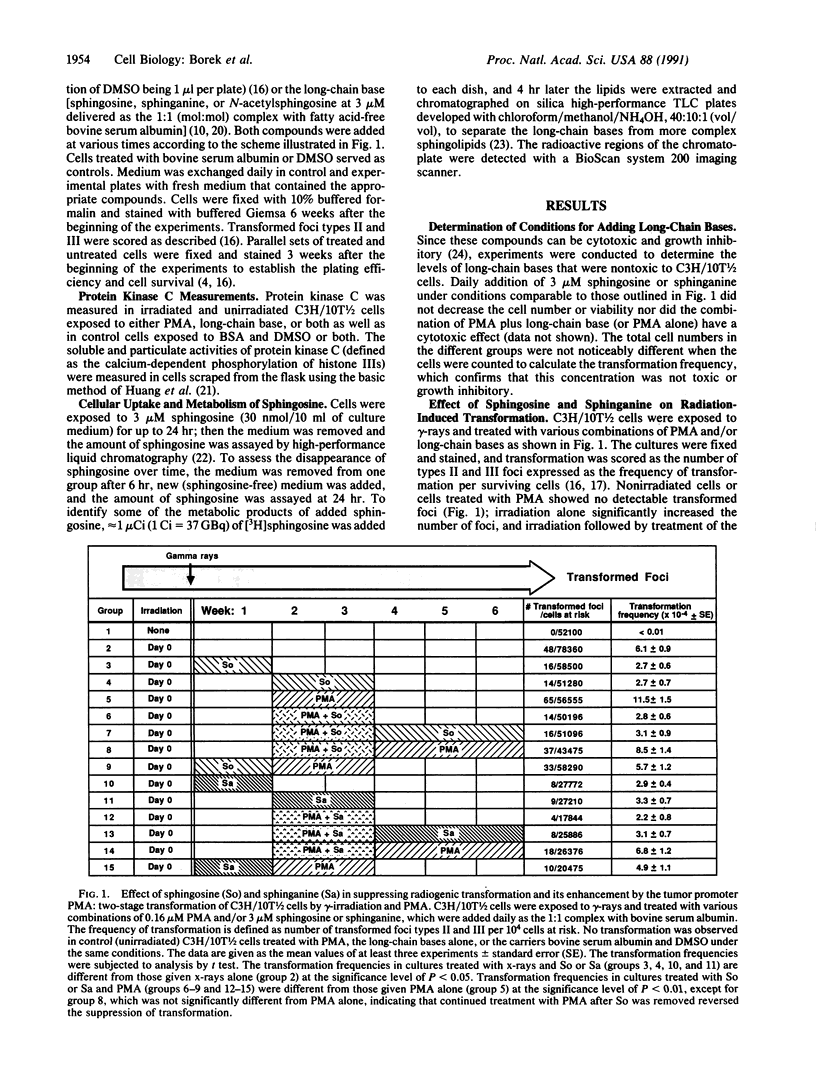
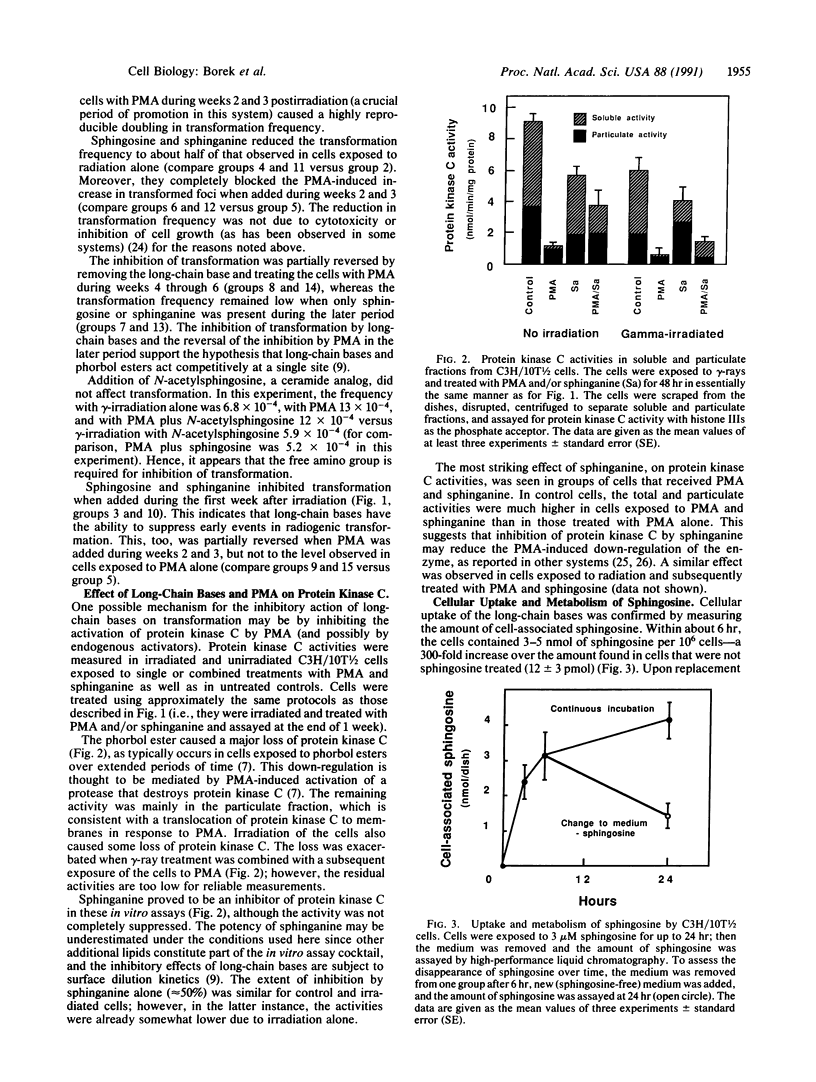
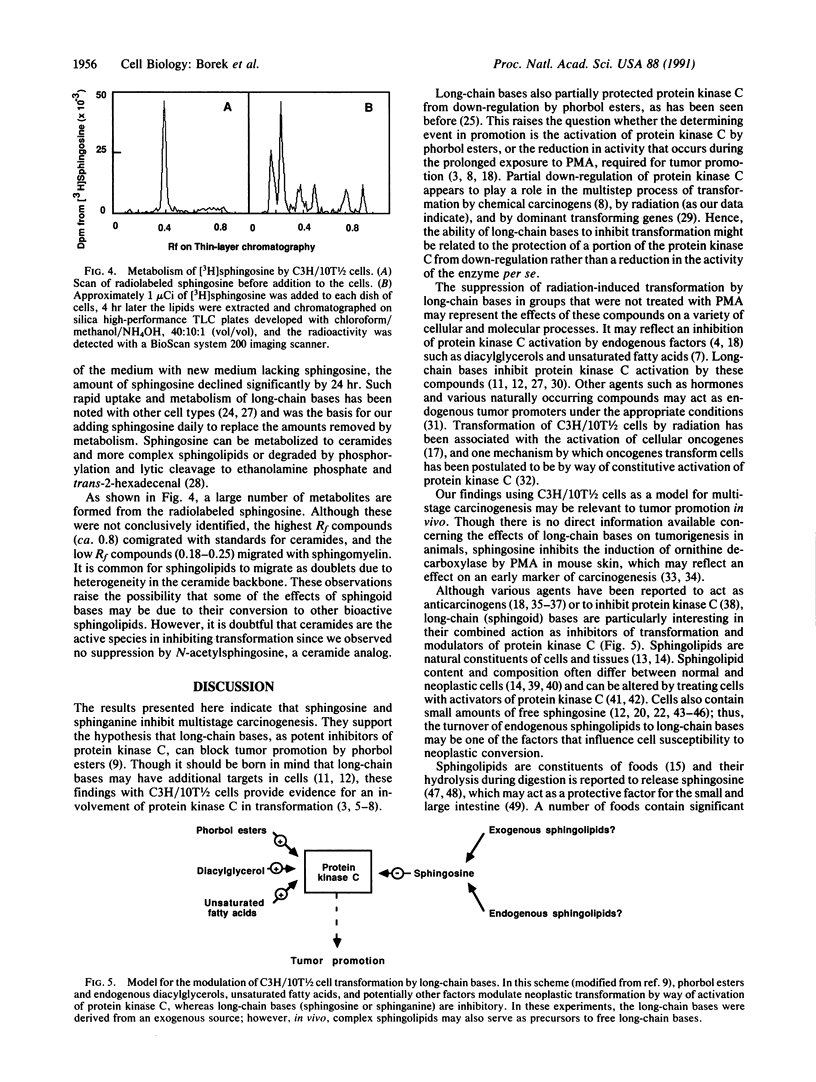
Abstract
Sphingosine and other long-chain (sphingoid) bases inhibit protein kinase C, the putative cellular receptor for the tumor promoter phorbol 12-myristate 13-acetate (PMA), and exert potent effects on diverse cell functions. We tested the ability of long-chain bases to modulate multistage carcinogenesis in mouse C3H/10T1/2 cells exposed to gamma-rays and PMA. Sphingosine and sphinganine completely blocked the enhancement of radiation-induced transformation by PMA (promotion) and partially suppressed transformation by radiation alone. N-Acetylsphingosine, a ceramide analog, did not inhibit transformation. Sphingosine was rapidly taken up by the cells and metabolized; hence, the long-chain bases were added daily to achieve prolonged inhibition. Long-chain bases inhibited protein kinase C activity in C3H/10T1/2 cells and suppressed the down-regulation of this enzyme by PMA. Our results establish that long-chain bases are highly effective inhibitors of carcinogenesis in this model. Our results also indicate that the suppressive effects may be mediated, in part, by inhibition of protein kinase C. The data suggest that sphingosine and other long-chain bases derived from complex sphingolipids may act as cancer-preventative agents.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Borek C., Guernsey D. L., Ong A., Edelman I. S. Critical role played by thyroid hormone in induction of neoplastic transformation by chemical carcinogens in tissue culture. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5749–5752. doi: 10.1073/pnas.80.18.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek C. In vitro cell cultures as tools in the study of free radicals and free radical modifiers in carcinogenesis. Methods Enzymol. 1984;105:464–479. doi: 10.1016/s0076-6879(84)05065-5. [DOI] [PubMed] [Google Scholar]
- Borek C., Ong A., Mason H. Distinctive transforming genes in x-ray-transformed mammalian cells. Proc Natl Acad Sci U S A. 1987 Feb;84(3):794–798. doi: 10.1073/pnas.84.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek C., Ong A., Mason H., Donahue L., Biaglow J. E. Selenium and vitamin E inhibit radiogenic and chemically induced transformation in vitro via different mechanisms. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1490–1494. doi: 10.1073/pnas.83.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek C., Pain C., Mason H. Neoplastic transformation of hamster embryo cells irradiated in utero and assayed in vitro. Nature. 1977 Mar 31;266(5601):452–454. doi: 10.1038/266452a0. [DOI] [PubMed] [Google Scholar]
- Borek C. Radiation and chemically induced transformation: free radicals, antioxidants and cancer. Br J Cancer Suppl. 1987 Jun;8:74–86. [PMC free article] [PubMed] [Google Scholar]
- Borek C. The induction and control of radiogenic transformation in vitro: cellular and molecular mechanisms. Pharmacol Ther. 1985;27(1):99–142. doi: 10.1016/0163-7258(85)90066-x. [DOI] [PubMed] [Google Scholar]
- Borek C., Troll W. Modifiers of free radicals inhibit in vitro the oncogenic actions of x-rays, bleomycin, and the tumor promoter 12-O-tetradecanoylphorbol 13-acetate. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1304–1307. doi: 10.1073/pnas.80.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner C., Eppenberger U., Wyss R., Fabbro D. Continuous synthesis of two protein-kinase-C-related proteins after down-regulation by phorbol esters. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2110–2114. doi: 10.1073/pnas.85.7.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady R. O., Borek C., Bradley R. M. Composition and synthesis of gangliosides in rat hepatocyte and hepatoma cell lines. J Biol Chem. 1969 Dec 10;244(23):6552–6554. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Enkvetchakul B., Merrill A. H., Jr, Birt D. F. Inhibition of the induction of ornithine decarboxylase activity by 12-O-tetradecanoylphorbol-13-acetate in mouse skin by sphingosine sulfate. Carcinogenesis. 1989 Feb;10(2):379–381. doi: 10.1093/carcin/10.2.379. [DOI] [PubMed] [Google Scholar]
- FOULDS L. The experimental study of tumor progression: a review. Cancer Res. 1954 Jun;14(5):327–339. [PubMed] [Google Scholar]
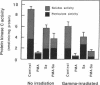
- Grove D. S., Mastro A. M. Prevention of the TPA-mediated down-regulation of protein kinase C. Biochem Biophys Res Commun. 1988 Feb 29;151(1):94–99. doi: 10.1016/0006-291x(88)90563-3. [DOI] [PubMed] [Google Scholar]
- Gupta A. K., Fisher G. J., Elder J. T., Nickoloff B. J., Voorhees J. J. Sphingosine inhibits phorbol ester-induced inflammation, ornithine decarboxylase activity, and activation of protein kinase C in mouse skin. J Invest Dermatol. 1988 Nov;91(5):486–491. doi: 10.1111/1523-1747.ep12476635. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989 Jan 27;243(4890):500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Merrill A. H., Jr, Bell R. M. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986 Sep 25;261(27):12604–12609. [PubMed] [Google Scholar]
- Hansen L. A., Monteiro-Riviere N. A., Smart R. C. Differential down-regulation of epidermal protein kinase C by 12-O-tetradecanoylphorbol-13-acetate and diacylglycerol: association with epidermal hyperplasia and tumor promotion. Cancer Res. 1990 Sep 15;50(18):5740–5745. [PubMed] [Google Scholar]
- Huang K. P., Nakabayashi H., Huang F. L. Isozymic forms of rat brain Ca2+-activated and phospholipid-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8535–8539. doi: 10.1073/pnas.83.22.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiguchi K., Henning-Chubb C., Huberman E. Alteration in glycosphingolipid pattern during phorbol-12-myristate-13-acetate-induced cell differentiation in human T-lymphoid leukemia cells. Cancer Res. 1986 Jun;46(6):3027–3033. [PubMed] [Google Scholar]
- Kobayashi T., Mitsuo K., Goto I. Free sphingoid bases in normal murine tissues. Eur J Biochem. 1988 Mar 15;172(3):747–752. doi: 10.1111/j.1432-1033.1988.tb13952.x. [DOI] [PubMed] [Google Scholar]
- Merrill A. H., Jr, Jones D. D. An update of the enzymology and regulation of sphingomyelin metabolism. Biochim Biophys Acta. 1990 May 1;1044(1):1–12. doi: 10.1016/0005-2760(90)90211-f. [DOI] [PubMed] [Google Scholar]
- Merrill A. H., Jr, Nimkar S., Menaldino D., Hannun Y. A., Loomis C., Bell R. M., Tyagi S. R., Lambeth J. D., Stevens V. L., Hunter R. Structural requirements for long-chain (sphingoid) base inhibition of protein kinase C in vitro and for the cellular effects of these compounds. Biochemistry. 1989 Apr 18;28(8):3138–3145. doi: 10.1021/bi00434a004. [DOI] [PubMed] [Google Scholar]
- Merrill A. H., Jr, Sereni A. M., Stevens V. L., Hannun Y. A., Bell R. M., Kinkade J. M., Jr Inhibition of phorbol ester-dependent differentiation of human promyelocytic leukemic (HL-60) cells by sphinganine and other long-chain bases. J Biol Chem. 1986 Sep 25;261(27):12610–12615. [PubMed] [Google Scholar]
- Merrill A. H., Jr, Stevens V. L. Modulation of protein kinase C and diverse cell functions by sphingosine--a pharmacologically interesting compound linking sphingolipids and signal transduction. Biochim Biophys Acta. 1989 Feb 9;1010(2):131–139. doi: 10.1016/0167-4889(89)90152-3. [DOI] [PubMed] [Google Scholar]
- Merrill A. H., Jr, Wang E. Biosynthesis of long-chain (sphingoid) bases from serine by LM cells. Evidence for introduction of the 4-trans-double bond after de novo biosynthesis of N-acylsphinganine(s). J Biol Chem. 1986 Mar 15;261(8):3764–3769. [PubMed] [Google Scholar]
- Merrill A. H., Jr, Wang E., Mullins R. E., Jamison W. C., Nimkar S., Liotta D. C. Quantitation of free sphingosine in liver by high-performance liquid chromatography. Anal Biochem. 1988 Jun;171(2):373–381. doi: 10.1016/0003-2697(88)90500-3. [DOI] [PubMed] [Google Scholar]
- Moskal J. R., Lockney M. W., Marvel C. C., Trosko J. E., Sweeley C. C. Effect of retinoic acid and phorbol-12-myristate-13-acetate on glycosyltransferase activities in normal and transformed cells. Cancer Res. 1987 Feb 1;47(3):787–790. [PubMed] [Google Scholar]
- Nilsson A. Metabolism of cerebroside in the intestinal tract of the rat. Biochim Biophys Acta. 1969 Jul 29;187(1):113–121. doi: 10.1016/0005-2760(69)90138-6. [DOI] [PubMed] [Google Scholar]
- Nilsson A. Metabolism of sphingomyelin in the intestinal tract of the rat. Biochim Biophys Acta. 1968 Dec 18;164(3):575–584. doi: 10.1016/0005-2760(68)90187-2. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and prospectives of the protein kinase c family for cellular regulation. Cancer. 1989 May 15;63(10):1892–1903. doi: 10.1002/1097-0142(19890515)63:10<1892::aid-cncr2820631005>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Slife C. W., Wang E., Hunter R., Wang S., Burgess C., Liotta D. C., Merrill A. H., Jr Free sphingosine formation from endogenous substrates by a liver plasma membrane system with a divalent cation dependence and a neutral pH optimum. J Biol Chem. 1989 Jun 25;264(18):10371–10377. [PubMed] [Google Scholar]
- Stevens V. L., Nimkar S., Jamison W. C., Liotta D. C., Merrill A. H., Jr Characteristics of the growth inhibition and cytotoxicity of long-chain (sphingoid) bases for Chinese hamster ovary cells: evidence for an involvement of protein kinase C. Biochim Biophys Acta. 1990 Jan 23;1051(1):37–45. doi: 10.1016/0167-4889(90)90171-9. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Bishop W. R., Bell R. M. Enzymatic quantification of sphingosine in the picomole range in cultured cells. Anal Biochem. 1989 Nov 15;183(1):177–189. doi: 10.1016/0003-2697(89)90186-3. [DOI] [PubMed] [Google Scholar]
- Weinstein I. B. The origins of human cancer: molecular mechanisms of carcinogenesis and their implications for cancer prevention and treatment--twenty-seventh G.H.A. Clowes memorial award lecture. Cancer Res. 1988 Aug 1;48(15):4135–4143. [PubMed] [Google Scholar]
- Weyman C. M., Taparowsky E. J., Wolfson M., Ashendel C. L. Partial down-regulation of protein kinase C in C3H 10T 1/2 mouse fibroblasts transfected with the human Ha-ras oncogene. Cancer Res. 1988 Nov 15;48(22):6535–6541. [PubMed] [Google Scholar]
- Wilson E., Olcott M. C., Bell R. M., Merrill A. H., Jr, Lambeth J. D. Inhibition of the oxidative burst in human neutrophils by sphingoid long-chain bases. Role of protein kinase C in activation of the burst. J Biol Chem. 1986 Sep 25;261(27):12616–12623. [PubMed] [Google Scholar]
- Wilson E., Wang E., Mullins R. E., Uhlinger D. J., Liotta D. C., Lambeth J. D., Merrill A. H., Jr Modulation of the free sphingosine levels in human neutrophils by phorbol esters and other factors. J Biol Chem. 1988 Jul 5;263(19):9304–9309. [PubMed] [Google Scholar]
- Wolfman A., Macara I. G. Elevated levels of diacylglycerol and decreased phorbol ester sensitivity in ras-transformed fibroblasts. Nature. 1987 Jan 22;325(6102):359–361. doi: 10.1038/325359a0. [DOI] [PubMed] [Google Scholar]
- Zeisel S. H., Char D., Sheard N. F. Choline, phosphatidylcholine and sphingomyelin in human and bovine milk and infant formulas. J Nutr. 1986 Jan;116(1):50–58. doi: 10.1093/jn/116.1.50. [DOI] [PubMed] [Google Scholar]
- el Touny S., Khan W., Hannun Y. Regulation of platelet protein kinase C by oleic acid. Kinetic analysis of allosteric regulation and effects on autophosphorylation, phorbol ester binding, and susceptibility to inhibition. J Biol Chem. 1990 Sep 25;265(27):16437–16443. [PubMed] [Google Scholar]