Abstract
Objectives:
Nociceptors are expressed at peripheral terminals of neurons. Recent studies have shown that TRPV1, a nociceptor, is expressed in bone tissue and regulates bone metabolism. We have demonstrated that a TRPV1 antagonist improved pain-like behavior in ovariectomized (OVX) mice. The aim of this study was to determine whether nociceptors, including TRPV1, acid-sensing ion channel (ASIC) and P2X2/3 are expressed in bone cells, and to examine the effects of nociceptor antagonists on bone metabolism.
Methods:
The expression of nociceptors in femoral bone tissue and cultured bone marrow cells in OVX and sham-operated mice were examined. The effects of nociceptor antagonists on the up-regulated expression of bone metabolic markers, Runx2, Osterix, osteocalcin and RANKL, were also examined.
Results:
TRPV1, ASIC 2 and 3, and P2X2 and 3, were expressed in bone tissue and bone marrow cells, and the expression levels of ASIC1 and 2, and P2X2 were significantly increased in OVX mice in comparison with those in sham mice. Treatment with nociceptor antagonists significantly inhibited the expression of bone metabolic markers in OVX mice.
Conclusion:
An array of nociceptors, TRPV1, ASICs and P2X2/3, could simultaneously regulate not only increases in skeletal pain but also bone turnover in OVX mice.
Keywords: Transient Receptor Potential Vanilloid Type 1 (TRPV1), Acid-Sensing Ion Channels (ASIC), P2X, Ovariectomized Mice, Bone Metabolic Marker
Introduction
Nociceptors, including transient receptor potential vanilloid type 1 (TRPV1)1,2, acid-sensing ion channels (ASICs)3 and P2X4 are expressed at the peripheral terminals of neurons, and transmit pain signals when activated in response to noxious chemical, mechanical or thermal stimuli4. The cell bodies of the primary afferent sensory nerves are located in the dorsal root ganglia and trigeminal ganglia1,5. Our recent studies have shown that treatment with the antagonists to TRPV1, acid-sensing ion channels (ASICs) (another acid-sensing nociceptor), P2X2/3 receptor (an ATP-ligand nociceptor), and an inhibitor of vacuolar H+-ATPase known as an proton pump, improved pain-like behavior in an ovariectomized (OVX) mouse model6,7. On the other hand, previous studies have shown that TRPV1 is also expressed in ostoclasts and osteoblast-like cells, and that it regulates bone metabolism8,9. Based on these results, we speculated that TRPV1 could regulate both the induction of the pain and bone turnover in osteoporosis. Those studies, therefore, encouraged us to examine whether other nociceptors, such as ASICs and P2X, are also expressed in bone marrow stromal cells, and whether the expression of these nociceptors are simultaneously regulated in the same manner as TRPV1 under pathological osteoporotic conditions.
ASICs and P2X, which belong to a novel class of ligand-gated cation channels, are activated by extracellular acidification and extracellular adenosine triphosphate (ATP), respectively3,10. P2X receptors, of which 7 subunits (P2X1-P2X7) have been cloned, are implicated in nociceptive signaling under both normal and pathologic pain states10. Regarding the expression of P2X receptors in bone, previous studies have generally shown that the P2X7 receptor, in particular, is predominantly expressed and functions in osteoclasts and osteoblasts, having significant roles in bone homeostasis or in association with the pathogenesis of osteoporosis11-13. However, there have been few studies on the expression and function of P2X2 or P2X3 receptors in bone. The P2X3 receptor, an ATP-sensitive ligand gated-ion channel, is selectively localized on the peripheral terminals and central processes of sensory afferent neurons where it participates in nociceptive signaling, and is natively expressed both as a functional homomeric and as a heteromultimeric combination with the P2X2 receptor14,15.
The aim of the present study was, therefore, to determine whether an array of nociceptors including the TRPV1, ASIC and P2X2/3 receptors, which are thought to be involved in the induction of skeletal pain, are expressed in bone cells, and to examine the changes in the expression of those receptor under high bone turnover conditions in OVX mice. In addition, we also examined the effect of nociceptor antagonists on the expression of bone metabolic markers.
Materials and methods
Animal model and bone marrow stromal cell culture
Seven-week-old female C57Bl6J mice weighing 20-25 g were obtained from Japan SLC (Hamamatsu, Japan). Fifty-two mice were randomly divided into two groups, 14 in the sham operation (sham) group and 38 in the OVX group. They were anesthetized at 8 weeks of age by an intraperitoneal injection of pentobarbital (0.5 mg/kg) and the OVX and sham operations were performed as previously reported6,7. The OVX mice demonstrated osteoporotic changes with increased bone resorption (supplementary Figure 1).
Figure 1.
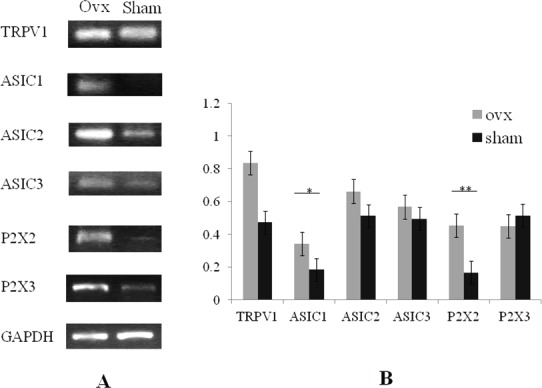
Analysis of the mRNA expression of TRPV1, and ASIC1, 2 and 3 in the femur bone tissue. An array of nociceptors (TRPV1, ASIC2 and 3, and P2X2 and 3) were expressed in bone tissue of OVX mice (A). The expression levels of ASIC1 (*p<0.01) and P2X2 (**p<0.05) were significantly increased in OVX mice (n=6) compared with those in sham mice (n=6). The expressions levels of TRPV1, ASIC2 and 3, and P2X3 were increased in OVX mice although there were no significant differences between the two groups (B). Expression levels were shown as the ratio of the gene of interest to the control gene (GAPDH). The RT-PCR picture (A) demonstrated a representative data and the error bars (B) meant variation of the samples from different mice in independent femurs.
Bone marrow stromal cells were obtained from excised bilateral femurs at 6 weeks after OVX (n=3) or the sham (n=3) operation by flushing the shafts. The cells were plated in Dulbecco’s modified Eagle’s medium (GIBCO, Grand Island, NY, USA) containing 10% fetal bovine serum, 4 mM L-glutamine, and penicillin (100 U/ml) / streptomycin (100 ng/ml). After 8 hours, non-adherent cells were washed out twice with fresh medium. The adherent bone marrow stromal cells on the culture plate from each mouse were individually incubated in fresh medium at 37°C and 5% CO2, and were isolated at 48 hours after plating for RNA isolation.
Reagents
The TRPV1 antagonist N-(3-methoxyphenyl)-4-chlorocinnamide (SB366791; BIOMOL International; Plymouth Meeting, PA, USA), a selective blocker to the ASIC3 channel APETx2 (PEPTIDE Institute, Inc., Osaka, Japan), and the selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors A317491 (Sigma-Aldrich Japan, Yokohama, Japan) were purchased as shown. SB366791 was administered intraperitoneally to OVX mice at a dose of 1.0 mg/kg16. APETx2 was administered intramuscularly at a dose of 20 µM into the left gastrocnemius muscle17. A-317491 was administered subcutaneously at a dose of 10 mg/kg18. Mice were given a single injection at 6 weeks after OVX or the sham operation, and the femur and serum were isolated at 6 hours after administration.
Evaluation of bone metabolic markers expression
For RNA isolation and reverse transcription-polymerase chain reaction, the excised bilateral femurs from OVX (n=15) and sham (n=6) mice were individually homogenized in 1ml TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) 10 times at 3000 rpm/30s using a bead crusher (TITEC, Tokyo, Japan). The homogenates were centrifuged at 15000 × g for 15 min at 4°C and the supernatants were then extracted. Total RNA was individually isolated from the whole femur or cultured bone marrow stromal cells each mouse, and then reverse transcribed into cDNA by polymerase chain reaction (PCR) using an RNA PCR kit (Quiagen, Takara BIO Inc. Japan) according to the manufacturer’s protocol. Primers are shown in Table 1. Reactions were carried out by PCR as follows; 40 cycles at 95°C for 40s, 55°C for 40s and 72°C for 40s for TRPV1and GAPDH; 45 cycles at 94°C for 15s, 47°C for 30s and 72°C for 30s for ASIC1, 2 and 3; 40 cycles at 94°C for 30s, 62°C for 30s and 72°C for 30s for P2X2; 40 cycles at 94°C for 30s, 60°C for 30s and 72°C for 30s for P2X3; 35 cycles at 95°C for 30 s, 64°C for 30 s and 72°C for 30 s for RANKL; and 35 cycles at 95°C for 30s, 64°C for 30s and 72°C for 30s for Runx2, Osterix and osteocalcin. Reaction products were analyzed by electrophoresis, and a computer-assisted image analyzer (Luminous Imager, AISIN, Japan) was used to measure the values of the target gene bands, which were normalized to the level of GAPDH gene expression in the same sample as semi-quantitative measurement. Values in graphs are the means ± SD obtained from 3 independent experiments.
Table 1.
Primer sequences.
| Forward primer | Reverse primer | |
|---|---|---|
| TRPVl | AAGGCTTGCCCCCCTATAA | CACCAGCATGAA CAGTGACTGC |
| P2X2 | AACAGCATCCACTATCCCAAG | GGTGGTGCCGTTTATCTTGT |
| P2X3 | AGGTGTCCCATCTCCTTTTTG | AGAGTTGAGTTGAGGGAGGAGA |
| ASIC1 | AGATGGCTGATGAAAAGCA | AAGTGGCACGAGAGAAGCAT |
| ASIC2 | TGACATTGGTGGTCAAATOG | ATCATGGCTCCCTTCCTCTT |
| ASIC3 | AGGGAGAAGTCCCAAAGCAT | GACACTCCATTCCCAGGAGA |
| Rıınx2 | GCTTGATGACTCTAAACCTA | AAAAAGGGCCCAGTTCTGAA |
| Osteiix | AGGCACAAAGAAGCCATAC | AATGAGTGAGGGAAGGGT |
| Osteocalcin | CTCACTCTGCTGGCCCTG | CCGTAGATGCGTTTGTAGGC |
| RANKL | ATCAGAAGACAGCACTCACT | ATCTAGGACATCCATGCTAATGTTC |
| GAPDH | TGAAGGTCGGTGTGAACGAATT | GCTTTCTCCATGGTGGTGAAGA |
For the measurement of femur and serum tartrate-resistant acid-phosphatase 5b (TRAP5b) levels, femur and serum samples were collected from mice at 6 weeks after OVX (n=20) or the sham (n=5) operation. Femur and serum concentrations of TRAP5b, a bone resorption marker, were determined using a mouse TRAP5b enzyme -linked immune sorbent assay kit (Immunodiagnostic Systems, London, UK) in accordance with the manufacturer’s recommendations.
All data are presented as mean ± standard deviation. To determine difference between groups, RT-PCR analysis and TRAP 5b measurement were repeated at least three times and the statistical significance was determined using a Student’s t test and ANOVA. Differences with p values of < 0.05 were considered to be statistically significant.
Results
Expression of nociceptors in bone tissue and bone marrow stromal cells
An array of nociceptors (TRPV1, ASIC 2 and 3, and P2X2 and 3) were expressed in bone tissue. In addition, the expression levels of ASIC1 and P2X2 were significantly increased in OVX mice (n=6) in comparison with those in sham mice (n=6) (Figures 1A and B). The expression of other nociceptor including TRPV1, ASIC2 and 3, and P2X3 were also increased in OVX mice, although the differences between two groups were not significant (Figures 1A and B). We also showed that TRPV1, ASIC 2 and 3, and P2X2 and 3 were expressed in bone marrow stromal cells. This, with regard to expression in OVX mice (n=3), the levels of ASIC1 and 2 were significantly increased compared with those in sham mice (n=3) (Figures 2A and B), while the expression of TRPV1, ASIC3 and P2X2 and 3 tended to be increased in comparison with those in sham mice (Figures 2A and B).
Figure 2.
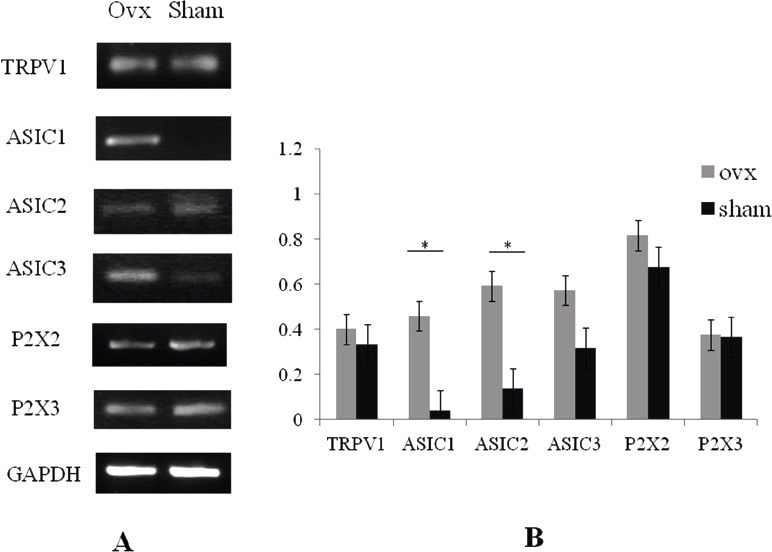
Analysis of the mRNA expression of TRPV1, and ASIC1, 2 and 3 in bone marrow stromal cells. An array of nociceptors (TRPV1, ASIC2 and 3, and P2X2 and 3) were expressed in bone marrow stromal cells of OVX mice (A). The expression levels of ASIC1 and 2 were significantly increased in OVX mice (n=3) (*p<0.01), compared with those in sham mice (n=3). The expression levels of TRPV1, ASIC3 and P2X2 and 3 were increased in OVX mice although there were no significant differences between the two groups (B). Expression levels are shown as the ratio of the gene of interest to the control gene (GAPDH). The RT-PCR picture (A) demonstrated a representative data and the error bars (B) meant variation of the samples from different mice in independent culture of bone marrow stromal cells.
Changes in bone metabolic marker expression by treatment with nociceptor antagonists
We examined in vivo effects of treatment with nociceptor antagonists on the expression of bone metabolic markers in OVX mice. The expression levels of Runx2 (Figures 3A and B), Osterix (Figures 3A and C), osteocalcin (Figures 3A and D) and RANKL (Figures 3A and E) in the bone tissue of OVX mice (n=3) were significantly inhibited by treatment with TRPV1 (n=3), ASIC3 (n=3) or P2X2/3 (n=3) antagonists, except that the P2X2/3 antagonist had no inhibitory effect on RANKL expression in OVX mice (Figure 3).
Figure 3.
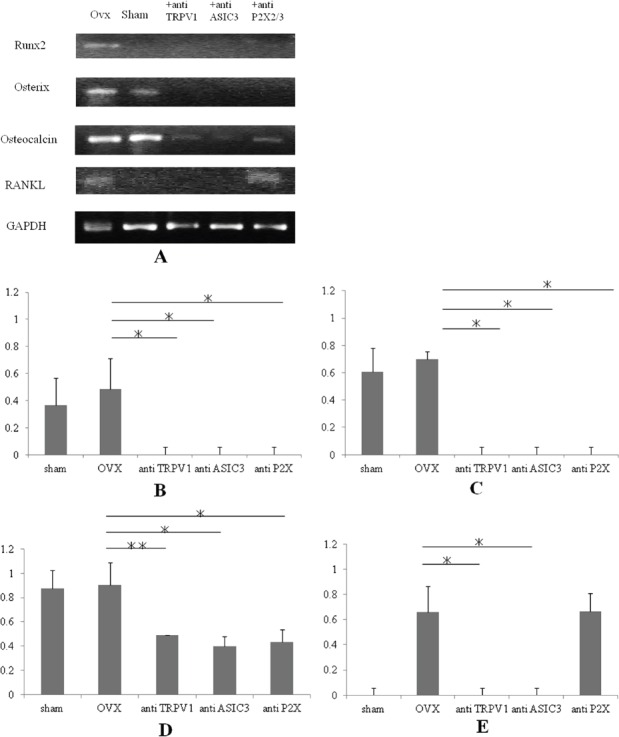
Changes in the expression of Runx2, Osterix, osteocalcin and RANKL in OVX mice by treatment with TRPV1, ASIC3, and P2X2/3 antagonists. The Runx2 (A, B) and Osterix (A, C) expression was completely inhibited by treatment with TRPV1 (+anti-TRPV1, n=3), ASIC3 (+anti-ASIC3, n=3) or P2X2/3 (+anti-P2X2/3, n=3) antagonists. The osteocalcin expression was significantly inhibited by treatment with TRPV1 (+anti-TRPV1), ASIC3 (+anti-ASIC3) or P2X2/3 (+anti-P2X2/3) antagonists (A, D). The up-regulation of RANKL expression was completely inhibited by treatment with TRPV1 (+anti-TRPV1) and ASIC3 (+anti-ASIC3) antagonists, although no inhibitory effect was observed for the P2X2/3 antagonist (A, E). Expression levels are shown as the ratio of the gene of interest to the control gene (GAPDH). *P<0.01, **P<0.05. The RT-PCR picture (A) demonstrated a representative data and the error bars (B, C, D, E) meant variation of the samples from different mice in independent femurs.
Changes in TRAP5b level by treatment with nociceptor antagonists
TRAP5b level in the femur tissue was significantly higher in the OVX group (n=5, 61.9±18.9 U/L) than in the sham group (n=5, 30.6±8.9 U/L) at 6 weeks after surgery. This increase was inhibited by treatment with the TRPV1 antagonist (45.0±20.1 U/L, p=0.06) (n=5) or ASIC3 blocker (42.0±19.6 U/L, p=0.08) (n=5), although the differences were not statistically significant (Figure 4). The P2X antagonist (61.1±28.4 U/L) (n=5) has no inhibitory effect on the increase in TRAP5b level in the OVX group (61.9±18.9 U/L). Overall, we could not detect any significant changes in serum TRAP5b level by treatment with the antagonists (data not shown).
Discussion
Recently, we indicated that antagonists to TRPV1, ASIC3 and P2X2/3 improved the pain-like behavior in OVX mice due to the inhibition of activated nociceptors at the peripheral terminals of neurons in bone tissue7. In this study, we demonstrated that an array of nociceptors, including TRPV1, ASIC1, 2 and 3, and P2X2 and 3, were simultaneously expressed in bone tissue and bone marrow stromal cells. In addition, the expression levels of ASIC1 and 2 as well as P2X2 were increased in OVX mice compared with those in sham mice. These results indicated that treatment with the respective antagonists could improve pain-like behavior due to the inhibition of nociceptors not only on the terminal of neurons but also in the activated osteoclasts which formed the acidic environment in the bone. We, therefore, speculate that nociceptors such as TRPV1, ASICs, and P2X2 and 3 have potential roles in the regulation of pain signal transmissions and bone metabolism. To the best our knowledge, there are few reports demonstrating the simultaneous expression of an array of nociceptors in bone tissue or bone marrow stromal cells, as well as the increased expression of several nociceptors such as ASIC1 and 2, and P2X2 in OVX mice.
TRPV1 is a member of a family of polymodal and nonselective cation channels that are predominantly expressed by sensory nerve fibers of the somatic and autonomic afferent neurons1. Recent research has demonstrated that TRPV1 directly regulates osteoblast and osteoclast differentiation and function both in vitro and in vivo, and TRPV1 blockade protects against OVX-induced bone loss in mice9. These results support our data with regard to the likely roles of TRPV1, and encouraged us to pursue further experiments to elucidate whether antagonists to nociceptors such as ASIC and P2X receptor might have some effect on the regulation of bone metabolism.
ASICs form a novel class of ligand-gated cation channels with 6 subunits (1a, 1b, 2a, 2b, 3 and 4) identified to date. These subunits are activated by a fall in extracellular PH and are cation-selective19. A previous study has shown that ASICs are expressed in bone cells20; however, it is uncertain whether the expression is affected under a pathogenic state leading to bone turnover and whether the bone cell receptors have any effect on the regulation of bone metabolism. In this study, we demonstrated that the expression levels of ASIC1 and 2 in bone tissue and bone marrow stromal cells are increased in OVX mice in comparison with those in sham mice. Furthermore, antagonists to ASIC 3 markedly inhibited the Runx2, Osterix, osteocalcin and RANKL expression in OVX mice. A recent study has also demonstrated that proton concentration is a major contributor to the modification of osteoclast and osteoblast differentiation21. Taken together these findings indicate that ASICs expressed in bone cells in response to extracellular acidification might have a role in the regulation of bone metabolism.
In the bone microenvironment, ATP is locally released and regulates bone remodeling as extracellular signaling molecules via P2 receptors22. Although evidence has shown that all seven P2X ion channel receptor subtypes (P2X1-7) are expressed in bone and cartilage cells, most studies have focused on analyzing the roles of P2X7 receptor in bone homeostasis or musculoskeletal diseases such as osteoporosis11-13. In this study, we demonstrated that the P2X2/3 receptor is expressed in bone tissues and bone marrow stromal cells, and that this expression was regulated in accordance with changes in bone turnover, with P2X2/3 expression increased in OVX mice in comparison with that in sham mice. To the best of our knowledge, there are few studies that show an increase in P2X2/3 receptor expression in bone tissue and bone marrow stromal cells in OVX mice. Additionally, P2X2/3 antagonists inhibited the Runx2, Osterix and osteocalcin expression in OVX mice, whereas it had no effect on RANKL expression. Based on these results, P2X2/3 is speculated to have a role in the regulation of bone metabolism. With regard to the effects of nociceptor antagonists on a high bone turnover state, the antagonists to TRPV1 and ASIC3 inhibited the elevation of TRAP5b level in the bone tissue of OVX mice, although this change was not statistically significant. These results also supported the notion that the nociceptors might regulate bone turnover.
Thus, we believe that an array of nociceptors, such as TRPV1, ASICs and P2X2/3, might regulate the physiological and pathogenic processes associated with bone metabolism. Recently, we have demonstrated that the antagonist to TRPV1 improved pain-like behavior induced under a high bone turnover state with relation to osteoclast activation in OVX mice6. In addition, we and other previous studies have also shown that complex regional pain syndrome (CRPS) reveals severe skeletal pain accompanied with regional osteoporotic changes, the pathophysiological conditions of which were related to high bone turnover23-25. According to these studies, we are encouraged to examine whether the antagonists to TRPV1, ASICs and P2X2/3 could be candidates therapeutic agents in the treatment of patients with refractory severe pain and regional osteoporotic changes, such as observed in CRPS.
The present study has several limitations. First, except for ASIC3 and P2X2/3, we did not evaluate the effects of antagonists to other subtypes of ASIC and P2X. Second, we could not demonstrate the expression of the nociceptors at a protein level in bone tissue. Third, we did not evaluate the effects of antagonists to the nociceptors on bone mineral density or bone morphometric changes in OVX mice. Additional studies, therefore, are needed to further elucidate whether the nociceptors play significant roles in the physiological and pathogenic processes associated with bone metabolism.
In conclusion, we demonstrated that an array of nociceptors, including TRPV1, ASIC and P2X2/3 receptors, were simultaneously expressed in bone tissue and bone marrow stromal cells, and that the expressions levels of these receptor changed under a high bone turnover state in OVX mice. In addition, the antagonists to these nociceptors inhibited the expression of bone metabolic markers such as Runx2, Osterix, osteocalcin and RANKL.
Acknowledgements
We thank Dr. Yasuhisa Abe (Department of Orthopaedic Surgery, Sapporo Medical University School of Medicine) for his advice and invaluable contributions to this study. This study was partially supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan.
Footnotes
The authors declare that they have no conflict of interest. All procedures performed in studies involving animals were in accordance with the ethical standards of the Experimental Animal Care Committee of Sapporo Medical University and were followed the ethical guidelines of the National Institute of Health.
Edited by: M. Hamrick
References
- 1.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–13. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 2.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–10. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 3.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–7. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 4.Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371:516–9. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- 5.Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics:recent advances and setbacks. Brain Res Rev. 2009;60:267–77. doi: 10.1016/j.brainresrev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Abe Y, Iba K, Sasaki K, Chiba H, Kanaya K, Kawamata T, Oda K, Amizuka N, Sasaki M, Yamashita T. Inhibitory effect of bisphosphonate on osteoclast funvction contributes to improved skeletal pain in ovariectomized mice. J Bone Miner Metab. 2015;33:125–134. doi: 10.1007/s00774-014-0574-x. [DOI] [PubMed] [Google Scholar]
- 7.Kanaya K, Iba K, Abe Y, et al. Acid-sensing ion channel 3 or P2X2/3 is involved in the pain-like behavior under a high bone turnover state in ovariectomized mice. J Orthop Res. doi: 10.1002/jor.23047. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Rossi F, Siniscalco D, Luongo L, et al. The endovanilloid /endocannabinoid system in human osteoclasts:possible involvement in bone formation and resorption. Bone. 2011;44:476–84. doi: 10.1016/j.bone.2008.10.056. [DOI] [PubMed] [Google Scholar]
- 9.Idris AI, Landao-Bassonga E, Ralston SH. The TRPV1 ion channel antagonist capazepine inhibits osteoclast and osteoblast differentiation in vitro and ovariectomy induced bone loss in vivo. Bone. 2010;46:1089–99. doi: 10.1016/j.bone.2010.01.368. [DOI] [PubMed] [Google Scholar]
- 10.Chizh BA, Illes P. P2X receptors and nociception. Pharm Rev. 2001;53:553–68. [PubMed] [Google Scholar]
- 11.Grol MW, Panupinthu N, Korcok J, Sims SM, Dixon SJ. Expression, signaling, and function of P2X7 receptors in bone. Purinergic Signal. 2009;5:205–21. doi: 10.1007/s11302-009-9139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoebertz A, Townsend-Nicholson A, Glass R, Burnstock G, Arnett TR. Expression of P2 receptors in bone and cultured bone cells. Bone. 2000;27:503–10. doi: 10.1016/s8756-3282(00)00351-3. [DOI] [PubMed] [Google Scholar]
- 13.Pellegatti P, Falzoni S, Donvito G, Lemaire I, Di Virgilio F. P2X receptor drives osteoclast fusion by increasing the extracellular adenosine concentration. FASEB J. 2011;25:1264–74. doi: 10.1096/fj.10-169854. [DOI] [PubMed] [Google Scholar]
- 14.Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Brunstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–31. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- 15.Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–5. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- 16.Niiyama Y, Kawamata T, Yamamoto J, Furuse S, Namiki A. SB366791, a TRPV1 antagonist, potentiates analgesic effects of systemic morphine in a murine model of bone cancer pain. Br J Anaesth. 2009;102:251–8. doi: 10.1093/bja/aen347. [DOI] [PubMed] [Google Scholar]
- 17.Karczewski J, Spencer RH, Garsky VM, Liang A, Leitl MD, Cato MJ, Cook SP, Kane S, Urban MO. Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br J Pharmacol. 2010;161:950–60. doi: 10.1111/j.1476-5381.2010.00918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu G, Whiteside GT, Lee G, Nolan S, Niosi M, Pearson MS, Ilyin VI. A-317491, a selective P2X3/P2X2/3 receptor antagonist, reverses inflammatory mechanical hyperalgesia through action at peripheral receptors in rats. Eul J Pharmacol. 2004;504:45–53. doi: 10.1016/j.ejphar.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 19.Xu T, Xiong ZG. Dynamic regulation of acid-sensing ion channels by extracellular and intracellular modulators. Curr Med Chem. 2007;14:1753–63. doi: 10.2174/092986707781058977. [DOI] [PubMed] [Google Scholar]
- 20.Jahl H, van Driel M, van Osch GJ, Weinans H, van Leeuwen JP. Identification of acid-sensing ion channels in bone. Biochem Biophys Res Commun. 2005;337:349–54. doi: 10.1016/j.bbrc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 21.Kato K, Matsushita M. Proton concentrations can be a major contributor to the modification of osteoclast and osteoblast differentiation, working independently of extracellular bicarbonate ions. J Bone Miner Metab. 2014;32:17–28. doi: 10.1007/s00774-013-0462-9. [DOI] [PubMed] [Google Scholar]
- 22.Grol MW, Pereverxev A, Sims SM, Dixon SJ. P2 receptor networks regulate signaling duration over a wide dynamic range of ATP concentrations. J Cell Sci. 2013;126:3615–26. doi: 10.1242/jcs.122705. [DOI] [PubMed] [Google Scholar]
- 23.Abe Y, Iba K, Takada J, Wada T, Yamashita T. Improvement of pain and regional osteoporotic changes in the foot and ankle by low-dose bisphosphonate therapy for complex regional pain syndrome type I:a case series. J Med Case Rep. 2011;5:349. doi: 10.1186/1752-1947-5-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozin F. Reflex sympathetic dystrophy syndrome. Curr Opin Rheumatol. 1994;6:210–6. doi: 10.1097/00002281-199403000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Kubalek I, Fain O, Paries J, Kettaneh A, Thomas M. Treatment of reflex sympathetic dystrophy with pamidronate:29 cases. Rheumatology. 2001;40:1394–7. doi: 10.1093/rheumatology/40.12.1394. [DOI] [PubMed] [Google Scholar]


