Abstract
Vitamin B6 includes six water-soluble vitamers: pyridoxal (PL), pyridoxamine (PM), pyridoxine (PN), and their phosphorylated forms. Pyridoxal 5′-phosphate (PLP) is an important cofactor for many metabolic enzymes. Several lines of evidence demonstrate that blood levels of PLP are significantly lower in patients with inflammation than in control subjects and that vitamin B6 has anti-inflammatory effects, with therapeutic potential for a variety of inflammatory diseases. Although one of our group previously demonstrated that PL inhibits the NF-κB pathway, the molecular mechanism by which vitamin B6 suppresses inflammation is not well understood. Here, we showed that both PL and PLP suppressed the expression of cytokine genes in macrophages by inhibiting Toll-like receptor (TLR)-mediated TAK1 phosphorylation and the subsequent NF-κB and JNK activation. Furthermore, PL and PLP abolished NLRP3-dependent caspase-1 processing and the subsequent secretion of mature IL-1β and IL-18 in LPS-primed macrophages. In contrast, PM and PN had little effect on IL-1β production. PLP, but not PL, markedly reduced the production of mitochondrial reactive oxygen species (ROS) in peritoneal macrophages. Importantly, PL and PLP reduced IL-1β production induced by LPS and ATP, or by LPS alone, in mice. Moreover, PL and PLP protected mice from lethal endotoxic shock. Collectively, these findings reveal novel anti-inflammatory activities for vitamin B6 and suggest its potential for preventing inflammatory diseases driven by the NLRP3 inflammasome.
Keywords: endotoxin, inflammasome, inflammation, interleukin 1 (IL-1), macrophage, NF-kappa B (NF-KB), NLRP3, vitamin, Vitamin B6, caspase-1, IL-1B, mouse
Introduction
Vitamin B6 is ingested from a variety of foods and can also be taken as a dietary supplement or clinical drug. The B6 vitamer pyridoxal 5′-phosphate (PLP)2 is an essential cofactor for many enzymes involved in amino acid metabolism. There is growing evidence that vitamin B6 has anti-inflammatory activity. Epidemiological evidence indicates that patients with inflammation have significantly lower blood levels of PLP than control subjects (1, 2). In patients with rheumatoid arthritis, high-dose vitamin B6 supplementation (100 mg/day) suppresses plasma IL-6 and TNF-α levels (3). Both human and animal studies have demonstrated an inverse relationship between vitamin B6 and colon cancer (4, 5). Recent clinical trials found that in Alzheimer's patients, B-vitamin supplementation (folic acid, vitamin B6, and vitamin B12) slowed the shrinkage of the whole brain and decreased atrophy in specific regions of the brain (6). A low vitamin B6 intake is associated with an increased risk of Parkinson's disease (7). Because inflammatory mechanisms are implicated in these diseases, vitamin B6 may be useful in preventing inflammatory diseases. Notably, B6 vitamer pyridoxal (PL) inhibits the LPS-induced activation of NF-κB, which is an important transcription factor for many inflammation-related genes in mouse macrophage RAW264.7 cells (8). However, the anti-inflammatory mechanisms of vitamin B6 are poorly understood.
The pleiotropic inflammatory cytokine IL-1β, which is primarily produced by myeloid cells such as monocytes and macrophages, induces the proliferation and/or production of other inflammatory cytokines, leukocyte adhesion molecules, or acute-phase proteins in leukocytes, myeloid cells, endothelial cells, hepatocytes, and so forth (9). IL-1β is synthesized as a precursor form (pro-IL-1β) that has to be proteolytically processed into a mature form to gain biological activity (10). The former step (pro-IL-1β synthesis) is mainly regulated by a cytoplasmic signaling pathway that activates NF-κB (signal 1), which is triggered, for example, by TLRs. The latter step (IL-1β processing) can be catalyzed by several proteases; of these, caspase 1 is the most important, as shown by the severe defects in mature IL-1β production in caspase-1-deficient mice (11, 12).
Caspase 1 is also synthesized as an inactive precursor and is fully activated by autoprocessing. The cytoplasmic signaling pathway that activates caspase 1 (signal 2) has been extensively studied recently, revealing that the inflammasome, which is a cytoplasmic multiprotein complex consisting of sensor proteins (such as NLRP3, NLRC4, or AIM2), adaptor proteins (ASC), and caspase 1, forms a platform to activate caspase 1 (10, 13). Of the sensor proteins, NLRP3 has been studied most intensively because it responds (directly or indirectly) not only to pathogen-associated molecules (such as bacterial ionophores, pore-forming toxins, and bacterial and viral RNA), but also to a variety of environmental and endogenous inflammatory substances (including asbestos, silica, ATP, urate crystals, β-amyloids, cholesterol crystals, and even fatty acids) (14–22). Accordingly, NLRP3 has been implicated in a variety of inflammatory diseases, including inflammatory bowel disease, gout, Alzheimer's disease, arteriosclerosis, and diabetes (19, 21, 23–28). In addition, NLRP3 mutations are known to cause autoinflammatory syndromes that are collectively called cryopyrin-associated periodic syndrome (29).
In this study, we demonstrate a novel role of vitamin B6 in suppressing IL-1β production by inhibiting the activation of the NLRP3 inflammasome. Furthermore, we show that vitamin B6 prevented LPS-induced endotoxic shock in vivo, suggesting that the NLRP3 inflammasome is an important target for vitamin B6 anti-inflammatory activity.
Results
Vitamin B6 Inhibited LPS-induced NF-κB and JNK Activation and Gene Expression
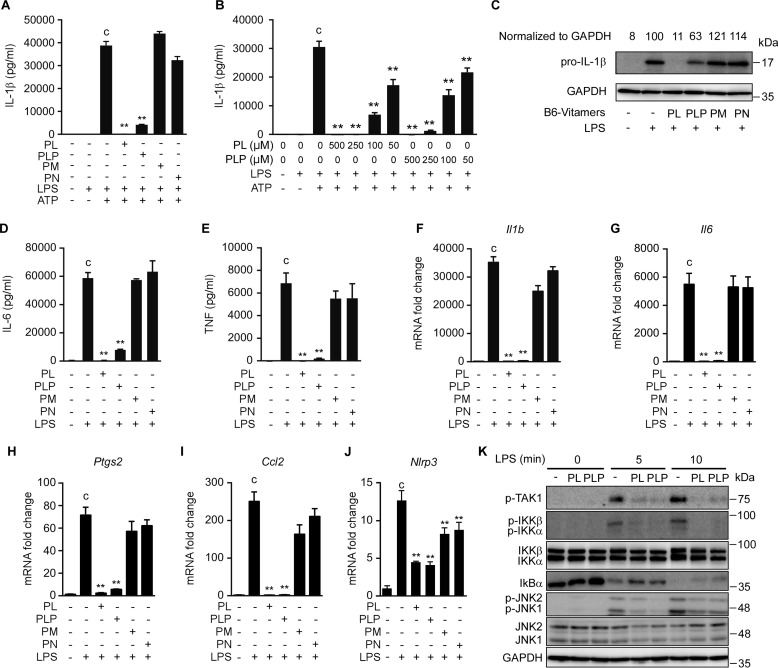
We initially investigated the overall effect of vitamin B6 on IL-1β secretion from macrophages stimulated with LPS plus ATP. To this end, thioglycollate-induced peritoneal macrophages were cultured for 24 h with or without B6 vitamer supplementation (500 μm), and then sequentially stimulated with LPS (TLR4 agonist) and ATP (NLRP3 activator) to induce IL-1β secretion. Under these conditions, IL-1β secretion was strongly inhibited by PL or PLP but not by pyridoxamine (PM) or pyridoxine (PN) (Fig. 1A). Titration experiments indicated that as little as 50 μm PL or PLP significantly suppressed IL-1β secretion (Fig. 1B). The LPS-induced intracellular accumulation of pro-IL-1β and secretion of IL-6 and TNF-α were also inhibited by PL or PLP, but not by PM or PN (Fig. 1, C–E). Furthermore, the LPS-induced expression of Il1b, Il6, Ptgs2, and Ccl2 mRNAs in peritoneal macrophages was inhibited by PL or PLP, but not by PM or PN (Fig. 1, F–I). PL and PLP, as well as PM and PN (although to a lesser extent), suppressed Nlrp3 mRNA expression (Fig. 1J). These results were consistent with the previous findings that PL inhibited LPS-induced NF-κB activation and expression of NF-κB target genes (Nos2 and Ptgs2) in the Raw264.7 mouse macrophage cell line (8). Actually, Western blotting analyses indicated that events upstream of LPS-induced NF-κB activation, including the phosphorylation of TAK1 and IκB kinases and the degradation of IκBα, were severely suppressed by PL or PLP (Fig. 1K). PL and PLP also inhibited the LPS-induced JNK phosphorylation (Fig. 1K), which occurs downstream of TAK1 and contributes to IL-1β gene expression (30, 31).
FIGURE 1.
PL and PLP suppress signal 1. A and B, peritoneal macrophages were treated with the indicated B6 vitamers (500 μm in A, and the indicated concentrations in B) for 24 h, stimulated with LPS for 16 h, and finally exposed to 5 mm ATP for 6 h. The IL-1β concentration in culture supernatants was determined by ELISA. C–E, peritoneal macrophages were treated with B6 vitamers for 24 h, and then stimulated with LPS for 16 h. ProIL-1β and GAPDH (loading control) were detected by Western blotting (C). The IL-6 and TNF-α concentrations in culture supernatants were determined by ELISA (D and E). F–J, peritoneal macrophages were treated with B6 vitamers for 24 h, and then stimulated with LPS for 16 h, after which the Il1b, Il6, Ptgs2, Ccl2, and Nlrp3 mRNAs were quantified by real-time PCR. K, peritoneal macrophages were treated with PL or PLP for 2 h, and then stimulated with LPS for the indicated times. The total and/or phosphorylated (p-) forms of TAK1, IKKs, IκBα, and JNKs were detected by Western blotting. A, B, and D–J, data show mean ± S.D.; n = 3. Asterisks indicate significant differences (**, p < 0.01) from the control group (c). All experiments were repeated at least three times, and representative data are shown.
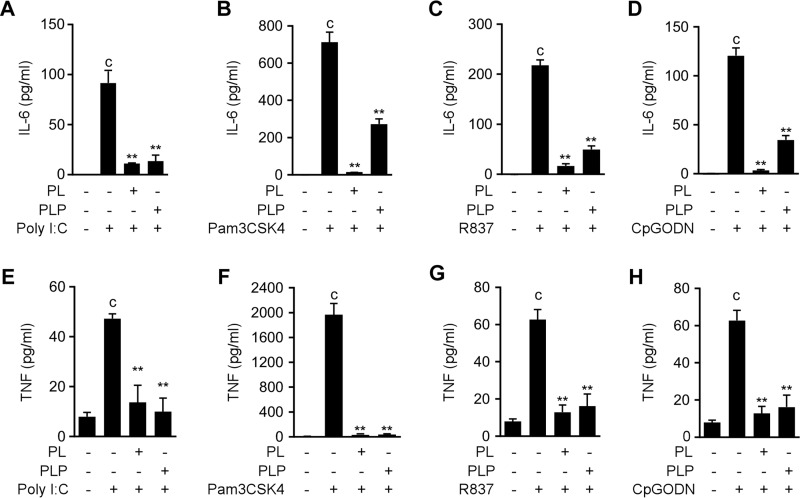
In addition, PL and PLP inhibited IL-6 and TNF-α production induced by other TLR ligands, including the TLR3 ligand poly(I:C), the TLR2 ligand Pam3CSK4, the TLR7 ligand imiquimod (R837), and CpG oligodeoxynucleotides, which are TLR9 ligands (Fig. 2). Taken together, our results demonstrate that both PL and PLP negatively regulate the TLR-mediated activation of NF-κB and MAPK pathways by inhibiting Tak1 phosphorylation, and thereby inhibit the expression of cytokine genes including Il1b in primary macrophages.
FIGURE 2.
PL and PLP suppress IL-6 and TNF-α production induced by TLR ligands. A–H, peritoneal macrophages were incubated with PL or PLP for 2 h, and then stimulated with poly I:C (20 μg/ml), Pam3CSK4 (10 μg/ml), R837 (10 μg/ml), or CpG oligodeoxynucleotides (CpGODN, 1 μm) for 6 h. IL-6 and TNF were measured by ELISA. Data show mean ± S.D.; n = 3. Asterisks indicate significant differences (**, p < 0.01) from the control group (c). All experiments were repeated at least three times, and representative data are shown.
Vitamin B6 Suppressed NLRP3 Inflammasome Activation
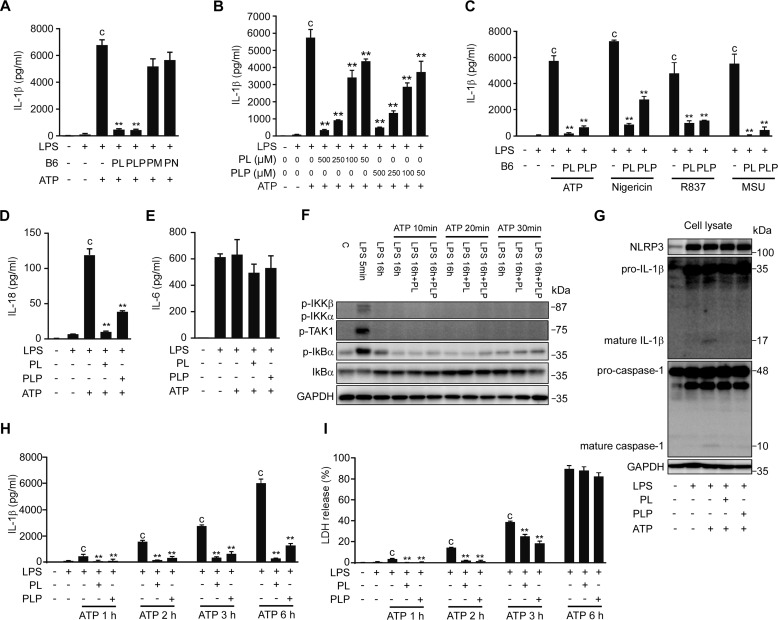
To further investigate the effect of vitamin B6 on signal 2, which leads to the proteolytic maturation of IL-1β, peritoneal macrophages were cultured with LPS for 16 h to fully induce intracellular pro-IL-1β accumulation, and then treated with B6 vitamers and stimulated with ATP for 6 h to activate the NLRP3 inflammasome. Under these conditions, IL-1β secretion was again inhibited by as little as 50 μm PL or PLP, but not by 500 μm PM or PN (Fig. 3, A and B). IL-1β secretion induced by other NLRP3 activators such as nigericin, R837, and monosodium urate (MSU) crystals was also inhibited by PL and PLP (Fig. 3C). The secretion of IL-18, which, like IL-1β undergoes proteolytic maturation catalyzed by caspase-1, was also suppressed by PL and PLP (Fig. 3D). In contrast, neither PL nor PLP added after LPS treatment affected the secretion of IL-6, an NF-κB-dependent but caspase-1-independent cytokine (Fig. 3E). In addition, ATP treatment after LPS priming induced no more phosphorylation of TAK1 and IκB kinases and degradation of IκBα, and PL and PLP did not affect these events (Fig. 3F). These data indicated that the suppression of IL-1β secretion by PL and PLP under these conditions was not due to the inhibition of NF-κB.
FIGURE 3.
PL and PLP suppress signal 2. A–E, peritoneal macrophages were sequentially incubated with LPS for 16 h, B6 vitamers for 2 h, and ATP (5 mm), nigericin (5 μm), R837 (10 μg/ml), or MSU (150 μg/ml) for 6 h. IL-1β, IL-18, and IL-6 in culture supernatants were quantified by ELISA. F, mouse peritoneal macrophages were exposed to LPS for 16 h, incubated with PL or PLP for 2 h, and finally stimulated with ATP for 10, 20, or 30 min. Whole cell lysates were collected and subjected to Western blotting using antibodies against phosphorylated (p-) forms of IKKα/β, TAK1, and IκBα, and total IκBα. GAPDH served as a loading control. G, peritoneal macrophages were treated with LPS for 16 h, and then incubated with PL or PLP for 2 h and finally exposed to ATP (5 mm) for 30 min. Pro-IL-1β, mature IL-1β, caspase-1, and GAPDH in cell lysates were detected by Western blotting. H and I, peritoneal macrophages were sequentially incubated with LPS for 16 h, with B6 vitamers for 2 h, and with ATP (5 mm) for 1, 2, 3, or 6 h. IL-1β in culture supernatants was quantified by ELISA (H). Cell death was evaluated by assaying LDH release (I). A–E, H, and I, data show mean ± S.D.; n = 3. Asterisks indicate significant differences (**, p < 0.01) from the control group (c). All experiments were repeated at least three times, and representative data are shown.
Western blotting analyses indicated that PL and PLP inhibited the generation of mature IL-1β (p17) and of the p10 fragment of mature caspase-1 when these vitamers were added after LPS treatment and before ATP stimulation (Fig. 3G). However, the LPS-induced NLRP3 and pro-IL-1β expression and the constitutive caspase-1 expression at both mRNA and protein levels were not inhibited by PL or PLP (data not shown and Fig. 3G), consistent with the notion that NF-κB-dependent gene expression was not inhibited under these conditions.
Inflammasome activation induces pyroptosis, a caspase-1-dependent programmed cell death. Pyroptotic cells rupture rapidly, releasing lactate dehydrogenase (LDH) and other cytoplasmic contents. Pyroptosis would also facilitate the IL-1β release from macrophages. PL and PLP inhibited LDH release from macrophages at 1, 2, and 3 h, but not 6 h after ATP stimulation (Fig. 3I). These results indicate that PL and PLP delayed pyroptosis; however, their suppression of IL-1β secretion at 6 h was not due to the inhibition of pyroptosis (Fig. 3H). Taken together, these results indicate that PL and PLP can inhibit the signal 2 mediated by the NLRP3 inflammasome.
Vitamin B6 Did Not Affect the Signal 2 Mediated by the NLRC4 and AIM2 Inflammasomes
The NLRP3, NLRC4, and AIM2 inflammasomes can be specifically activated by different bacterial species under certain conditions. For example, Staphylococcus aureus and Salmonella typhimurium at the logarithmic growth phase activate mainly the NLRP3 and NLRC4 inflammasome, respectively (32–34). In contrast, the infection of unprimed macrophages with Listeria monocytogenes followed by penicillin G treatment, which causes intracellular releases of bacterial DNA, induces the AIM2-dependent secretion of IL-1β (35).
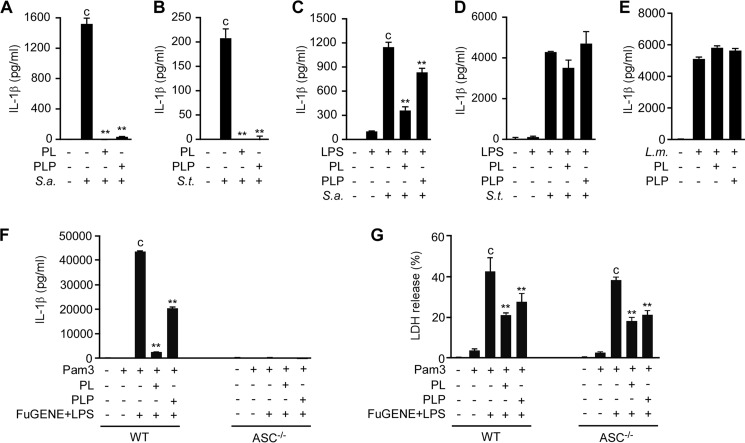
To investigate whether PL and PLP inhibit IL-1β production induced by S. aureus and S. typhimurium, unprimed macrophages and LPS-primed macrophages were treated with PL or PLP and then infected with the bacteria. In unprimed macrophages, the signal 1 for pro-IL-1β production depended on the bacterial infection. Under such conditions, PL and PLP inhibited IL-1β production induced by either of these bacterial species (Fig. 4, A and B). However, in LPS-primed macrophages (in which pro-IL-1β had already been produced), PL and PLP inhibited IL-1β production induced by S. aureus but not by S. typhimurium (Fig. 4, C and D).
FIGURE 4.
PL and PLP selectively suppress the NLRP3 inflammasome. A and B, peritoneal macrophages were incubated with PL or PLP for 24 h, and then infected with S. aureus (S. a.) with a multiplicity of infection (MOI) of 100 or with S. typhimurium (S. t.) with a MOI of 20. C and D, peritoneal macrophages were primed with LPS for 16 h, and then incubated with PL or PLP for 2 h and finally infected with S. aureus (MOI 50) or S. typhimurium (MOI 20). E, peritoneal macrophages were infected with L. monocytogenes (L. m., MOI 2) for 4 h, and then incubated with PL or PLP for 2 h, after which penicillin G (100 units/ml) was added to facilitate intracellular bacterial DNA release. F and G, WT and ASC−/− peritoneal macrophages were primed with Pam3CSK4 (1 μg/ml) for 5 h, incubated with PL or PLP for 1 h, and finally transfected with LPS (2 μg/ml) using FuGENE HD for 18 h. IL-1β in culture supernatants was quantified by ELISA (A–F). Cell death was evaluated by assaying LDH release (G). Data show mean ± S.D.; n = 3. Asterisks indicate significant differences (**, p < 0.01) from the control group (c). All experiments were repeated at least three times, and representative data are shown.
To further investigate the effect of PL and PLP on the AIM2 inflammasome, macrophages were infected with L. monocytogenes, which provides signal 1 through TLR2-dependent recognition of cell wall components (36). The infected cells were cultured with PL or PLP for 2 h, and then treated with penicillin G, leading to bacterial cell lysis and DNA release into the cytoplasm followed by inflammasome activation that largely depends on AIM2 (35). Under these conditions, PL and PLP did not affect the secretion of IL-1β (Fig. 4E). These results indicate that PL and PLP commonly inhibit the signal 1 induced by different bacteria, but specifically inhibit the signal 2 mediated by the NLRP3 inflammasome and not by the NLRC4 or the AIM2 inflammasome.
Vitamin B6 Suppressed Noncanonical IL-1β Secretion and Pyroptosis Induced by LPS Transfection
We also investigated the effect of PL and PLP on noncanonical NLRP3 inflammasome-dependent IL-1β secretion induced by LPS transfection in Pam3CSK4-primed peritoneal macrophages. Both PL and PLP suppressed IL-1β secretion (Fig. 4F) under these conditions. As this response requires NLRP3 inflammasome (37), IL-1β production was not observed in ASC-deficient macrophages (Fig. 4F). Under the same conditions, pyroptosis is induced by caspase-11-dependent but NLRP3 inflammasome-independent manner (37). Consistently, ASC−/− macrophages released LDH to a similar extent as wild-type macrophages (Fig. 4G). Importantly, PL and PLP reduced LDH release, suggesting that PL and PLP could inhibit caspase-11-dependent pyroptosis (Fig. 4G).
Vitamin B6 Inhibited Signal 1 and Signal 2 for IL-1β Production in Human Cells
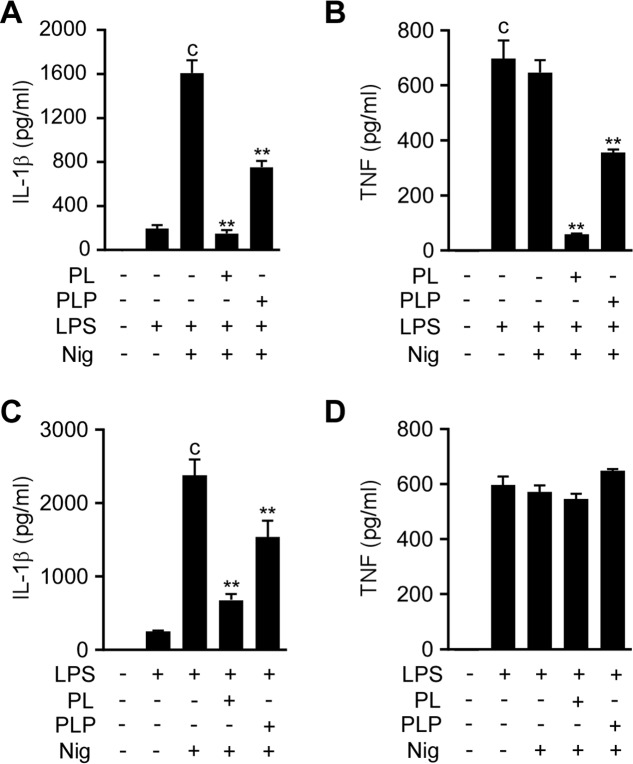
To test the inhibitory effects of vitamin B6 on IL-1β secretion requiring signal 1 and signal 2 and on TNF-α secretion that requires only signal 1 in human cells, we used macrophagic cells differentiated from the THP-1 human monocytic cell line by PMA treatment (THP-1 macrophages). The THP-1 macrophages were treated with PL or PLP before or after LPS treatment, and then stimulated with nigericin to activate the NLRP3 inflammasome. As expected, potent IL-1β secretion was observed after sequential stimulation with LPS and nigericin, whereas TNF-α secretion was fully induced by LPS stimulation alone; nigericin did not affect TNF-α production (Fig. 5, A–D). When the THP-1 macrophages were treated with PL or PLP before LPS was added, the secretion of both IL-1β and TNF-α was inhibited (Fig. 5, A and B). In contrast, when PL or PLP were added after LPS treatment, only IL-1β secretion was inhibited; TNF-α secretion was unaffected (Fig. 5, C and D). These results, which were consistent with our results using mouse peritoneal macrophages, suggest that PL and PLP inhibit both signal 1 and signal 2 in human cells.
FIGURE 5.
PL and PLP suppress signal 1 and signal 2 in human cells. A and B, THP-1 macrophages were treated with PL or PLP for 2 h, incubated with LPS for 4 h, and finally stimulated with nigericin (Nig, 5 μm) for 0.5 h. C and D, THP-1 macrophages were primed with LPS for 4 h, treated with PL or PLP for 2 h, and finally stimulated with nigericin (5 μm) for 0.5 h. A–D, IL-1β and TNF-α in culture supernatants were measured by ELISA. Data show mean ± S.D.; n = 3. Asterisks indicate significant differences (**, p < 0.01) from the control group (c). All experiments were repeated at least three times, and representative data are shown.
Vitamin B6 Suppressed ASC Speck Formation and Oligomerization
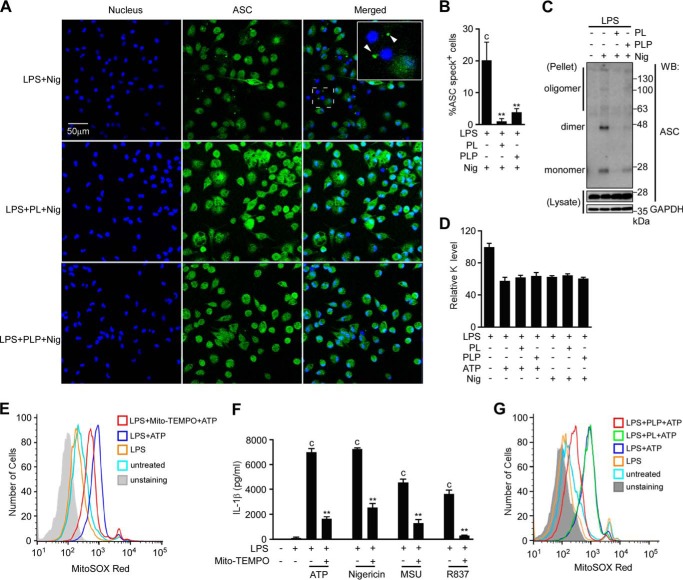
ASC forms large aggregates called “specks” when inflammasomes are activated. ASC speck formation is suggested to be involved in efficient caspase-1 activation and IL-1β processing. Therefore, we investigated whether PL and PLP inhibited ASC speck formation. ASC specks formed when LPS-primed peritoneal macrophages were stimulated with nigericin (Fig. 6A, upper panels, and 6B). However, treating LPS-primed macrophages with PL or PLP prior to nigericin stimulation strongly inhibited the ASC speck formation (Fig. 6A, middle and lower panels, and 6B). Consistently, PL and PLP inhibited ASC oligomerization under the same conditions (Fig. 6C). Taken together with our observation that PL and PLP selectively inhibit the NLRP3 inflammasome, these results suggest that PL and PLP inhibit signal 2 by targeting NLRP3 or upstream events that induce the NLRP3 inflammasome.
FIGURE 6.
Effects of PL and PLP in ASC speck formation, ASC oligomerization, K+ efflux, and mitochondrial ROS generation. A and B, peritoneal macrophages cultured on glass coverslips in 24-well plates were primed with LPS for 16 h, treated with PL or PLP for 2 h, and finally exposed to nigericin (5 μm) for 1 h. ASC specks (FITC) and nuclei (DAPI) were visualized using a confocal fluorescence microscope (A). Total cells and cells with ASC aggregates (specks) were counted in four microscope fields per group, and data show mean ± S.D. (B). C, peritoneal macrophages were primed with LPS for 16 h, treated with PL or PLP for 2 h, and finally exposed to nigericin (5 μm) for 1 h. Cells were lysed with 0.5% Triton X-100 in TBS, and then centrifuged. The supernatants (lysates) and disuccinimidyl suberate-crosslinked pellets were subjected to Western blotting (WB) using antibodies against ASC and GAPDH. D, peritoneal macrophages were primed with LPS for 16 h, treated with PL or PLP for 2 h, and finally exposed to ATP (5 mm) or nigericin (5 μm) for 0.5 h. Intracellular K+ levels relative to those in cells treated with LPS only were determined using inductively coupled plasma mass spectrometry. E and G, peritoneal macrophages primed with LPS for 16 h were detached from the culture plate, and the single-cell suspension was treated with Mito-TEMPO (500 μm, E) or PL or PLP (G) for 2 h, after which the cells were exposed to ATP (5 mm) in the presence of MitoSOX (5 μm) for 10 min. Fluorescence intensities were analyzed by flow cytometry. F, peritoneal macrophages were primed with LPS for 16 h, incubated with Mito-TEMPO (500 μm) for 2 h, and finally exposed to ATP (5 mm), 5 μm nigericin, 150 μg/ml MSU, or 10 μg/ml R837 for 6 h. Supernatant IL-1β content was measured by ELISA. B, D, and F, data show mean ± S.D.; n = 3. Asterisks indicate significant differences (**, p < 0.01) from the control group (c). All experiments were repeated at least three times, and representative data are shown.
Mitochondrial Reactive Oxygen Species (ROS) Production Was Inhibited by PLP but Not by PL
Because the NLRP3 inflammasome is activated by structurally diverse molecules, it has been postulated that different activators induce common intracellular events that eventually cause NLRP3 inflammasomes to form. The efflux of K+ and the resulting decrease in intracellular K+ concentration has been proposed as a common upstream event in NLRP3 inflammasome formation (10, 38). Therefore, we measured cellular potassium levels using inductively coupled plasma mass spectrometry. Consistent with previous findings (38), treating LPS-primed macrophages with ATP or nigericin decreased the cellular potassium level. This event was not affected by PL or PLP treatment (Fig. 6D).
Mitochondrial ROS generation has also been proposed as a common upstream event in NLRP3 activation (39). Consistently, treating LPS-primed macrophages with ATP enhanced the MitoSOX Red fluorescence, indicating elevated mitochondrial ROS generation (Fig. 6E). Furthermore, Mito-TEMPO, a mitochondria-targeted antioxidant that inhibited ATP-induced mitochondrial ROS elevation, suppressed IL-1β production in LPS-primed macrophages (Fig. 6, E and F). Mito-TEMPO also inhibited IL-1β production induced by other NLRP3 activators (Fig. 6F). Because vitamin B6 also acts as an antioxidant (40), we examined whether PL and PLP could inhibit mitochondrial ROS generation. Interestingly, this event was markedly inhibited by PLP but not by PL (Fig. 6G). These results indicate that PLP has a potential to suppress mitochondrial ROS generation, which can, at least in part, explain the ability of PLP to inhibit NLRP3-dependent IL-1β production.
Vitamin B6 Inhibited IL-1β Production in Mice and Protected Mice against LPS-induced Endotoxic Shock
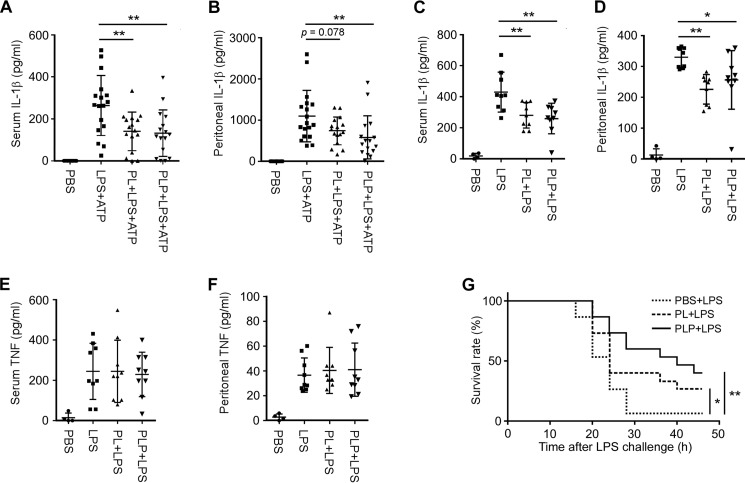
Finally, we examined the in vivo effects of PL and PLP. We induced IL-1β production in ICR mice by i.p. injections of a low dose of LPS (2 μg/kg body weight (bw)) followed by ATP (50 μmol/kg bw) (41, 42), or in C57BL/6 mice by a high dose of LPS (20 mg/kg bw) alone (43). In both experimental systems, serum and/or peritoneal IL-1β levels were suppressed by injecting PL or PLP at 20 mg/kg bw (Fig. 7, A–D). In contrast, PL or PLP did not significantly suppress serum or peritoneal TNF-α levels (Fig. 7, E and F).
FIGURE 7.
PL and PLP suppress IL-1β production in vivo. A and B, ICR mice aged 8 weeks were i.p. injected first with PBS (solvent control), PL, or PLP, then 2 h later with PBS or LPS (2 μg/kg bw), and then 90 min after that with PBS or ATP (50 μmol/kg bw). After 1 h, serum and peritoneal lavage samples were collected, and the IL-1β was quantified by ELISA (n = 11 mice for the PBS group; n = 16–18 mice for the other groups). C–F, C57BL/6 mice aged 10 weeks were i.p. injected with PBS or LPS (20 mg/kg bw) alone or with PL or PLP, and serum and peritoneal lavage samples were collected 3 h later and analyzed by ELISA for IL-1β (C and D) or TNF-α (E and F); n = 4 mice for the PBS group; n = 9 mice for the other groups. G, C57BL/6 mice aged 8 weeks were i.p. injected with PBS, PL, or PLP, and then challenged with LPS (50 mg/kg bw) 2 h later (n = 15 mice for each group). A–F, data of individual mice and mean ± S.D. are shown. A–G, *, p < 0.05; **, p < 0.01. All experiments were repeated at least three times, and cumulative data are shown.
Injecting a high dose of LPS induces lethal endotoxic shock in mice. Components of the NLRP3 inflammasome (i.e. NLRP3 and ASC) play essential roles in this disease model (44–46), although IL-1β and IL-18 are dispensable (47, 48). To test whether PL and PLP can rescue mice from lethal endotoxic shock, C57BL/6 mice pretreated with PBS (control), PL, or PLP were given an injection of LPS at 50 mg/kg bw. Mice pretreated with PBS (n = 15) died within 2 days after LPS injection; notably, the survival was improved in mice pretreated with PL or PLP (n = 15 each group) (Fig. 7G). Taken together, these results suggest that PL and PLP may inhibit the activity of the NLRP3 inflammasome in vivo.
Discussion
In the present study, we showed that the B6 vitamers PL and PLP inhibit both TLR-induced NF-κB activation (signal 1) and NLRP3-mediated caspase-1 activation (signal 2), thereby abolishing IL-1β production in macrophages. PLP is a required cofactor for many metabolic enzymes. However, the PLP concentration necessary to inhibit IL-1β production was higher than its physiological concentrations in humans and mice, suggesting that PLP's inhibition of IL-1β production is likely to be a pharmacological effect.
The inhibitory effect of PL and PLP on signal 1 was not TLR4-specific, because PL and PLP inhibited IL-6 and TNF-α production induced by ligands for other TLRs (Fig. 2). Although TLR4 activates both the MyD88-dependent and the TRIF-dependent NF-κB activation pathways, TLR2, TLR7, and TLR9 specifically activate the MyD88-dependent pathway, and TLR3 only activates the TRIF-dependent pathway (49). Thus, it is likely that the target of PL and PLP in the NF-κB pathway is a common downstream component of the MyD88- and TRIF-dependent pathways. In this context, it is worth noting that these pathways commonly induce the phosphorylation cascade of TAK1-IKK-IκBα, thereby degrading IκBα and activating NF-κB (49). TAK1 also activates MAP kinases such as JNK and p38, which in turn activate the AP-1 transcription factor involved in IL-1β gene expression together with NF-κB (49). Our results (Fig. 1K) indicate that PL and PLP inhibited the LPS-induced TAK1 phosphorylation, IKK-IκBα pathway, and JNK phosphorylation. Taken together, it is likely that PL and PLP inhibit signal 1 by targeting TAK1 or a molecule upstream of TAK1 but commonly found downstream of TLRs. Further study is required to determine whether PL and PLP directly target TAK1 itself, or a molecule upstream of TAK1.
Our experiments using bacterial species that selectively activate NLRP3, NLRC4, or AIM2 suggested that PL and PLP specifically inhibited the NLRP3 inflammasome. Thus, it is likely that PL and PLP target NLRP3 or a more upstream event in signal 2. Consistent with this possibility, PL and PLP sharply inhibited ASC speck formation, which occurs immediately downstream of NLRP3 activation. K+ efflux and mitochondrial ROS generation have been suggested as common upstream events of the activation of NLRP3 by various activators. Our results indicated that PLP inhibited ATP-induced mitochondrial ROS generation, which may contribute to PLP's inhibition of signal 2. However, PL did not affect mitochondrial ROS generation. In addition, neither PL nor PLP inhibited ATP- and nigericin-induced K+ efflux.
Because it was previously reported that NLRP3 is recruited to mitochondria upon activation (50), we investigated whether PL and PLP affect the mitochondrial localization of NLRP3 in peritoneal macrophages treated with LPS and/or ATP. In our study, a portion of NLRP3 localized at the mitochondria after the induction of NLRP3 expression by LPS, and ATP stimulation did not change the amount of mitochondrial NLRP3. Furthermore, neither PL nor PLP affected the amount of mitochondrial NLRP3, when PL or PLP was added after LPS stimulation but before ATP stimulation (data not shown).
We also sought to determine whether PL and PLP affected the interaction of NLRP3 and ASC or post-translational modification of NLRP3 such as ubiquitination or tyrosine phosphorylation (51). However, in our study, these responses of endogenous NLRP3 in peritoneal macrophages were at barely detectable levels (data not shown) so that we could not obtain conclusive answers to these questions at this moment. Thus, further study is required to determine the direct target of PL in inhibiting signal 2.
Importantly, vitamin B6 suppressed IL-1β production in vivo and protected mice from LPS-induced endotoxic shock. In our experiments on LPS-induced IL-1β production, PL and PLP were administered with the LPS injection. However, PL and PLP did not significantly suppress TNF-α production, which requires only signal 1, suggesting that PL and PLP suppressed the in vivo IL-1β production primarily by inhibiting signal 2. In addition, it has been demonstrated that components of the NLRP3 inflammasome play important roles in LPS toxicity (44–46), whereas IL-1β and IL-18 are dispensable for it (47, 48). HMGB1, an alarmin released from dead cells, was recently revealed to play an important role in LPS toxicity (52). Because HMGB1 is released by pyroptosis, the delay of pyroptosis by PL and PLP might also have played a role in protecting mice from LPS toxicity. Finally, because NLRP3 has been suggested to play pathological roles in various inflammation-related diseases, PL and PLP might have clinical value in treating these diseases.
Experimental Procedures
Mice
ICR mice and C57BL/6J mice were purchased from Japan SLC (Shizuoka, Japan). All protocols for animal studies were approved by the Kanazawa University Committee on Animal Welfare.
Reagents
PL hydrochloride, PN hydrochloride, PLP (Nacalai Tesque, Kyoto, Japan), PM dihydrochloride (Calbiochem), LPS from Escherichia coli K235 and from E. coli 0111:B4, ATP, nigericin, poly(I:C) (Sigma-Aldrich), Pam3CSK4, R837 (InvivoGen, San Diego, CA), CpG oligodeoxynucleotides (Genset Oligos, La Jolla, CA), and MSU (Wako, Osaka Japan) were purchased.
Macrophage Preparation and Stimulation
C57BL/6J mice aged 8–12 weeks were injected i.p. with 3% thioglycollate solution, and peritoneal exudate cells were collected 4 days later. The cells (5 × 104 cells/well) were cultured in a 96-well plate for 3 h, after which non-adherent cells were removed by aspiration to enrich for macrophages. Human THP-1 cells were treated with 100 nm PMA for 3 h. The PMA-treated THP-1 cells were seeded in a 96-well plate (5 × 104 cells/well) and cultured overnight without PMA to allow them to differentiate into macrophagic cells. Cells were cultured in RPMI 1640 medium supplemented with 10% FCS, 100 units/ml penicillin, and 100 μg/ml streptomycin (Meiji Seika, Tokyo, Japan).
To test vitamin B6's inhibition of signal 1, mouse macrophages were cultured with a B6 vitamer (500 μm unless otherwise specified) for 24 h and then with LPS (from E. coli K235, 0.5 μg/ml for all in vitro stimulations) for 16 h. To test the inhibitory activity of vitamin B6 on other TLR ligands, mouse macrophages were cultured with a B6 vitamer for 2 h and then with various TLR agonists for 6 h. THP-1 macrophages were treated sequentially with a B6 vitamer for 2 h, LPS for 4 h, and nigericin (5 μm) for 30 min.
To test the inhibitory activity of vitamin B6 on signal 2, mouse macrophages were treated with LPS for 16 h, with a B6 vitamer for 2 h, and with various inflammasome activators for 6 h. THP-1 macrophages were treated with LPS for 4 h, with a B6 vitamer for 2 h, and with nigericin for 30 min.
To induce noncanonical NLRP3 inflammasome formation, peritoneal macrophages were primed with 1 μg/ml Pam3CSK4 for 5 h, washed once with fresh medium, and then treated with or without 500 μm PL or PLP for 1 h. Finally, cells were transfected with LPS (2 μg/ml) using FuGENE HD (Promega, Madison WI) and cultured for 18 h.
Bacterial Infection
S. aureus (Smith strain, kindly provided by Dr. Nakanishi, Kanazawa University, Kanazawa, Ishikawa, Japan) and S. typhimurium (ATCC 14028) in the log phase were used for infection. L. monocytogenes (EGD, serovar 1/2a) was cultured in brain-heart infusion broth (Eiken Chemical, Tokyo, Japan), collected in the log phase, washed with PBS, suspended in PBS supplemented with 10% glycerol, and stored in aliquots at −80 °C. Bacterial stocks were thawed and diluted in RPMI 1640 medium just prior to infecting macrophages (35).
The peritoneal macrophages were placed in antibiotic-free medium in 96-well plates and infected with the bacteria. The plates were briefly centrifuged to improve interactions between the cells and bacteria. Penicillin G (100 units/ml), streptomycin (100 μg/ml), and gentamycin (50 μg/ml, Thermo Fisher Scientific) were added 1 h after infection with S. aureus or S. typhimurium, and the cells were further cultured for 5 h. To activate AIM2, peritoneal macrophages were infected with L. monocytogenes. Finally, penicillin G (100 units/ml) was added to the culture to facilitate intracellular bacterial DNA release, and the cells were further cultured for 3 h.
Real-time PCR
Total RNA was purified from mouse macrophages using TRIZOL reagent (Thermo Fisher Scientific), and cDNA was synthesized using the First Strand cDNA Synthesis kit (Toyobo, Osaka, Japan). Real-time PCR was performed using the StepOne Real-Time PCR system (Thermo Fisher Scientific) with the THUNDERBIRD SYBR quantitative PCR mix (Toyobo) and the following primers: Il1b, 5′-TGGGCCTCAAAGGAAAGA-3′ and 5′-GGTGCTGATGTACCAGTT-3′; Il6, 5′-AGACAAAGCCAGAGTCCTTCAG-3′ and 5′-TGCCGAGTAGATCTCAAAGTGA-3′; Ccl2, 5′-GGTCCCTGTCATGCTTCTGG-3′ and 5′-CCTTCTTGGGGTCAGCACAG-3′; Ptgs2, 5′-GCCAGGCTGAACTTCGAAACA-3′ and 5′-GCTCACGAGGCCACTGATACCTA-3′; Nlrp3, 5′-GTGGTGACCCTCTGTGAGGT-3′ and 5′-TCTTCCTGGAGCGCTTCTAA-3′; and Gapdh, 5′-CAATGACCCCTTCATTGACC-3′ and 5′-TGGAAGATGGTGATGGGATT-3′.
ELISA and LDH Analysis
The concentrations of mouse and human IL-1β, IL-6, and TNF-α in culture supernatants were determined using OptEIA ELISA kits (BD Pharmingen) according to the manufacturer's protocols. The mouse IL-18 ELISA kit was purchased from MBL (Nagoya, Japan). Cell death was determined by measuring LDH activity in the culture medium using the CytoTox96 NonRadioactive Cytotoxicity Assay (Promega).
Western Blotting Analysis
Cells were lysed in Tris-buffered saline containing 1% Nonidet P-40 and Complete protease inhibitor cocktail (Roche Diagnostics GmbH, Mannheim, Germany) for 30 min on ice. Lysates were centrifuged at 12,000 × g for 10 min to remove debris and then boiled in Laemmli sample buffer for 5 min. Proteins were separated by SDS-PAGE and transferred to PVDF membranes. Membranes were incubated for 1 h in a blocking buffer (5% skim milk, 0.05% Tween 20 in Tris-buffered saline) or in Blocking One-P (for phosphoprotein detection, Nacalai Tesque), followed by the addition of a primary antibody against IL-1β (Genzyme TECHNE, Minneapolis, MN), caspase-1 (Santa Cruz Biotechnology, Santa Cruz, CA), NLRP3 (Enzo Life Science, Villeurbanne, France), IκBα, phospho-TAK1, JNK, phospho-JNK, IKKα, IKKβ, phospho-IKKα/β (Cell Signaling, Danvers, MA), or GAPDH (Novus Biologicals, Littleton, CO). Antibodies were detected by a horseradish-peroxidase-conjugated goat anti-mouse or anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) using enhanced chemiluminescence (Thermo Fisher Scientific).
To detect ASC oligomerization, cells were lysed with TBS containing 0.5% Triton X-100, and then centrifuged at 6,000 × g at 4 °C for 15 min. Supernatants (lysates) were transferred to new tubes. The pellets were washed with TBS twice and then crosslinked at 37 °C for 45 min by disuccinimidyl suberate (2 mm, Thermo Fisher Scientific). The crosslinked pellets were spun down at 6,000 × g for 15 min, dissolved in SDS-containing sample buffer, and subjected to SDS-PAGE and Western blotting using anti-ASC antibody (Santa Cruz Biotechnology).
Immunofluorescence Confocal Microscopy
Mouse ASC was detected using a rat mAb (46) followed by FITC-conjugated goat anti-rat IgG (American Qualex, San Clemente, CA). Nuclei were stained by DAPI (Dojindo, Kumamoto, Japan). The stained cells were examined under a laser scanning microscope (LSM 510 META with EC Plan-Neofluar 40×/0.75; Carl Zeiss, Jena, Germany) equipped with 488-nm argon, 543-nm HeNe, 633-nm HeNe, and Blue Diode 405 lasers. Images were acquired and analyzed using Zen2009 software.
Determination of Intracellular Potassium Levels
Cells (6 × 105 cells/well in a 24-well plate) were lysed in 3% ultrapure HNO3 (600 μl) for 30 min on ice. Lysates were diluted 10 times with ultrapure H2O, and potassium concentrations were determined by inductively coupled plasma mass spectrometry (SPQ-9000, Seiko Instruments, Chiba, Japan) using KCl solution as a standard.
Determination of Mitochondrial ROS Levels
Mitochondrial ROS levels were measured using MitoSOX Red (Thermo Fisher Scientific) according to the manufacturer's protocol. Data were acquired with a FACSCanto II (BD Biosciences) and analyzed with FlowJo software (FlowJo, Ashland, OR).
In Vivo Experiments
The Food and Nutrition Board of the Institute of Medicine proposed a tolerable upper limit of 100 mg/day of vitamin B6 for adult humans (53). Based on this and the conversion between human doses and animal-equivalent doses according to body surface area (54), we estimated a tolerable vitamin B6 dose of 20 mg/kg bw/day for mice; this was the dose used for the in vivo experiments in the present study. ICR mice were i.p. injected first with PL or PLP, injected 2 h later with LPS (from E. coli 0111:B4, 2 μg/kg bw), and injected 90 min later with 10 ml/kg bw of 5 mm ATP (50 μmol/kg bw). Serum and peritoneal lavage samples were collected 1 h after the ATP injection. C57BL/6 mice were i.p. injected with LPS (20 mg/kg bw) with or without PL or PLP, and serum and peritoneal lavage samples were collected 3 h later. Lethal endotoxic shock was induced in C57BL/6 mice by i.p. LPS injection (50 mg/kg bw, from E. coli 0111:B4, Sigma-Aldrich).
Statistical Analysis
Data were analyzed using GraphPad Prism 6.05 (GraphPad Software, La Jolla, CA). Difference between a control and an experimental group was examined by one-way analysis of variance and Dunnett's test. Difference between mouse survival curves was evaluated by the log-rank (Mantel-Cox) test. p < 0.05 was considered significant.
Author Contributions
P. Z. contributed to the experimental design, performed most of the experiments, and wrote the manuscript; K. T. performed or supervised experiments involving bacterial infection; T. K. and H. K. provided technical assistance; S. S. helped collect samples; M. H. and H. I. performed and supervised inductively coupled plasma mass spectrometry analyses; N. K. contributed to the experimental design and critical review of the manuscript; and T. S. designed and supervised the research project and edited the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgment
We thank Dr. Nakanishi from Kanazawa University for providing S. aureus (Smith strain) used in bacterial infection experiments.
This work was supported by Grant-in-Aid for Challenging Exploratory Research 15K15078 and by Grant-in-Aid for Scientific Research on Innovative Areas 26110002 from the Japan Society for the Promotion of Science. The authors declare that they have no conflicts of interest with the contents of this article.
- PLP
- pyridoxal 5′-phosphate
- PL
- pyridoxal
- PM
- pyridoxamine
- PN
- pyridoxine
- LDH
- lactate dehydrogenase
- MOI
- multiplicity of infection
- MSU
- monosodium urate
- ROS
- reactive oxygen species
- TLR
- Toll-like receptor
- IKK
- IκB kinase
- PMA
- phorbol 12-myristate 13-acetate
- bw
- body weight.
References
- 1. Saibeni S., Cattaneo M., Vecchi M., Zighetti M. L., Lecchi A., Lombardi R., Meucci G., Spina L., and de Franchis R. (2003) Low vitamin B6 plasma levels, a risk factor for thrombosis, in inflammatory bowel disease: role of inflammation and correlation with acute phase reactants. Am. J. Gastroenterol. 98, 112–117 [DOI] [PubMed] [Google Scholar]
- 2. Sakakeeny L., Roubenoff R., Obin M., Fontes J. D., Benjamin E. J., Bujanover Y., Jacques P. F., and Selhub J. (2012) Plasma pyridoxal-5-phosphate is inversely associated with systemic markers of inflammation in a population of U.S. adults. J. Nutr. 142, 1280–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang S. C., Wei J. C., Wu D. J., and Huang Y. C. (2010) Vitamin B6 supplementation improves pro-inflammatory responses in patients with rheumatoid arthritis. Eur. J. Clin. Nutr. 64, 1007–1013 [DOI] [PubMed] [Google Scholar]
- 4. Komatsu S. I., Watanabe H., Oka T., Tsuge H., Nii H., and Kato N. (2001) Vitamin B-6-supplemented diets compared with a low vitamin B-6 diet suppress azoxymethane-induced colon tumorigenesis in mice by reducing cell proliferation. J. Nutr. 131, 2204–2207 [DOI] [PubMed] [Google Scholar]
- 5. Galluzzi L., Vacchelli E., Michels J., Garcia P., Kepp O., Senovilla L., Vitale I., and Kroemer G. (2013) Effects of vitamin B6 metabolism on oncogenesis, tumor progression and therapeutic responses. Oncogene 32, 4995–5004 [DOI] [PubMed] [Google Scholar]
- 6. Douaud G., Refsum H., de Jager C. A., Jacoby R., Nichols T. E., Smith S. M., and Smith A. D. (2013) Preventing Alzheimer's disease-related gray matter atrophy by B-vitamin treatment. Proc. Natl. Acad. Sci. U.S.A. 110, 9523–9528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murakami K., Miyake Y., Sasaki S., Tanaka K., Fukushima W., Kiyohara C., Tsuboi Y., Yamada T., Oeda T., Miki T., Kawamura N., Sakae N., Fukuyama H., Hirota Y., Nagai M., and Fukuoka Kinki Parkinson's Disease Study Group (2010) Dietary intake of folate, vitamin B-6, vitamin B-12 and riboflavin and risk of Parkinson's disease: a case-control study in Japan. Br. J. Nutr. 104, 757–764 [DOI] [PubMed] [Google Scholar]
- 8. Yanaka N., Koyama T. A., Komatsu S., Nakamura E., Kanda M., and Kato N. (2005) Vitamin B6 suppresses NF-κB activation in LPS-stimulated mouse macrophages. Int. J. Mol. Med. 16, 1071–1075 [PubMed] [Google Scholar]
- 9. Garlanda C., Dinarello C. A., and Mantovani A. (2013) The interleukin-1 family: back to the future. Immunity 39, 1003–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Franchi L., Muñoz-Planillo R., and Núñez G. (2012) Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 13, 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li P., Allen H., Banerjee S., Franklin S., Herzog L., Johnston C., McDowell J., Paskind M., Rodman L., Salfeld J., et al. (1995) Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell 80, 401–411 [DOI] [PubMed] [Google Scholar]
- 12. Kayagaki N., Warming S., Lamkanfi M., Vande Walle L., Louie S., Dong J., Newton K., Qu Y., Liu J., Heldens S., Zhang J., Lee W. P., Roose-Girma M., and Dixit V. M. (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 [DOI] [PubMed] [Google Scholar]
- 13. Schroder K., and Tschopp J. (2010) The inflammasomes. Cell 140, 821–832 [DOI] [PubMed] [Google Scholar]
- 14. Mariathasan S., Weiss D. S., Newton K., McBride J., O'Rourke K., Roose-Girma M., Lee W. P., Weinrauch Y., Monack D. M., and Dixit V. M. (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 [DOI] [PubMed] [Google Scholar]
- 15. Craven R. R., Gao X., Allen I. C., Gris D., Bubeck Wardenburg J., McElvania-Tekippe E., Ting J. P., and Duncan J. A. (2009) Staphylococcus aureus α-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS ONE 4, e7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harder J., Franchi L., Muñoz-Planillo R., Park J. H., Reimer T., and Núñez G. (2009) Activation of the Nlrp3 inflammasome by Streptococcus pyogenes requires streptolysin O and NF-κB activation but proceeds independently of TLR signaling and P2X7 receptor. J. Immunol. 183, 5823–5829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kanneganti T. D., Ozören N., Body-Malapel M., Amer A., Park J. H., Franchi L., Whitfield J., Barchet W., Colonna M., Vandenabeele P., Bertin J., Coyle A., Grant E. P., Akira S., and Núñez G. (2006) Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440, 233–236 [DOI] [PubMed] [Google Scholar]
- 18. Dostert C., Pétrilli V., Van Bruggen R., Steele C., Mossman B. T., and Tschopp J. (2008) Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martinon F., Pétrilli V., Mayor A., Tardivel A., and Tschopp J. (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 [DOI] [PubMed] [Google Scholar]
- 20. Halle A., Hornung V., Petzold G. C., Stewart C. R., Monks B. G., Reinheckel T., Fitzgerald K. A., Latz E., Moore K. J., and Golenbock D. T. (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol. 9, 857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duewell P., Kono H., Rayner K. J., Sirois C. M., Vladimer G., Bauernfeind F. G., Abela G. S., Franchi L., Nuñez G., Schnurr M., Espevik T., Lien E., Fitzgerald K. A., Rock K. L., Moore K. J., Wright S. D., Hornung V., and Latz E. (2010) NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wen H., Gris D., Lei Y., Jha S., Zhang L., Huang M. T., Brickey W. J., and Ting J. P. (2011) Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12, 408–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Villani A. C., Lemire M., Fortin G., Louis E., Silverberg M. S., Collette C., Baba N., Libioulle C., Belaiche J., Bitton A., Gaudet D., Cohen A., Langelier D., Fortin P. R., Wither J. E., et al. (2009) Common variants in the NLRP3 region contribute to Crohn's disease susceptibility. Nat. Genet. 41, 71–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schoultz I., Verma D., Halfvarsson J., Törkvist L., Fredrikson M., Sjöqvist U., Lördal M., Tysk C., Lerm M., Söderkvist P., and Söderholm J. D. (2009) Combined polymorphisms in genes encoding the inflammasome components NALP3 and CARD8 confer susceptibility to Crohn's disease in Swedish men. Am. J. Gastroenterol. 104, 1180–1188 [DOI] [PubMed] [Google Scholar]
- 25. Bauer C., Duewell P., Mayer C., Lehr H. A., Fitzgerald K. A., Dauer M., Tschopp J., Endres S., Latz E., and Schnurr M. (2010) Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 59, 1192–1199 [DOI] [PubMed] [Google Scholar]
- 26. Heneka M. T., Kummer M. P., Stutz A., Delekate A., Schwartz S., Vieira-Saecker A., Griep A., Axt D., Remus A., Tzeng T. C., Gelpi E., Halle A., Korte M., Latz E., and Golenbock D. T. (2013) NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Masters S. L., Dunne A., Subramanian S. L., Hull R. L., Tannahill G. M., Sharp F. A., Becker C., Franchi L., Yoshihara E., Chen Z., Mullooly N., Mielke L. A., Harris J., Coll R. C., Mills K. H., et al. (2010) Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 11, 897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stienstra R., Joosten L. A., Koenen T., van Tits B., van Diepen J. A., van den Berg S. A., Rensen P. C., Voshol P. J., Fantuzzi G., Hijmans A., Kersten S., Müller M., van den Berg W. B., van Rooijen N., Wabitsch M., et al. (2010) The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 12, 593–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuemmerle-Deschner J. B. (2015) CAPS: pathogenesis, presentation and treatment of an autoinflammatory disease. Semin. Immunopathol. 37, 377–385 [DOI] [PubMed] [Google Scholar]
- 30. Lee J., Mira-Arbibe L., and Ulevitch R. J. (2000) TAK1 regulates multiple protein kinase cascades activated by bacterial lipopolysaccharide. J. Leukoc. Biol. 68, 909–915 [PubMed] [Google Scholar]
- 31. Sato S., Sanjo H., Takeda K., Ninomiya-Tsuji J., Yamamoto M., Kawai T., Matsumoto K., Takeuchi O., and Akira S. (2005) Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 6, 1087–1095 [DOI] [PubMed] [Google Scholar]
- 32. Muñoz-Planillo R., Franchi L., Miller L. S., and Núñez G. (2009) A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J. Immunol. 183, 3942–3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miao E. A., Alpuche-Aranda C. M., Dors M., Clark A. E., Bader M. W., Miller S. I., and Aderem A. (2006) Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat. Immunol. 7, 569–575 [DOI] [PubMed] [Google Scholar]
- 34. Franchi L., Amer A., Body-Malapel M., Kanneganti T. D., Ozören N., Jagirdar R., Inohara N., Vandenabeele P., Bertin J., Coyle A., Grant E. P., and Núñez G. (2006) Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nat. Immunol. 7, 576–582 [DOI] [PubMed] [Google Scholar]
- 35. Tsuchiya K., Hara H., Kawamura I., Nomura T., Yamamoto T., Daim S., Dewamitta S. R., Shen Y., Fang R., and Mitsuyama M. (2010) Involvement of absent in melanoma 2 in inflammasome activation in macrophages infected with Listeria monocytogenes. J. Immunol. 185, 1186–1195 [DOI] [PubMed] [Google Scholar]
- 36. Flo T. H., Halaas O., Lien E., Ryan L., Teti G., Golenbock D. T., Sundan A., and Espevik T. (2000) Human Toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J. Immunol. 164, 2064–2069 [DOI] [PubMed] [Google Scholar]
- 37. Kayagaki N., Stowe I. B., Lee B. L., O'Rourke K., Anderson K., Warming S., Cuellar T., Haley B., Roose-Girma M., Phung Q. T., Liu P. S., Lill J. R., Li H., Wu J., et al. (2015) Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 [DOI] [PubMed] [Google Scholar]
- 38. Muñoz-Planillo R., Kuffa P., Martínez-Colón G., Smith B. L., Rajendiran T. M., and Núñez G. (2013) K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou R., Yazdi A. S., Menu P., and Tschopp J. (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 [DOI] [PubMed] [Google Scholar]
- 40. Mooney S., Leuendorf J. E., Hendrickson C., and Hellmann H. (2009) Vitamin B6: a long known compound of surprising complexity. Molecules 14, 329–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sun S., Xia S., Ji Y., Kersten S., and Qi L. (2012) The ATP-P2X7 signaling axis is dispensable for obesity-associated inflammasome activation in adipose tissue. Diabetes 61, 1471–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Griffiths R. J., Stam E. J., Downs J. T., and Otterness I. G. (1995) ATP induces the release of IL-1 from LPS-primed cells in vivo. J. Immunol. 154, 2821–2828 [PubMed] [Google Scholar]
- 43. Yan Y., Jiang W., Liu L., Wang X., Ding C., Tian Z., and Zhou R. (2015) Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160, 62–73 [DOI] [PubMed] [Google Scholar]
- 44. Mariathasan S., Newton K., Monack D. M., Vucic D., French D. M., Lee W. P., Roose-Girma M., Erickson S., and Dixit V. M. (2004) Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 [DOI] [PubMed] [Google Scholar]
- 45. Sutterwala F. S., Ogura Y., Szczepanik M., Lara-Tejero M., Lichtenberger G. S., Grant E. P., Bertin J., Coyle A. J., Galán J. E., Askenase P. W., and Flavell R. A. (2006) Critical role for NALP3/CIAS1/cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24, 317–327 [DOI] [PubMed] [Google Scholar]
- 46. Imamura R., Wang Y., Kinoshita T., Suzuki M., Noda T., Sagara J., Taniguchi S., Okamoto H., and Suda T. (2010) Anti-inflammatory activity of PYNOD and its mechanism in humans and mice. J. Immunol. 184, 5874–5884 [DOI] [PubMed] [Google Scholar]
- 47. Shornick L. P., De Togni P., Mariathasan S., Goellner J., Strauss-Schoenberger J., Karr R. W., Ferguson T. A., and Chaplin D. D. (1996) Mice deficient in IL-1β manifest impaired contact hypersensitivity to trinitrochlorobenzone. J. Exp. Med. 183, 1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sakao Y., Takeda K., Tsutsui H., Kaisho T., Nomura F., Okamura H., Nakanishi K., and Akira S. (1999) IL-18-deficient mice are resistant to endotoxin-induced liver injury but highly susceptible to endotoxin shock. Int. Immunol. 11, 471–480 [DOI] [PubMed] [Google Scholar]
- 49. Kawai T., and Akira S. (2007) Signaling to NF-κB by Toll-like receptors. Trends Mol. Med. 13, 460–469 [DOI] [PubMed] [Google Scholar]
- 50. Subramanian N., Natarajan K., Clatworthy M. R., Wang Z., and Germain R. N. (2013) The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell 153, 348–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Juliana C., Fernandes-Alnemri T., Kang S., Farias A., Qin F., and Alnemri E. S. (2012) Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem. 287, 36617–36622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alves J. N., Pires K. M., Lanzetti M., Barroso M. V., Benjamim C. F., Costa C. A., Resende A. C., Santos J. C., Ribeiro M. L., Porto L. C., and Valença S. S. (2013) Critical role for CCR2 and HMGB1 in induction of experimental endotoxic shock. Arch. Biochem. Biophys. 537, 72–81 [DOI] [PubMed] [Google Scholar]
- 53. Institute of and Medicine, U. S. (1998) Vitamin B6. in Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline, pp. 150–195, National Academies Press, Washington, D. C. [PubMed] [Google Scholar]
- 54. U. S. Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER) (2005) Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers, pp. 6–7, U. S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research, Rockville, MD [Google Scholar]