal moment arm within both the jump and lead legs were measured prone with the hip and knee extended and ankle in neutral (i.e. 90° angle between the foot and shank). A portable diagnostic ultrasound machine (MyLab™ 25 Gold; Esaote, Florence, Italy) equipped with a 10-18 MHz real-time linear array transducer was used to obtain images of the AT. To acquire tendon resting length, the ultrasound transducer was initially positioned longitudinally over the posterior aspect of the heel to locate the insertion of the AT to the calcaneus. A marker casting a hypoechoic shadow was positioned on the skin overlying the tendon attachment site. The transducer was subsequently moved proximally to locate the musculotendinous junction of the medial gastrocnemius muscle and a second marker casting a hypoechoic shadow was positioned over the corresponding skin location. The distance between the two skin markers was measured in triplicate using a tape measure to acquire AT resting length (mm).
For tendon CSA, the ultrasound transducer was positioned perpendicular to the tendon to acquire tomographic images at 2, 4, and 6 cm proximal to the distal skin marker (i.e. tendon insertion), as previously described6. The outline of the AT was traced and analyzed using the ultrasound imaging system’s software to acquire CSA (m2) at each imaged level, with the average of the three measures per tendon used in calculations of tendon mechanical and material properties.
The AT internal moment arm was estimated using a combination of ultrasonography and anthropometry, as previously described13. The distance between the midpoint of the AT directly horizontal to the medial malleolus and the skin overlying the posterior surface of the AT was measured ultrasonographically. This value was deducted from the horizontal distance between the medial malleolus (approximate joint center) and skin overlying the posterior surface of the AT, with the latter distance obtained using a tape measure. The net outcome was estimated AT internal moment arm.
Tendon mechanical and material properties
To enable derivation of AT mechanical and material properties, subjects were positioned prone on a Biodex System 2 isokinetic dynamometer (Biodex Medical Systems Inc., Shirley, NY) with their hip and knee extended and foot in neutral. The lateral malleolus was aligned with the axis of rotation of the dynamometer and the foot was firmly secured to the foot plate using a ratchet tie down strap to ensure that there was no movement of the heel during contractions. Additional straps over the thorax and hips were used to prevent forward displacement of the body during testing. Subjects were asked to perform multiple slow, ramped, incremental plantar flexion contractions to 25%, 50%, 75% and 100% of maximal isometric force. Subjects held the contraction at each increment for 2-3 sec before relaxing for 4-5 seconds and proceeding to the next contraction level. A warm-up trial was performed followed by 1 min rest and a data collection trial.
During the data collection trial, the ultrasound transducer was manually positioned over the proximal skin marker so that a hypoechoic shadow was visibsle at the musculotendinous junction of the medial gastrocnemius muscle. Static ultrasound images of the musculotendinous junction were taken during each incremental contraction and the simultaneous external isometric plantar flexion torque (Nm) was recorded. Tendon elongation (mm) during each incremental contraction was measured as the distance of the musculotendinous junction from its starting position (i.e. hypoechoic shadow of the proximal skin marker) (Figure 1). AT force (N) was derived as the quotient of external torque and internal moment arm. Assessments were performed bilaterally to obtain measures in both the jumping and lead legs.
Figure 1.
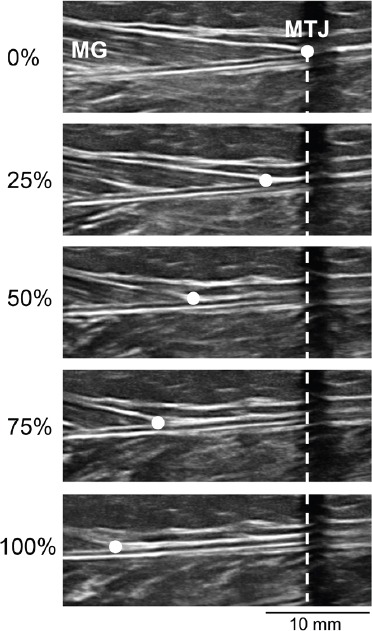
Ultrasound images of the musculotendinous junction (MTJ) of the medial gastrocnemius (MG) muscle at rest (0%), 25%, 50%, 75% and 100% of maximal voluntary contraction in a representative subject. A skin marker that cast a hypoechoic shadow was positioned over the MTJ when the MG was at rest (0%). Tendon elongation (mm) during each incremental contraction was measured as the distance of the MTJ (dot) from the hypoechoic shadow (dashed line).
For each individual tendon, tendon force and elongation during each incremental contraction was plotted on a force versus displacement graph. An exponential curve (as opposed to a linear, quadratic, cubic or logarithmic curve) was fit to the data as it provided the best fit (i.e. strongest correlation) for the greatest number of tendons. The linear slope between the 75% and 100% points of the curve (i.e. last 25% of the curve) was calculated to obtain a measure of tendon stiffness (N/mm). This region of the curve was used as it had the greatest slope. Tendon strain (%) during each incremental contraction was calculated by dividing the elongation of the tendon by resting length, whereas tendon stress (MPa) during each incremental contraction was derived by dividing the calculated tendon force with the average CSA of the tendon. The tendon strain and stress data were plotted and fit with an exponential curve, as described above. The linear slope between the 75% and 100% points of the curve (i.e. region with the greatest slope) was calculated to obtain a measure of Young’s modulus (MPa).
Statistical analyses
Two-tailed analyses with α=0.05 were performed with IBM SPSS Statistics (v21; SPSS Inc., Chicago, IL). Participant characteristics were compared between jumpers and controls using independent sample t-tests. Group effects on jumping performance and tendon measures were determined using two-way, one-repeated measure analysis of variance (ANOVA), with activity (jumpers vs. controls) and leg (jump vs. lead) being the between- and within-individual independent variables, respectively. In the advent of a non-significant ANOVA interaction, main effects for each independent variable were explored. Significant ANOVA interactions were explored using simple effects tests to assess for the effect of activity (jumpers vs. controls) on properties in the jump and lead legs (independent sample t-tests) and effect of leg (jump vs. lead) on properties within the jumpers and controls (paired sample t-tests). A false discovery rate (FDR) threshold set at q=0.05 was used to correct for multiple comparisons7.
Results
Participant characteristics are detailed in Table 1. Jumpers had greater total and lean mass and less percent fat mass than controls (all p<0.01). There were significant interactions for jump force and height between the activity (jumpers vs. controls) and leg (jump vs. lead) groups during jump performance testing (all p<0.05; Figure 2). The interactions resulted from jumpers being able to generate 9.5% (0.29 BW; 95% CI, 0.13 to 0.45 BW) greater ground reaction jump force and jump 13.6% (8.1 cm; 95% CI, 1.8 to 14.4 cm) higher with their jump leg compared to their contralateral lead leg (all p<0.02; Figure 2). In contrast, there were no side-to-side differences for jump force or height during jumping performance assessment in controls (all p=0.40 to 0.53; Figure 2).
Table 1.
Demographic and anthropometric characteristics of jumpers and controlsa.
| Tendon property | Controls | Jumpers |
|---|---|---|
| Demographics | ||
| - Age (yr) | 22.4±3.3 | 20.9±2.1 |
| - Age starting competitive jumping (yr) | - | 14.2±1.9 |
| - Year competing (yr) | - | 6.7±3.2 |
| - Jumping sport (long:high jump) | - | 10:6b |
| - Jump training per week (min) | - | 195±97 |
| - Jumps per week (n) | - | 70±39 |
| - Personal best: long jump (m) | - | 7.05±0.50 |
| - Personal best: high jump (m) | - | 1.97±0.18 |
| Whole-body anthropometry | ||
| - Height (m) | 1.77±0.08 | 1.82±0.10 |
| - Mass (kg) | 67.9±8.9 | 77.8±6.3* |
| - BMI (kg/m2) | 21.6±1.8 | 23.5±2.0* |
| - Lean mass (kg)c | 48.0±5.8 | 58.1±4.8* |
| - Fat mass (%)c | 17.1±3.1 | 14.0±1.4* |
Data indicate mean ± SD (except for frequencies)
6 jumpers participated in both long and high jump
Obtained via dual-energy x-ray absorptiometry
p<0.05 (independent sample t-test: controls vs. jumpers)
Figure 2.
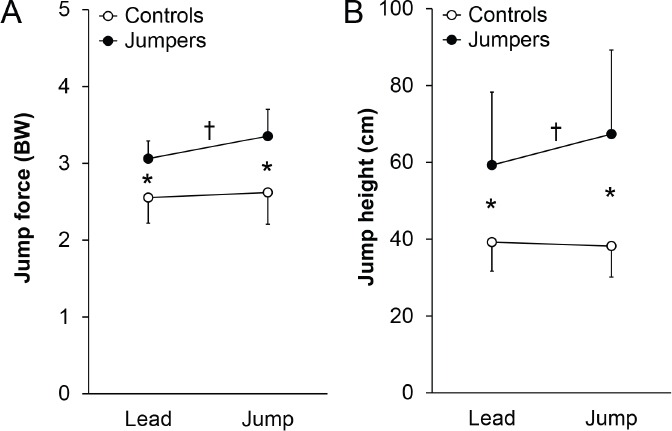
Jump. A) force and B) height during a counter-movement jump off of the preferred (jump) and non-preferred (lead) jumping legs in jumpers and controls. There were significant activity X leg interactions on both jump force and height, as determined by two-way, one-repeated measure ANOVA. Jumpers exerted more force and jumped higher off their jump leg vs. lead leg (†p<0.05, paired t-test), and exerted more force and jumped higher off both their jump and lead legs compared to the corresponding leg in controls (*p<0.05, unpaired t-test). There were no jump vs. lead leg differences in jump force or height within controls. Data represent mean ± SD.
There were no statistical interactions between activity (jumpers vs. controls) and leg (jump vs. lead) groups during tendon property testing for tendon resting length, CSA, force, elongation, stress or strain, indicating that side-to-side differences in jumpers were equivalent with those associated with limb dominance in controls (all p≥0.24; Table 2). There were no main effects for either activity or leg group on tendon resting length, CSA, elongation or strain, indicating that these tendon properties did not differ between jumpers and controls or between jump and lead legs (all p≥0.25; Table 2). However, bilateral tendons in jumpers were exposed to 42.9% (742 N; 95% CI, 67 to 1417 N) more force and 45.9% (13.1 MPa, 95% CI, 3.6 to 22.6 MPa) more stress during tendon property testing than in controls (all p ≤0.03; Table 2). Similarly, there was a significant main effect for leg group on both tendon force and stress, with jump leg tendons in jumpers and controls being exposed to 9.2% (185 N; 95% CI, 3 to 368 N) more force and 10.0% (3.3 MPa, 95% CI, 0.2 to 6.5 MPa) more stress during plantarflexion testing than in lead leg tendons (all p<0.05; Table 2).
Table 2.
Achilles tendon morphological, material, and mechanical propertiesa.
| Controls | Jumpers | Two-way ANOVA results | |||||
|---|---|---|---|---|---|---|---|
| Tendon property | Lead leg | Jump leg | Lead leg | Jump leg | Activity | Leg | Interaction |
| Resting length (mm) | 234±32 | 235±29 | 239±27 | 238±27 | NS | NS | NS |
| CSA (mm2) | 61.0±12.0 | 60.9±10.8 | 59.3±8.6 | 61.1±10.9 | NS | NS | NS |
| Force (N) | 1650±728 | 1809±659 | 2366±922 | 2578±633 | <0.05 | 0.03 | NS |
| Elongation (mm) | 13.7±4.2 | 14.2±3.5 | 16.2±3.2 | 15.3±3.8 | NS | NS | NS |
| Stress (MPa) | 27.1±10.9 | 29.9±9.6 | 39.6±12.8 | 43.5±8.6 | 0.01 | 0.04 | NS |
| Strain (%) | 5.93±1.85 | 6.23±1.87 | 6.85±1.52 | 6.37±1.99 | NS | NS | NS |
Data indicate mean ± SD
There were significant interactions between activity (jumpers vs. controls) and leg (jump vs. lead) groups for both stiffness and Young’s modulus, indicating that side-to-side differences in jumpers differed from that associated with limb dominance in controls (all p≤0.04; Figure 3A,B). The interactions resulted from jumpers having 17.8% (37.8 N/mm; 95% CI, 2.9 to 72.7 N/mm) and 24.4% (245 MPa; 95% CI, 4 to 486 MPa) greater tendon stiffness and Young’s modulus in the jump leg compared to the contralateral lead leg, respectively (all p<0.05; Figure 3A-C). Jumpers also had 35.3% (65.7 N/mm; 95% CI, 26.7 to 72.7 N/mm) and 76.7% (544 MPa; 95% CI, 227 to 861 MPa) greater tendon stiffness and Young’s modulus in their jump leg compared to in the jump leg of controls, respectively (all p<0.01; Figure 3A,B). In contrast, there were no side-to-side differences for tendon stiffness (-4.4 N/mm; 95% CI, -20.7 to 12.0 N/mm) or Young’s modulus (-32 MPa; 95% CI, -170 to 106 MPa) in controls (all p =0.56 to 0.61; Figure 3A,B).
Figure 3.
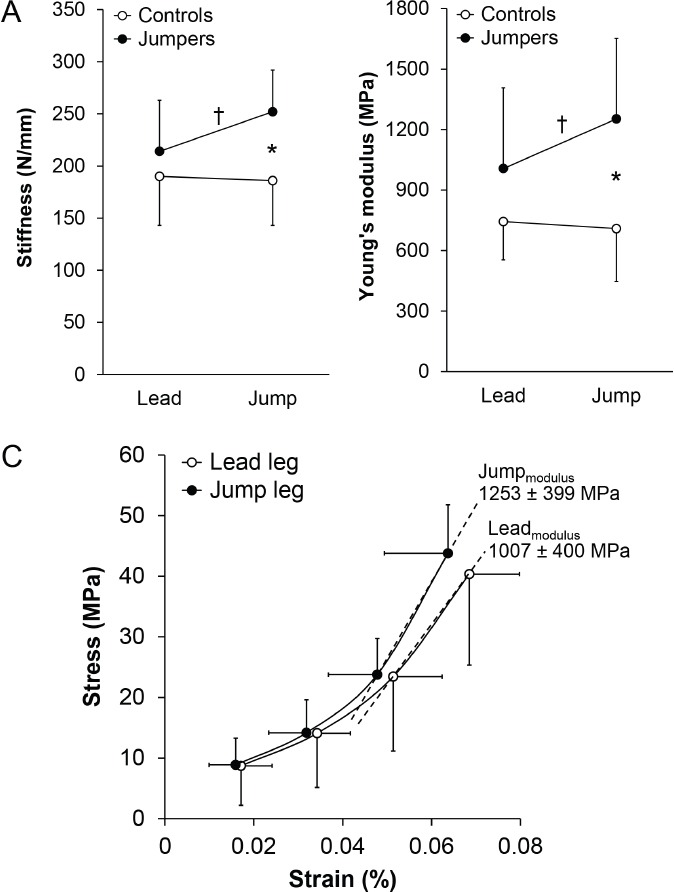
Achilles tendon. A) stiffness and B) Young’s modulus in the preferred (jump) and non-preferred (lead) jumping legs in jumpers and controls. There were significant activity X leg interactions on both stiffness and Young’s modulus, as determined by two-way, one-repeated measure ANOVA. Jumpers had greater Achilles tendon stiffness and Young’s modulus in their jump leg compared to their lead leg (†p<0.05, paired t-test), and compared to the jump leg in controls (*p<0.05, unpaired t-test). There were no jump vs. lead leg differences in Achilles tendon stiffness or Young’s modulus within controls, or between lead legs in jumpers and controls. Data represent mean ± SD. C) Mean (± SD) stress and strain at 25%, 50%, 75% and 100% of maximal voluntary contraction in the jump and lead legs of jumpers, and mean (± SD) Young’s modulus. Young’s modulus was calculated from the linear slope between the 75% and 100% points of an exponential curve fit to the data of each individual tendon.
Discussion
This within-subject controlled, cross-sectional cohort study found collegiate-level athletes with a history of competing in unilateral dominant jumping sports (long and/or high jump) had greater AT stiffness and Young’s modulus in their jump leg compared to their contralateral lead leg. The assessment of jumping effects on AT material and mechanical properties within-subject by using the lead leg as an internal control protected the cross-sectional nature of this study against the impact of potential systemic factors, including genetics/natural selection. There were no jump-to-lead leg differences in a cohort of athletic controls (collegiate-level cross country athletes), indicating that jump-to-lead leg differences in jumpers were not due to habitual loading associated with limb dominance10. To our knowledge, only one previous study has used a within-subject controlled, cross-sectional cohort study design to explore tendon adaptation as a result of prolonged loading, with findings limited to the patellar tendon15.
Adaptation of the AT to its mechanical environment follows teleologically given the mechanical role of the tendon. The current data suggests that the AT adapted to the repeated mechanical loading associated with jumping principally via a change in its material properties, as opposed to a change in its morphology. There were no measurable differences in AT CSA between the jump and lead legs in jumping athletes or between jumpers and controls. In contrast, jumpers had 24.4% and 76.7% greater Young’s modulus in their jump leg compared to in their lead leg and the jump leg of controls, respectively. As Young’s modulus reflects the stiffness of the constituent material independent of tendon morphology (i.e. material stiffness), a difference in Young’s modulus represents a difference in material properties.
In addition to having greater Young’s modulus, the AT in the jump leg of jumpers had 17.8% and 35.3% greater stiffness than in their lead leg and the jump leg of controls, respectively. These differences in AT stiffness further support a difference in material properties associated with jumping. Stiffness is a mechanical property that represents the ability of a structure to resist deformation (i.e. structural stiffness). It is dependent upon both the quality (i.e. amount and type) of material present and how the material is arranged (i.e. structure). As there were no measurable group differences in AT CSA, the greater structural stiffness of the AT in the jump leg of jumpers reflects a difference in material properties. This finding is in agreement with the conclusion of Bohm et al.9 who performed a systematic review and meta-analysis to conclude that intervention-induced changes in tendon stiffness seem to be more attributed to adaptations of the material rather than morphological properties.
The current data contribute to the growing body of work exploring the adaptation of the AT to mechanical loading. Pooling data via systematic review, both Bohm et al.9 and Wiesinger et al.39 recently reported great variability between the results of studies exploring the effect of mechanical loading on AT morphometric, mechanical and material properties. Part of the variability was attributed to methodological heterogeneity between studies9,39; however, Wiesinger et al.39 ultimately hypothesized that material and morphological adaptation of the AT to mechanical loading occur on the same continuum. Mechanical loading of the AT has been shown to trigger a tendon remodelling response that is measureable within a few days24. Wiesinger et al.39 hypothesized this initial remodelling response increases tissue density contributing to early increases in tendon stiffness and Young’s modulus. Once a critical density is reached, Wiesinger et al.39 hypothesized tendon hypertrophy occurs at a slower rate to normalize stresses.
The current data are not in full agreement with Wiesinger et al.’s39 hypothesis as there were no differences in tendon CSA, despite tendons being repetitively or forcibly loaded over a period of years. One explanation for our disparate finding may be the fact that all tendons in both our jumper and control groups were exposed to repetitive loading over a prolonged period of time. This may have resulted in morphometric changes in all tendons, with any superimposed effect of jumping being negligible or too small to be detected. In contrast, previous studies reporting exercise effects on AT CSA may have potentiated differences or changes by comparing individuals with vastly differing levels of mechanical loading4,20,26,32 or exploring changes in AT CSA over time in individuals exposed to a novel loading regime that varied greatly from usual loads3,11,28.
The greater AT Young’s modulus and stiffness in the jump leg of jumpers represents a favourable adaptation. During explosive activities, such as jumping, a structurally and materially stiffer tendon enables an improved ability to transmit muscle-generated forces12. The functional consequence is improved explosive activity performance12, which was reflected in the current study by greater jump force and height in the jump leg of jumpers. From a pathological standpoint, a stiffer tendon is advantageous as it may be more resistant to accumulating damage and failing. Stiffer tendons have reduced strain per given applied force3,4. As strain is hypothesized as the primary mechanical parameter governing tendon damage accumulation and injury40, and it is intuitive that tendons experience failure in a relatively set strain range22, a stiffer tendon that experiences less strain per unit force has a greater safety factor between functional and injurious tendon strains.
A stiffer tendon is exposed to greater stress which may be considered potentially hazardous. However, tendon ultimate stress (i.e. the stress at which a tendon fails) is directly correlated to the tendon Young’s modulus22. Thus, the increase in Young’s modulus observed in jump leg of jumpers would be associated with the tendon being able to tolerate more stress before failure. Further support for the benefits of the observed greater Young’s modulus and stiffness in the jump leg of jumpers are the observations that these properties are reduced in dysfunctional ATs6, and decrease with both disuse31 and aging35.
Our study had a number of strengths, including the comparison of AT mechanical and material properties within-subject to control for selection bias and the inclusion of an athletic comparative group that did not unilaterally exposed one AT to elevated loads. However, our study also possessed a number of limitations. A relatively small sample size was recruited which may have reduced our power to detect subtle group differences in some measures, such as CSA. CSA was assessed using ultrasonography as the average of three pre-selected regions, consistent with previous protocols6,13,16,28,37. However, adaptation of AT morphometry to jumping-related mechanical loads may be region specific3,11,20,26 and occurred outside the regions assessed. Alternatively, there may simply have been an absence of side-to-side differences in CSA, with other studies observing a similar lack of effect of loading on tendon size5,16,17,37. We only imaged motion of the musculotendinous junction during isometric contraction and not simultaneous motion of the calcaneus. Calcaneus motion occurs during isometric contraction of the triceps surae muscle and can impact measures of AT elongation33. Greater calcaneal motion is likely to occur in legs capable of generating more plantarflexion force, which would overestimate AT elongation and strain and lead to an underestimation of stiffness and Young’s modulus. However, the jump leg of jumpers had greater stiffness and Young’s modulus than in the contralateral lead leg despite an ability to generate more plantarflexion force in the former. Finally, our data are limited to males, with adaptive responses and their magnitude potentially differing in females38.
In summary, the current data demonstrate that chronic exposure of the AT to elevated loads results in adaptation of its mechanical and material properties. Using a within-subject controlled study design to protect against the influence of selection bias, the AT in the jump leg of male collegiate-level jumping athletes had 17.8% and 24.4% greater stiffness and Young’s modulus compared to in the contralateral lead (non-jump) leg, respectively. The side-to-side differences in jumpers were greater than observed in a cohort of athletic controls, suggesting that they are not simply due to limb dominance.
Footnotes
The authors have no conflict of interest.
Edited by: A. Ireland
References
- 1.Alexander RM. Energy-saving mechanisms in walking and running. J Exp Biol. 1991;160:55–69. doi: 10.1242/jeb.160.1.55. [DOI] [PubMed] [Google Scholar]
- 2.Andarawis-Puri N, Flatow EL. Tendon fatigue in response to mechanical loading. J Musculoskelet Neuronal Interact. 2011;11:106–14. [PMC free article] [PubMed] [Google Scholar]
- 3.Arampatzis A, Karamanidis K, Albracht K. Adaptational responses of the human Achilles tendon by modulation of the applied cyclic strain magnitude. J Exp Biol. 2007;210:2743–53. doi: 10.1242/jeb.003814. [DOI] [PubMed] [Google Scholar]
- 4.Arampatzis A, Karamanidis K, Morey-Klapsing G, De Monte G, Stafilidis S. Mechanical properties of the triceps surae tendon and aponeurosis in relation to intensity of sport activity. J Biomech. 2007;40:1946–52. doi: 10.1016/j.jbiomech.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Arampatzis A, Peper A, Bierbaum S, Albracht K. Plasticity of human Achilles tendon mechanical and morphological properties in response to cyclic strain. J Biomech. 2010;43:3073–9. doi: 10.1016/j.jbiomech.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Arya S, Kulig K. Tendinopathy alters mechanical and material properties of the Achilles tendon. J Appl Physiol. 2010;108:670–5. doi: 10.1152/japplphysiol.00259.2009. [DOI] [PubMed] [Google Scholar]
- 7.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate:a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B. 1995;57:289–300. [Google Scholar]
- 8.Biewener AA, Roberts TJ. Muscle and tendon contributions to force, work, and elastic energy savings:a comparative perspective. Exerc Sport Sci Rev. 2000;28:99–107. [PubMed] [Google Scholar]
- 9.Bohm S, Mersmann F, Arampatzis A. Human tendon adaptation in response to mechanical loading:a systematic review and meta-analysis of exercise intervention studies on healthy adults. Sports Med Open. 2015;1:7. doi: 10.1186/s40798-015-0009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohm S, Mersmann F, Marzilger R, Schroll A, Arampatzis A. Asymmetry of Achilles tendon mechanical and morphological properties between both legs. Scand J Med Sci Sports. 2015;25:e124–32. doi: 10.1111/sms.12242. [DOI] [PubMed] [Google Scholar]
- 11.Bohm S, Mersmann F, Tettke M, Kraft M, Arampatzis A. Human Achilles tendon plasticity in response to cyclic strain:effect of rate and duration. J Exp Biol. 2014;217:4010–7. doi: 10.1242/jeb.112268. [DOI] [PubMed] [Google Scholar]
- 12.Bojsen-Moller J, Magnusson SP, Rasmussen LR, Kjaer M, Aagaard P. Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of the tendinous structures. J Appl Physiol. 2005;99:986–94. doi: 10.1152/japplphysiol.01305.2004. [DOI] [PubMed] [Google Scholar]
- 13.Bryant AL, Clark RA, Bartold S, Murphy A, Bennell KL, Hohmann E, et al. Effects of estrogen on the mechanical behavior of the human Achilles tendon in vivo. J Appl Physiol. 2008;105:1035–43. doi: 10.1152/japplphysiol.01281.2007. [DOI] [PubMed] [Google Scholar]
- 14.Coh M, Supej M. Biomechanical model of the take-off action in the high jump:a case study. New Stud Athlet. 2008;23:63–73. [Google Scholar]
- 15.Couppe C, Kongsgaard M, Aagaard P, Hansen P, Bojsen-Moller J, Kjaer M, et al. Habitual loading results in tendon hypertrophy and increased stiffness of the human patellar tendon. J Appl Physiol. 2008;105:805–10. doi: 10.1152/japplphysiol.90361.2008. [DOI] [PubMed] [Google Scholar]
- 16.Fouré A, Nordez A, Cornu C. Plyometric training effects on Achilles tendon stiffness and dissipative properties. J Appl Physiol. 2010;109:849–54. doi: 10.1152/japplphysiol.01150.2009. [DOI] [PubMed] [Google Scholar]
- 17.Hansen P, Aagaard P, Kjaer M, Larsson B, Magnusson SP. Effect of habitual running on human Achilles tendon load-deformation properties and cross-sectional area. J Appl Physiol. 2003;95:2375–80. doi: 10.1152/japplphysiol.00503.2003. [DOI] [PubMed] [Google Scholar]
- 18.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–98. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 19.Komi PV, Fukashiro S, Jarvinen M. Biomechanical loading of Achilles tendon during normal locomotion. Clin Sports Med. 1992;11:521–31. [PubMed] [Google Scholar]
- 20.Kongsgaard M, Aagaard P, Kjaer M, Magnusson SP. Structural Achilles tendon properties in athletes subjected to different exercise modes and in Achilles tendon rupture patients. J Appl Physiol. 2005;99:1965–71. doi: 10.1152/japplphysiol.00384.2005. [DOI] [PubMed] [Google Scholar]
- 21.Kubo K, Ikebukuro T, Yaeshima K, Yata H, Tsunoda N, Kanehisa H. Effects of static and dynamic training on the stiffness and blood volume of tendon in vivo. J Appl Physiol. 2009;106:412–7. doi: 10.1152/japplphysiol.91381.2008. [DOI] [PubMed] [Google Scholar]
- 22.LaCroix AS, Duenwald-Kuehl SE, Lakes RS, Vanderby R., Jr Relationship between tendon stiffness and failure:a metaanalysis. J Appl Physiol. 2013;115:43–51. doi: 10.1152/japplphysiol.01449.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langberg H, Rosendal L, Kjaer M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol. 2001;534:297–302. doi: 10.1111/j.1469-7793.2001.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langberg H, Skovgaard D, Petersen LJ, Bulow J, Kjaer M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol. 1999;521(Pt 1):299–306. doi: 10.1111/j.1469-7793.1999.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luhtanen P, Komi PV. Mechanical power and segmental contribution to force impulses in long jump take-off. Eur J Appl Physiol Occup Physiol. 1979;41:267–74. doi: 10.1007/BF00429743. [DOI] [PubMed] [Google Scholar]
- 26.Magnusson SP, Kjaer M. Region-specific differences in Achilles tendon cross-sectional area in runners and non-runners. Eur J Appl Physiol. 2003;90:549–53. doi: 10.1007/s00421-003-0865-8. [DOI] [PubMed] [Google Scholar]
- 27.Mero A, Komi PV. Force-, EMG-, and elasticity-velocity relationships at submaximal, maximal and supramaximal running speeds in sprinters. Eur J Appl Physiol Occup Physiol. 1986;55:553–61. doi: 10.1007/BF00421652. [DOI] [PubMed] [Google Scholar]
- 28.Milgrom Y, Milgrom C, Altaras T, Globus O, Zeltzer E, Finestone AS. Achilles tendons hypertrophy in response to high loading training. Foot Ankle Int. 2014;35:1303–8. doi: 10.1177/1071100714550651. [DOI] [PubMed] [Google Scholar]
- 29.Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, et al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021–33. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muraki Y, Ae M, Koyama H, Yokozawa T. Joint torque and power of the takeoff leg in the long jump. Int J Sport Health Sci. 2008;6:21–32. [Google Scholar]
- 31.Reeves ND, Maganaris CN, Ferretti G, Narici MV. Influence of 90-day simulated microgravity on human tendon mechanical properties and the effect of resistive countermeasures. J Appl Physiol. 2005;98:2278–86. doi: 10.1152/japplphysiol.01266.2004. [DOI] [PubMed] [Google Scholar]
- 32.Rosager S, Aagaard P, Dyhre-Poulsen P, Neergaard K, Kjaer M, Magnusson SP. Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand J Med Sci Sports. 2002;12:90–8. doi: 10.1034/j.1600-0838.2002.120205.x. [DOI] [PubMed] [Google Scholar]
- 33.Seynnes OR, Bojsen-Moller J, Albracht K, Arndt A, Cronin NJ, Finni T, et al. Ultrasound-based testing of tendon mechanical properties:a critical evaluation. J Appl Physiol. 2015;118:133–41. doi: 10.1152/japplphysiol.00849.2014. [DOI] [PubMed] [Google Scholar]
- 34.Seynnes OR, Erskine RM, Maganaris CN, Longo S, Simoneau EM, Grosset JF, et al. Training-induced changes in structural and mechanical properties of the patellar tendon are related to muscle hypertrophy but not to strength gains. J Appl Physiol. 2009;107:523–30. doi: 10.1152/japplphysiol.00213.2009. [DOI] [PubMed] [Google Scholar]
- 35.Stenroth L, Peltonen J, Cronin NJ, Sipila S, Finni T. Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. J Appl Physiol. 2012;113:1537–44. doi: 10.1152/japplphysiol.00782.2012. [DOI] [PubMed] [Google Scholar]
- 36.Urlando A, Hawkins D. Achilles tendon adaptation during strength training in young adults. Med Sci Sports Exerc. 2007;39:1147–52. doi: 10.1249/mss.0b013e31805371d1. [DOI] [PubMed] [Google Scholar]
- 37.Waugh CM, Korff T, Fath F, Blazevich AJ. Effects of resistance training on tendon mechanical properties and rapid force production in prepubertal children. J Appl Physiol. 2014;117:257–66. doi: 10.1152/japplphysiol.00325.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Westh E, Kongsgaard M, Bojsen-Moller J, Aagaard P, Hansen M, Kjaer M, et al. Effect of habitual exercise on the structural and mechanical properties of human tendon, in vivo, in men and women. Scand J Med Sci Sports. 2008;18:23–30. doi: 10.1111/j.1600-0838.2007.00638.x. [DOI] [PubMed] [Google Scholar]
- 39.Wiesinger HP, Kosters A, Muller E, Seynnes OR. Effects of increased loading on in vivo tendon properties:a systematic review. Med Sci Sports Exerc. 2015 doi: 10.1249/MSS.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wren TA, Lindsey DP, Beaupre GS, Carter DR. Effects of creep and cyclic loading on the mechanical properties and failure of human Achilles tendons. Ann Biomed Eng. 2003;31:710–7. doi: 10.1114/1.1569267. [DOI] [PubMed] [Google Scholar]


