Abstract
Objectives:
Effects of collagen hydrolysate (CHD) on the oxidative capacity of the tibialis anterior muscle and the cortical and trabecular density of the femur were investigated in senescence-accelerated mouse prone 6 (SAMP6).
Methods:
Sixteen-week-old male SAMP6 mice were divided into control (CON) and CHD groups. The CON group was given normal water, while the CHD group was given water containing CHD. Fibre cross-sectional areas (CSAs), fibre succinate dehydrogenase (SDH) staining intensity, and SDH activity of the tibialis anterior muscle were determined at 42 and 60 weeks of age. The cortical and trabecular density of the femur and serum osteocalcin levels were also determined.
Results:
The fibre SDH staining intensity and muscle SDH activity were higher in the CHD group at 60 weeks of age than in the age-matched CON group. The cortical and trabecular density and serum osteocalcin levels were greater in the CHD group at 60 weeks of age than in the age-matched CON group.
Conclusion:
CHD inhibited the age-induced decrease in muscle oxidative capacity and bone density of SAMP6 mice. There is a possibility that CHD is effective for inhibition of age-induced degeneration in the musculoskeletal system.
Keywords: Bone Density, Collagen Hydrolysate, Muscle Oxidative Capacity, Osteocalcin, Senescence-accelerated Mouse Prone 6
Introduction
Degenerative changes in the musculoskeletal system are observed with age1-3, in metabolic diseases4,5, after decreased neuromuscular activity6,7, and following exposure to microgravity8,9. Muscle atrophy and osteoporosis, which are caused by a decrease in cell size and loss of muscle fibres1-3 and the over-resorption of bone10, respectively, are markedly induced with age.
Collagen hydrolysate (CHD) is produced by digestion of gelatine manufactured from bones or hides of fish and animals. Most of the protein in these raw materials is comprised of type I collagen. A recent study11 has reported that CHD improves skin conditions in experimental animals with altered muscle-related gene expression. Although the safety of CHD in advanced treatments makes it attractive for long-term use in a chronic disorder like osteoporosis, data concerning the effects of CHD on osteoporosis are limited. Previous studies12-16 were carried out under excessively limited nutrition and with a relatively high concentration dose. In addition, these studies used ovariectomized animals and measured the bone density by X-ray with a single- or dual-energy photon. The values obtained from these measurements do not reflect a volumetric density.
Senescence-accelerated mouse prone 6 (SAMP6) is accepted as a murine model of senile osteoporosis17-20. SAMP6 mice at 60 weeks of age show osteoporosis and muscle atrophy compared to those at 40 weeks of age3,21, indicating that degenerative changes in the musculoskeletal system of SAMP6 mice are induced dramatically at early stages compared to age-matched normal mice. This study examined whether CHD prevents age-induced degenerative changes in the musculoskeletal system of SAMP6 mice. This study is unique because peripheral quantitative computer tomography (pQCT) analysis, which provides simultaneous information on geometric property and volumetric density of appendicular bone22, was performed on aged SAMP6 mice. In addition, the pQCT analysis was performed to separately assess cortical and trabecular density23.
Materials and methods
Ethics approval
All experimental procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals published by the Institutional Animal Care and Use Committee of Kyoto University (Kyoto, Japan).
Animals and CHD treatment
Fifteen-week-old male SAMP6 mice were used in this study. After 1 week of acclimatization to the laboratory environment, SAMP6 mice were divided into the control (CON, n=10) and CHD (n=10) groups. They were sacrificed at 42 and 60 weeks of age (n=5/group). All mice were individually housed in cages of the same size and were kept in a controlled environment with fixed 12:12 h light:dark cycles (lights off from 20:00 to 08:00) and temperature maintained at 22±2°C with 45-55% relative humidity. Mice in the CON and CHD groups had free access to food (MF, Oriental East Inc., Tokyo, Japan). CHD, which was extracted from chicken feet, desalted, degraded by proteinase to various sizes of peptide, and thereafter sterilized and dried, was supplied by NH Foods Ltd. (Tsukuba, Japan). The composition of dried CHD was 4.0% moisture, 94.0% protein, 1.7% carbohydrate, and 0.3% ash hydrolysate. The concentration of CHD in water was set at 1.3 g/kg body weight. The mice in the CON group had free access to water. The volume of water that the mice in the CON group consumed was measured every day. The volume of water that the mice in the CHD group consumed was adjusted to the same volume that was delivered to the CON group.
Procedures
The mice were weighed and anesthetized with the administration of sodium pentobarbital (50 mg/kg body weight). Blood was taken from the heart. The tibialis anterior muscles of both legs were removed bilaterally, cleaned of excess fat and connective tissue, and wet-weighed. The muscles were pinned on a corkboard, stretched to their approximate in vivo length, rapidly frozen in isopentane that had been cooled in a mixture of dry ice and acetone, and then stored in a deep freezer until biochemical and histochemical analyses. The femurs were removed bilaterally and cleaned of connective and soft tissues. The femurs were wet weighted and then stored in 70% ethanol until pQCT analysis.
Muscle succinate dehydrogenase (SDH) activity
The tibialis anterior muscle of the right leg was used for the measurement of SDH activity24,25. The muscle was homogenized in a glass tissue homogenizer with five volumes of ice-cold 0.3 M phosphate buffer, pH 7.4. Sodium succinate was added to yield a final concentration of 17 mM. The final concentrations of the components of the reaction mixture were as follows: 17 mM sodium succinate, 1 mM sodium cyanide, 0.4 mM aluminium chloride, and 0.4 mM calcium chloride. This reaction mixture was transferred to a spectrophotometer, and the reduction in cytochrome c was determined by observing the increase in extinction at 550 nm. The SDH activity was calculated from the ferricytochrome c concentration and protein content.
Muscle fibre property
Serial 10-µm thick transverse sections of the tibialis anterior muscle of the left leg were cut in a cryostat set at -20°C. The sections were brought to room temperature, air-dried for 30 min, and then stained to evaluate SDH staining intensity, an indicator of mitochondrial oxidative enzyme activity3. The cross-sectional areas (CSAs) and SDH staining intensity of approximately 50 muscle fibres from the deep, middle, and superficial regions of the muscle were examined using a computer-assisted image processing system (Neuroimaging System, Kyoto, Japan). These regions were selected for analysis because the tibialis anterior muscle shows an increasing gradient of fibres with a high oxidative enzyme activity from the superficial to the deep regions of the muscle.
Sectional images were digitized as greyscale images. Each pixel was quantified as 1 of 256 grey levels; a grey level of 0 was equivalent to 100% light transmission, whereas a grey level of 255 was equivalent to 0% light transmission. The mean optical density (OD) of all pixels, which was converted to a grey level value within a fibre, was determined using a calibration photographic tablet with 21 steps of gradient-density ranges and the corresponding diffused density values.
pQCT analysis
The femur was analysed using pQCT (XCT Research SA+, Stratec Medizintechnik GmbH, Pforzheim, Germany)21. The distal site of the bone was chosen for analysis of cortical and trabecular density (mg/cm3).
Serum calcium and osteocalcin levels
Serum calcium levels were measured by the optimized pH (OCOP) method (Calcium C-Test; Wako Pure Chemical Industries, Osaka, Japan). The levels of serum osteocalcin, which is a marker of bone formation, were measured using a sandwich ELISA Kit (Biomedical Technologies Inc., MA, USA) according to the manufacturer’s instruction.
Statistics
Values were expressed as mean ± standard deviation (SD) of five animals. Two-way analysis of variance (ANOVA) was performed to determine the age-induced changes (42 and 60 weeks of age) and differences between the two age-matched groups (CON and CHD groups). When changes or differences were found to be significant by ANOVA, individual group comparisons were made by performing the Scheffé’s post hoc test. Statistical significance was set at P<0.05.
Results
Body and muscle weights
There were age-induced changes in the body weight (Figure 1A). In the case of the CON group, the body weight was greater at 60 weeks of age than at 42 weeks of age. There were no differences in the body weight between the CON and age-matched CHD groups.
Figure 1.
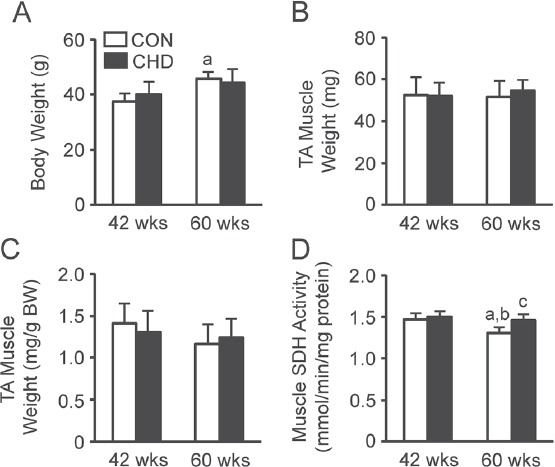
Body weights (A), tibialis anterior (TA) muscle weights (B), relative muscle weights (C), and muscle succinate dehydrogenase (SDH) activity (D) of the control (CON) and collagen hydrolysate (CHD) groups at 42 and 60 weeks of age. Values are means ± SD for 5 animals. BW, body weight. aP<0.05 compared with the CON group at 42 weeks of age; bP<0.05 compared with the CHD group at 42 weeks of age; cP<0.05 compared with the CON group at 60 weeks of age.
There were no age-induced changes in the absolute (Figure 1B) or relative (Figure 1C) tibialis anterior muscle weights. In addition, there were no differences in the absolute or relative tibialis anterior muscle weights between the CON and age-matched CHD groups.
Muscle SDH activity
There were age-induced changes in the muscle SDH activity (Figure 1D). The muscle SDH activity was lower in the CON group at 60 weeks of age than in the CON and CHD groups at 42 weeks of age. In addition, there were differences in the muscle SDH activity between the CON and age-matched CHD groups. The muscle SDH activity was higher in the CHD group at 60 weeks of age than in the age-matched CON group.
Muscle fibre property
Figure 2 shows the transverse sections of the tibialis anterior muscles, which were stained to evaluate SDH activity, of mice in the CON and CHD groups at 42 and 60 weeks of age.
Figure 2.
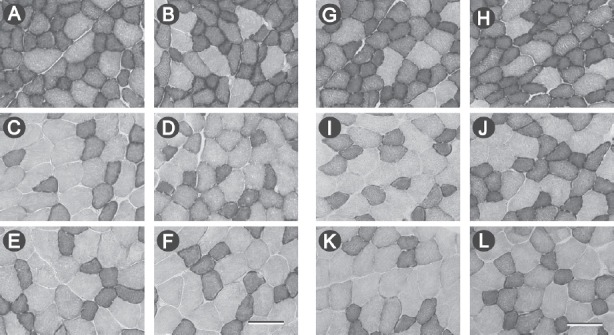
Transverse sections of the deep (A, B, G, H), middle (C, D, I, J), and superficial (E, F, K, L) regions in mice of the control (A, C, E, G, I, K) and collagen hydrolysate (B, D, F, H, J, L) groups at 42 (A–F) and 60 (G–L) weeks of age, stained to evaluate succinate dehydrogenase (SDH) activity. Scale bar in F and L=100 µm.
There were no age-induced changes in the fibre CSA, irrespective of the muscle region (Figures 3A–C). In addition, there were no differences in the fibre CSA between the CON and age-matched CHD groups, irrespective of the muscle region.
Figure 3.
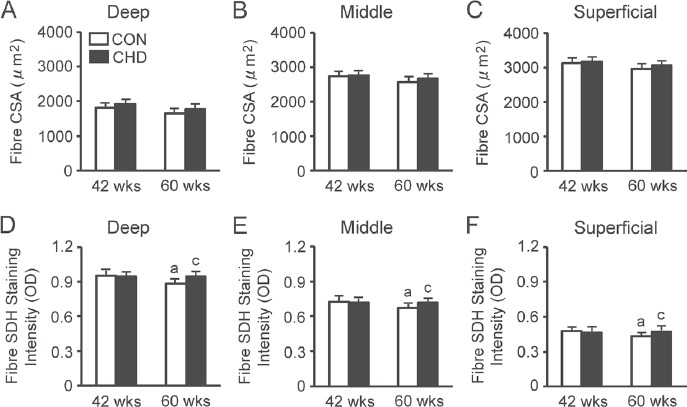
Fibre cross-sectional areas (CSAs) and succinate dehydrogenase (SDH) staining intensity of the deep (A, D), middle (B, E), and superficial (C, F) regions in the tibialis anterior muscle of the control (CON) and collagen hydrolysate (CHD) groups at 42 and 60 weeks of age. Values are means ± SD for 5 animals. OD, optical density. aP<0.05 compared with the CON group at 42 weeks of age; cP<0.05 compared with the CON group at 60 weeks of age.
There were age-induced changes in the fibre SDH staining intensity, irrespective of the muscle region (Figures 3D–F). In the case of the CON group, the SDH staining intensity was lower at 60 weeks of age than at 42 weeks of age. In addition, there were differences in the fibre SDH staining intensity between the CON and age-matched CHD groups, irrespective of the muscle region. The fibre SDH staining intensity was higher in the CHD group at 60 weeks of age than in the age-matched CON group.
Bone weight
There were age-induced changes in the absolute bone weight (Figure 4A). The absolute bone weight was greater in the CON and CHD groups at 60 weeks of age than in the CHD group at 42 weeks of age. There were no differences in the absolute bone weight between the CON and age-matched CHD groups.
Figure 4.
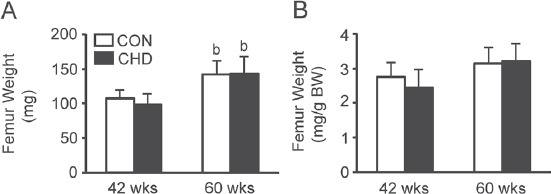
Femur weights (A) and relative weights (B) of the control (CON) and collagen hydrolysate (CHD) groups at 42 and 60 weeks of age. Values are means ± SD for 5 animals. BW, body weight. bP<0.05 compared with the CHD group at 42 weeks of age.
There were no age-induced changes in the relative bone weight (Figure 4B). In addition, there were no differences in the relative bone weight between the CON and age-matched CHD groups.
Cortical and trabecular density
There were age-induced changes in the cortical (Figure 5A) and trabecular (Figure 5B) density. The cortical and trabecular density was lower in the CON group at 60 weeks of age than in the CON and CHD groups at 42 weeks of age. In addition, there were differences in the cortical and trabecular density between the CON and age-matched CHD groups. The cortical and trabecular density was higher in the CHD group at 60 weeks of age than in the age-matched CON group.
Figure 5.
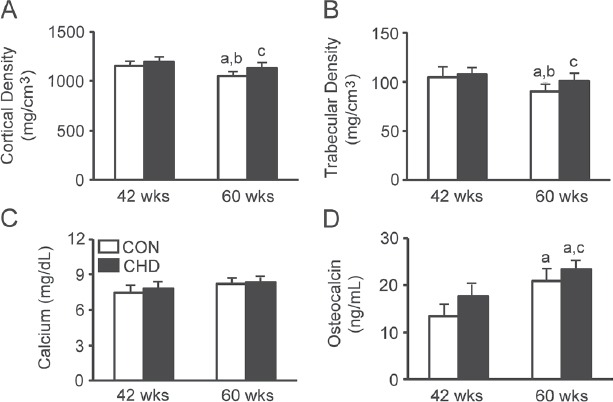
Cortical (A) and trabecular (B) density of the femur and serum calcium (C) and osteocalcin (D) levels of the control (CON) and collagen hydrolysate (CHD) groups at 42 and 60 weeks of age. Values are means ± SD for 5 animals. aP<0.05 compared with the CON group at 42 weeks of age; bP<0.05 compared with the CHD group at 42 weeks of age; cP<0.05 compared with the CON group at 60 weeks of age.
Serum calcium and osteocalcin levels
There were no age-induced changes in calcium levels (Figure 5C). In addition, there were no differences in calcium levels between the CON and age-matched CHD groups.
There were age-induced changes in osteocalcin levels (Figure 5D). Osteocalcin levels were higher in the CON and CHD groups at 60 weeks of age than in the CON group at 42 weeks of age. In addition, there were differences in osteocalcin levels between the CON and age-matched CHD groups. Osteocalcin levels were higher in the CHD group at 60 weeks of age than in the age-matched CON group.
Discussion
Effects of age on the musculoskeletal system in SAMP6 mice
This study demonstrated that the oxidative capacity of the tibialis anterior muscle (Figure 1D) and its fibres, irrespective of the muscle region (Figures 3D–F), were lower in the CON group at 60 weeks of age than in the CON group at 42 weeks of age. In addition, the cortical (Figure 5A) and trabecular (Figure 5B) density of the femur was lower in the CON group at 60 weeks of age than in the CON group at 42 weeks of age, indicating muscle atrophy and osteoporosis in SAMP6 mice at 60 weeks of age. These results were consistent with our previous findings that SAMP6 mice at 60 weeks of age show muscle atrophy and senile osteoporosis3,21.
Effects of CHD on the musculoskeletal system
Effects of nutritional supplements on osteoporosis have not been well investigated, although the oral administration of a lactococcal strain recovered the cortical and trabecular density in senescence-accelerated mice26. Treatment with CHD showed a dose-dependent increase in proliferation of osteoblastic cells (MG63) and alkaline phosphatase activity in osteoblast-like cells (MC3T3). In addition, CHD increased collagen synthesis of MG63 cells and collagen type I alpha 1 (COL1A1) gene expression16. In contrast, CHD-induced COL1A1 gene expression was completely inhibited by an extracellular signal-regulated kinase (ERK) inhibitor16. These results suggest that the intake of CHD increases the biosynthesis of type I collagen and that this process is regulated at the transcription level. Previous studies27-29 observed that CHD is digested and circulates in the blood as collagen-derived hydroxyproline (Hyp)-containing dipeptides and tripeptides, and dipeptides like prolyl-Hyp (Pro-Hyp) particularly circulate in the blood. In fact, our previous study using CHD extracted from chicken feet28 showed that significant amounts of Pro-Hyp were found in the blood.
A previous study30 showed that MC3T3-E1 cells treated with Pro-Hyp had altered expression of muscle-related genes in mouse skin and increased activity of alkaline phosphatase, which is a differentiation marker of osteoblastic cells. When these cells were treated with Pro-Hyp, Runx2, Osterix, and COL1A gene expression was upregulated, indicating that Pro-Hyp promoted the differentiation of osteoblastic cells in this study. It has been suggested that Pro-Hyp, which is produced by the intake of CHD, accelerates an increase in bone density with differentiation and progression of osteoblastic cells. Recent studies11,31 showed that CHD improved skin conditions, e.g., epidermal barrier function and dermal skin elasticity, in mice with altered muscle-related gene expression.
Effects of CHD on the musculoskeletal system in SAMP6 mice
Bone metabolism and function are influenced by skeletal muscle, tendon, and periosteum32. It has been suggested that the decreased function in skeletal muscle leads to osteoporosis. There are few data, except for our previous studies3,21, that show the effects of age on musculoskeletal properties. Therefore, in this study, muscle properties, including muscle oxidative enzyme activity, fibre CSAs, and fibre oxidative capacity, as well as bone density and osteocalcin levels were examined. This study showed that CHD inhibits an age-induced decrease in muscle SDH activity (Figure 1D). In addition, the age-induced decrease in fibre SDH staining intensity, irrespective of the muscle region, was inhibited by CHD (Figures 3D-F). We did not elucidate the reason why CHD inhibits the age-induced decrease in skeletal muscle and fibre oxidative capacity. It has been suggested that enhanced oxidative metabolism, induced by CHD, maintains oxidative capacity in skeletal muscle and its fibres as well as skin conditions11.
Effects of age on osteocalcin levels in SAMP6 mice
Osteocalcin levels are highest during childhood, especially at adolescence when the increase in height is remarkable33. Thereafter, the osteocalcin levels decrease with growth and are maintained at a constant value until a certain age. Then, osteocalcin levels again increase with age in women and men aged 30 to 90 years old and increase markedly in men aged 80 to 90 years old34. In addition, osteocalcin levels are higher in women than in men, irrespective of age. Other studies35,36 showed that the increase in osteocalcin levels is remarkable in postmenopausal women because of excessive bone metabolism after menopause. It was concluded that an increase in osteocalcin levels in humans occurs with age when reduction in bone mass or postmenopausal osteoporosis becomes evident.
In a previous study using normal mice from 12 to 116 weeks of age37, it was shown that osteocalcin levels decrease after 24 weeks of age. Another study38 showed that osteocalcin levels in normal mice at 48 weeks of age were lower than those at 24 weeks of age. In contrast, there are no data available on age-induced changes in osteocalcin levels in aged SAMP6 mice more than 40 weeks of age. A previous study39 showed that SAMP6 mice from 20 to 40 weeks of age, which had a fractured bone, had increased osteocalcin levels.
In general, humans show increased osteocalcin levels with increased age. We did not elucidate whether the age-induced increase in osteocalcin levels observed in this study (Figure 5D) is specific to SAMP6 mice, because there are no data, except those from a few studies using normal mice37,38, available for age-induced changes in osteocalcin levels of aged mice.
In this study, we observed a decrease in cortical and trabecular density of the femur in SAMP6 mice during this period (Figures 5A, B), indicating a senile osteoporosis in SAMP6 mice during this period. It has been suggested that the age-induced increase in the osteocalcin levels in SAMP6 mice is a response to counter osteoporosis by inhibiting bone resorption with age. The increased osteocalcin levels in the CHD group at 60 weeks of age (Figure 5D) were consistent with the findings that the cortical (Figure 5A) and trabecular (Figure 5B) density of the femur was greater in the CHD group at 60 weeks of age than in the age-matched CON group.
Perspectives of this study
This study on SAMP6 mice, which showed age-induced osteoporosis at 60 weeks of age and the effect of CHD on osteoporosis, can be considered a preliminary experiment on the basis of its small sampling size (n=5 for each group). However, it is important to note that this is the first study showing the application of CHD treatment for degeneration of the musculoskeletal system in aged mice. We plan to extend our experiments to include bone formation and resorption and to collect data from more SAMP6 mice. We believe that this initial study presents a novel use of CHD treatment and is thus sufficiently valuable despite the small number of samples.
Conclusion
There is a possibility that CHD is effective for the inhibition in the degeneration of musculoskeletal system including reduced muscle and fibre oxidative capacity and cortical and trabecular bone density.
Acknowledgements
This work was supported in part by the NH Foods Ltd., Tsukuba, Japan.
Footnotes
The authors have no conflict of interest.
Edited by: M. Hamrick
References
- 1.Ishihara A, Naitoh H, Katsuta S. Effects of ageing on the total number of muscle fibers and motoneurons of the tibialis anterior and soleus muscles in the rat. Brain Res. 1987;435:355–8. doi: 10.1016/0006-8993(87)91624-6. [DOI] [PubMed] [Google Scholar]
- 2.Ishihara A, Araki H. Effects of age on the number and histochemical properties of muscle fibers and motoneurons in the rat extensor digitorum longus muscle. Mech Ageing Dev. 1988;45:213–21. doi: 10.1016/0047-6374(88)90003-6. [DOI] [PubMed] [Google Scholar]
- 3.Hirofuji C, Ishihara A, Roy RR, Itoh K, Itoh M, Edgerton VR, Katsuta S. SDH activity and cell size of tibialis anterior motoneurons and muscle fibers in SAMP6. Neuroreport. 2000;11:823–8. doi: 10.1097/00001756-200003200-00033. [DOI] [PubMed] [Google Scholar]
- 4.Nagatomo F, Gu N, Fujino H, Takeda I, Tsuda K, Ishihara A. Skeletal muscle characteristics of rats with obesity, diabetes, hypertension, and hyperlipidemia. J Atheroscler Thromb. 2009;16:576–85. doi: 10.5551/jat.1065. [DOI] [PubMed] [Google Scholar]
- 5.Nagatomo F, Fujino H, Kondo H, Gu N, Takeda I, Ishioka N, Tsuda K, Ishihara A. PGC-1αmRNA level and oxidative capacity of the plantaris muscle in rats with metabolic syndrome, hypertension, and type 2 diabetes. Acta Histochem Cytochem. 2011;44:73–80. doi: 10.1267/ahc.10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishihara A, Oishi Y, Roy RR, Edgerton VR. Influence of two weeks of non-weight bearing on rat soleus motoneurons and muscle fibers. Aviat Space Environ Med. 1997;68:421–5. [PubMed] [Google Scholar]
- 7.Ishihara A, Kawano F, Ishioka N, Oishi H, Higashibata A, Shimazu T, Ohira Y. Effects of running exercise during recovery from hindlimb unloading on soleus muscle fibers and their spinal motoneurons in rats. Neurosci Res. 2004;48:119–27. doi: 10.1016/j.neures.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Ishihara A, Ohira Y, Roy RR, Nagaoka S, Sekiguchi C, Hinds WE, Edgerton VR. Influence of spaceflight on succinate dehydrogenase activity and soma size of rat ventral horn neurons. Acta Anat. 1996;157:303–8. doi: 10.1159/000147892. [DOI] [PubMed] [Google Scholar]
- 9.Ishihara A, Ohira Y, Roy RR, Nagaoka S, Sekiguchi C, Hinds WE, Edgerton VR. Comparison of the response of motoneurons innervating perineal and hind limb muscles to spaceflight and recovery. Muscle Nerve. 2000;23:753–62. doi: 10.1002/(sici)1097-4598(200005)23:5<753::aid-mus13>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 10.Wronski TJ, Morey-Holton ER. Skeletal response to simulated weightlessness:a comparison of suspension techniques. Aviat Space Environ Med. 1987;58:63–8. [PubMed] [Google Scholar]
- 11.Shimizu J, Asami N, Kataoka A, Sugihara F, Inoue N, Kimira Y, Wada M, Mano H. Oral collagen-derived dipeptides, prolyl-hydroxyproline and hydroxyprolyl-glycine, ameliorate skin barrier dysfunction and alter gene expression profiles in the skin. Biochem Biophys Res Commun. 2015;456:626–30. doi: 10.1016/j.bbrc.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Koyama Y, Hirota A, Mori H, Takahara H, Kuwaba K, Kusubata M, Matsubara Y, Kasugai S, Itoh M, Irie S. Ingestion of gelatin has differential effect on bone mineral density and body weight in protein undernutrition. J Nutr Sci Vitaminol. 2001;47:84–6. doi: 10.3177/jnsv.47.84. [DOI] [PubMed] [Google Scholar]
- 13.Wu J, Fujioka M, Sugimoto K, Mu G, Ishimi Y. Assessment of effectiveness of oral administration of collagen peptide on bone metabolism in growing and mature rats. J Bone Miner Metab. 2004;22:547–53. doi: 10.1007/s00774-004-0522-2. [DOI] [PubMed] [Google Scholar]
- 14.Guillerminet F, Beaupied H, Fabien-Soule V, Tome D, Benhamou CL, Roux C, Blais A. Hydrolyzed collagen improves bone metabolism and biomechanical parameters in ovariectomized mice:an in vitro and in vivo study. Bone. 2010;46:827–34. doi: 10.1016/j.bone.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 15.Guillerminet F, Fabien-Soule V, Even PC, Tome D, Benhamou CL, Roux C, Blais A. Hydrolyzed collagen improves bone status and prevents bone loss in ovariectomized C3H/HeN mice. Osteoporos Int. 2012;23:1909–19. doi: 10.1007/s00198-011-1788-6. [DOI] [PubMed] [Google Scholar]
- 16.Kim HK, Kim MG, Leem KH. Osteogenic activity of collagen peptide via ERK/MAPK pathway mediated boosting of collagen synthesis and its therapeutic efficacy in osteoporotic bone by back-scattered electron imaging and microarchitecture analysis. Molecules. 2013;18:15474–89. doi: 10.3390/molecules181215474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushita M, Tsuboyama T, Kasai R, Okumura H, Yamamuro T, Higuchi K, Kohno A, Yonezu T, Utani A, Umezawa M, Takeda T. Age-related changes in bone mass in the senescence-accelerated mouse (SAM). SAM-R/3 and SAM-P/6 as new murine models for senile osteoporosis. Am J Pathol. 1986;125:276–83. [PMC free article] [PubMed] [Google Scholar]
- 18.Takeda T, Hosokawa M, Higuchi K. Senescence-accelerated mouse (SAM):a novel murine model of accelerated senescence. J Am Geriatr Soc. 1991;39:911–9. doi: 10.1111/j.1532-5415.1991.tb04460.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Zhou X, Emura S, Shoumura S. Site-specific bone loss in senescence-accelerated mouse (SAMP6):a murine model for senile osteoporosis. Exp Gerontol. 2009;44:792–8. doi: 10.1016/j.exger.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Kubo KY. Segmental variations in trabecular bone density and microstructure of the spine in senescence-accelerated mouse (SAMP6):a murine model for senile osteoporosis. Exp Gerontol. 2012;47:317–22. doi: 10.1016/j.exger.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Ishihara A, Roy RR, Ohira Y, Kawano F, Nonaka K, Yamamoto K, Edgerton VR. Effects of aging and exercise on density and cross-sectional area of femur in senescence-accelerated mouse prone 6. J Musculoskelet Neuronal Interact. 2003;3:162–9. [PubMed] [Google Scholar]
- 22.Lehmann R, Wapniarz M, Kvasnicka HM, Baedeker S, Klein K, Allolio B. Reproducibility of bone density measurements of the distal radius using a high resolution special scanner for peripheral quantitative computed tomography (Single Energy PQCT) Radiologe. 1992;32:177–81. [PubMed] [Google Scholar]
- 23.Lind PM, Lind L, Larsson S, Orberg J. Torsional testing and peripheral quantitative computed tomography in rat humerus. Bone. 2001;29:265–70. doi: 10.1016/s8756-3282(01)00576-2. [DOI] [PubMed] [Google Scholar]
- 24.Nagatomo F, Fujino H, Kondo H, Kouzaki M, Gu N, Takeda I, Tsuda K, Ishihara A. The effects of running exercise on oxidative capacity and PGC-1αmRNA levels in the soleus muscle of rats with metabolic syndrome. J Physiol Sci. 2012;62:105–14. doi: 10.1007/s12576-011-0188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagatomo F, Fujino H, Kondo H, Takeda I, Tsuda K, Ishihara A. High-fat diet-induced reduction of PGC-1αmRNA levels and oxidative capacity in the soleus muscle of rats with metabolic syndrome. Nutr Res. 2012;32:144–51. doi: 10.1016/j.nutres.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Kimoto-Nira H, Suzuki C, Kobayashi M, Sasaki K, Kurisaki J, Mizumachi K. Anti-ageing effect of a lactococcal strain:analysis using senescence-accelerated mice. Br J Nutr. 2007;98:1178–86. doi: 10.1017/S0007114507787469. [DOI] [PubMed] [Google Scholar]
- 27.Iwai K, Hasegawa T, Taguchi Y, Morimatsu F, Sato K, Nakamura Y, Higashi A, Kido Y, Nakabo Y, Ohtsuki K. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J Agric Food Chem. 2005;53:6531–6. doi: 10.1021/jf050206p. [DOI] [PubMed] [Google Scholar]
- 28.Nakatani S, Mano H, Sampei C, Shimizu J, Wada M. Chondroprotective effect of the bioactive peptide prolyl-hydroxyproline in mouse articular cartilage in vitro and in vivo. Osteoarthritis Cartilage. 2009;17:1620–7. doi: 10.1016/j.joca.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Taga Y, Kusubata M, Ogawa-Goto K, Hattori S. Highly accurate quantification of hydroxyproline-containing peptides in blood using a protease digest of stable isotope-labeled collagen. J Agric Food Chem. 2014;62:12096–102. doi: 10.1021/jf5039597. [DOI] [PubMed] [Google Scholar]
- 30.Kimira Y, Ogura K, Taniuchi Y, Kataoka A, Inoue N, Sugihara F, Nakatani S, Shimizu J, Wada M, Mano H. Collagen-derived dipeptide prolyl-hydroxyproline promotes differentiation of MC3T3-E1 osteoblastic cells. Biochem Biophys Res Commun. 2014;453:498–501. doi: 10.1016/j.bbrc.2014.09.121. [DOI] [PubMed] [Google Scholar]
- 31.Oba C, Ohara H, Morifuji M, Ito K, Ichikawa S, Kawahata K, Koga J. Collagen hydrolysate intake improves the loss of epidermal barrier function and skin elasticity induced by UVB irradiation in hairless mice. Photodermatol Photoimmunol Photomed. 2013;29:204–11. doi: 10.1111/phpp.12051. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Yao XF, Emura S, Shoumura S. Morphological changes of skeletal muscle, tendon and periosteum in the senescence-accelerated mouse (SAMP6):a murine model for senile osteoporosis. Tissue Cell. 2006;38:325–35. doi: 10.1016/j.tice.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Kanbur NO, Derman O, Sen TA, Kinik E. Osteocalcin. A biochemical marker of bone turnover during puberty. Int J Adolesc Med Health. 2002;14:235–44. doi: 10.1515/ijamh.2002.14.3.235. [DOI] [PubMed] [Google Scholar]
- 34.Epstein S, Poser J, McClintock R, Johnston CC, Jr, Bryce G, Hui S. Differences in serum bone GLA protein with age and sex. Lancet. 1984;1:307–10. doi: 10.1016/s0140-6736(84)90360-x. [DOI] [PubMed] [Google Scholar]
- 35.Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res. 1996;11:337–49. doi: 10.1002/jbmr.5650110307. [DOI] [PubMed] [Google Scholar]
- 36.Melton LJ, 3rd, Khosla S, Atkinson EJ, O'Fallon WM, Riggs BL. Relationship of bone turnover to bone density and fractures. J Bone Miner Res. 1997;12:1083–91. doi: 10.1359/jbmr.1997.12.7.1083. [DOI] [PubMed] [Google Scholar]
- 37.Hamrick MW, Ding KH, Pennington C, Chao YJ, Wu YD, Howard B, Immel D, Borlongan C, McNeil PL, Bollag WB, Curl WW, Yu J, Isales CM. Age-related loss of muscle mass and bone strength in mice is associated with a decline in physical activity and serum leptin. Bone. 2006;39:845–53. doi: 10.1016/j.bone.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Shahnazari M, Dwyer D, Chu V, Asuncion F, Stolina M, Ominsky M, Kostenuik P, Halloran B. Bone turnover markers in peripheral blood and marrow plasma reflect trabecular bone loss but not endocortical expansion in aging mice. Bone. 2012;50:628–37. doi: 10.1016/j.bone.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Histing T, Kuntz S, Stenger D, Scheuer C, Garcia P, Holstein JH, Klein M, Pohlemann T, Menger MD. Delayed fracture healing in aged senescence-accelerated P6 mice. J Invest Surg. 2013;26:30–5. doi: 10.3109/08941939.2012.687435. [DOI] [PubMed] [Google Scholar]


