Abstract
CRAF kinase maintains cell viability, growth, and proliferation by participating in the MAPK pathway. Unlike BRAF, CRAF requires continuous chaperoning by Hsp90 to retain MAPK signaling. However, the reason behind the continuous association of Hsp90 with CRAF is still elusive. In this study, we have identified the bipartite role of Hsp90 in chaperoning CRAF kinase. Hsp90 facilitates Ser-621 phosphorylation of CRAF and prevents the kinase from degradation. Co-chaperone Cdc37 assists in this phosphorylation event. However, after folding, the stability of the kinase becomes insensitive to Hsp90 inhibition, although the physical association between Hsp90 and CRAF remains intact. We observed that overexpression of Hsp90 stimulates MAPK signaling by activating CRAF. The interaction between Hsp90 and CRAF is substantially increased under an elevated level of cellular Hsp90 and in the presence of either active Ras (RasV12) or EGF. Surprisingly, enhanced binding of Hsp90 to CRAF occurs prior to the Ras-CRAF association and facilitates actin recruitment to CRAF for efficient Ras-CRAF interaction, which is independent of the ATPase activity of Hsp90. However, monomeric CRAF (CRAFR401H) shows abrogated interaction with both Hsp90 and actin, thereby affecting Hsp90-dependent CRAF activation. This finding suggests that stringent assemblage of Hsp90 keeps CRAF kinase equipped for participating in the MAPK pathway. Thus, the role of Hsp90 in CRAF maturation and activation acts as a limiting factor to maintain the function of a strong client like CRAF kinase.
Keywords: actin, autophosphorylation, heat shock protein 90 (Hsp90), protein stability, Raf kinase
Introduction
RAF kinases (ARAF, BRAF, and CRAF3) act as potential transducers of growth signal to activate the MAPK pathway (1). Proper regulation of RAF signaling acts as a molecular switch to maintain cellular growth and proliferation (2). Dysregulation of the RAF pathway is commonly observed in certain human cancers and developmental disorders due to mutations in CRAF and BRAF genes (3–6). Several molecules have been identified as common regulators of BRAF/CRAF kinases (1, 7–11). However, in contrast to BRAF, CRAF kinase is always assisted by a molecular chaperone, Hsp90. Therefore, inhibition of Hsp90 reduces CRAF kinase-mediated signaling by promoting its degradation (12, 13).
Hsp90 is evolutionary conserved (14) and is involved in various cellular processes including cell survival, regulation of cell cycle, kinase- and receptor-mediated signaling, and stress response (15–19). The function of Hsp90 relies on its ATPase cycling and is regulated by the associated co-chaperones (20–24). A specific set of proteins termed as “clients” requires Hsp90 for their nascent chain folding (14, 25, 26). Among the clientele, protein kinases majorly depend on Hsp90 to attain their proper conformation and activation (27, 28). In this process, co-chaperone Cdc37 delivers kinases to Hsp90, thereby promoting their maturation (29–33). The function of Hsp90 varies depending on the client protein. While Hsp90 assists in folding, maturation, and trafficking of certain clients like Src kinase, cystic fibrosis transmembrane conductance regulator (CFTR), and HER-2/ERBB2 (34–38), it promotes activation of some clients such as Lck kinase and AKT (39, 40). Thus, Hsp90-mediated modulation of client activation is imperative for precise regulation of various signaling pathways (41–45).
CRAF kinase activation is a complex regulatory process driven by phosphorylation/dephosphorylation events, translocation to the membrane, and subsequent heterodimerization. Additional known regulators of CRAF are Hsp90, Cdc37, and scaffold protein 14-3-3 (46, 47). Ser-259 and Ser-621, the two basal phosphorylation residues of CRAF, are the binding sites of 14-3-3 that keep CRAF in an inactive conformation at the cytosol (48–50). Dephosphorylation of the Ser-259 residue upon mitogenic stimulation relieves 14-3-3 inhibition and facilitates CRAF translocation to the plasma membrane for interaction with Ras via its Ras binding domain (RBD) and cysteine-rich domain (CRD) (51, 52). A complex cohort of phosphorylations at multiple residues, Ser-338 (53, 54), Tyr-341 (55–57), Thr-491, and Ser-494 (58), renders optimal activation of CRAF. Apart from 14-3-3-mediated regulation, both Hsp90 and Cdc37 are prerequisite for CRAF functioning (59), but the underlying mechanism by which Hsp90 maintains CRAF functioning is still elusive.
In this study, we have attempted to decipher the purpose behind the continuous dependence of CRAF on Hsp90. Impairment in both Hsp90 and Cdc37 down-regulates Ser-621 phosphorylation of CRAF, thereby reducing its stability and MAPK signaling. Surprisingly, after folding, CRAF kinase limits this phenomenon. However, folded CRAF remains bound with Hsp90. We found that enhanced binding of Hsp90 to CRAF recruits actin to the CRAF complex, which forms an effective transportosome during MAPK signaling. Interestingly, dimer-deficient CRAF mutant (CRAFR401H) lacks interaction with Hsp90 and is insensitive to Hsp90-mediated MAPK pathway activation. Additionally, we noticed that Hsp90 recruitment to CRAF is directed by the kinase dimer structure. Altogether, we report an essential bipartite function of Hsp90 in CRAF kinase maturation and activation.
Results
Stability of Folded CRAF Is Insensitive to Hsp90 Inhibition
Chemical inhibition of Hsp90 with geldanamycin (GA) accelerates CRAF degradation (12). A similar observation was made when we treated cells with GA. Notably, the effect of GA was observed at the 16th h of treatment (Fig. 1A). Comparing this observation with the half-life of endogenous CRAF (Fig. 1B, panel i), we anticipated that prolonged exposure to GA affects de novo folding of the kinase. Thus, we explored the effect of Hsp90 inhibition over folded CRAF by blocking protein synthesis with cycloheximide. Surprisingly, GA-induced CRAF degradation was not observed in the presence of cycloheximide; however, another strong client of Hsp90, Cdk2, showed gradual reduction in the aforementioned condition (Fig. 1B, panel ii). Additionally, cycloheximide chase from GA-preincubated samples (8 h) showed an intact CRAF level even in the presence of GA, confirming that folded CRAF becomes insensitive to Hsp90 inhibition (Fig. 1C). To mimic the effect of GA, a cycloheximide chase experiment was performed in the presence of ATP hydrolysis-deficient Hsp90 mutant, Hsp90DN (60). Overexpression of Hsp90DN reduced the CRAF level, but inhibition of protein synthesis with cycloheximide blocked Hsp90DN-mediated CRAF degradation (Fig. 1D). Having observed that CRAF is stable and active in a yeast model (Fig. 1, E–G), the effect of Hsp90 impairment on CRAF was further studied in previously described hsp90 temperature-sensitive (ts) mutant strains G313N and G170D (26). Pulse-chase assay showed faster turnover of newly made CRAF at non-permissive temperature (34 °C) (Fig. 1H, panel i); in contrast, unaltered turnover of CRAF in the presence of both GA and cycloheximide established the insensitivity of folded CRAF toward Hsp90 impairment (Fig. 1H, panel ii).
FIGURE 1.
Stability of mature CRAF is independent of Hsp90. A, prolonged GA treatment reduces de novo made CRAF kinase. The CRAF level was checked after treating HEK293T cells with 2 μm GA for the given time intervals by Western blotting analysis. The band intensity was quantified and expressed as a percentage of the 0-h sample mean value (n = 6). The error bars represent S.D. The significance was calculated by Mann-Whitney t test. The effect of GA was statistically significant for each time point (p < 0.001). BRAF, Hsp90, and Hsp70 levels are also shown. GAPDH was used as a loading control. B, mature CRAF is resistant to GA-mediated degradation. B, panel i, HEK293T cells were treated with 100 μm cycloheximide (CHX) for the indicated time intervals for evaluation of half-life of endogenous CRAF. B, panel ii, HEK293T cells were treated with cycloheximide along with 2 μm GA for the indicated time points. CRAF, BRAF, and CDK2 levels were examined by Western blotting analysis with respective antibodies. CDK2 is considered a positive control for GA-mediated degradation. C, inhibition of translation reduces turnover of GA-preincubated CRAF kinase. CRAF levels were examined in the presence and absence of cycloheximide at 12, 16, and 20 h after preincubating HEK293T cells with GA for 8 h. D, cycloheximide treatment antagonizes the effect of GA on CRAF signaling. ATP hydrolysis-deficient Hsp90βDN affects newly made CRAF. Hsp90βDN was overexpressed in HEK293T cells. CRAF levels were measured at the indicated time points after addition of cycloheximide (100 μm). GAPDH was used as a loading control. NT represents non-transfected. E–G, quality control study of CRAF kinase is suitable in yeast system. E, FLAG-tagged CRAF cDNA was cloned into yeast p416GPD vector and expressed in BY4741 yeast strain. CRAF expression was checked by Western blotting with anti-FLAG antibody. NT represents empty plasmid. Pgk1 was used as a loading control. F, CRAF is active in yeast. The kinase activity of CRAF was measured using purified MEK1 as substrate after immunoprecipitating CRAF with anti-FLAG antibody. Phospho-MEK1 was analyzed by Western blotting analysis. G, CRAF complements yeast MAPK Ste11 kinase. Activation of MAPK signaling was measured in yeast cells transformed with PRE fused with β-galactosidase reporter (PRE-LacZ) plasmid after induction with pheromone (α-factor). -Fold induction of β-galactosidase activity was measured without and with α-factor by MUG assay in WT, ste11Δ, and CRAF-transformed ste11Δ yeast strains. H, stability of mature CRAF does not require Hsp90 function in yeast. Pulse-chase with [35S]methionine (panel i) and cycloheximide chase of CRAF (panel ii) in hsp90 ts mutants G170D and G313N and its isogenic strain, p82, at non-permissive temperature (34 °C). CRAF turnover (red line for pulse-chase; blue line for cycloheximide chase) is shown in the associated graph. The error bars represent S.D. (n = 3). I, HEK293T cells were treated with GA (2 μm) alone or along with cycloheximide (100 μm) for 16 h. MEK phosphorylation was checked in cell lysates with p-MEK antibody. GAPDH was used as a loading control. NT represents non-treated. J and K, in vitro kinase activity of CRAF was checked after treating the cells with GA alone (J) and in combination with cycloheximide (K) for 8 or 16 h, respectively. FLAG-CRAF was immunoprecipitated, and its capacity to phosphorylate MEK1 was analyzed by Western blotting analysis. For each sample, MEK phosphorylation was normalized with CRAF pulled down and is represented in the associated graph. The error bars are the S.D. of three independent experiments. L, prolonged GA treatment increases Hsp90 binding to CRAF in the absence of cycloheximide. HEK293T cells were treated with GA in the absence and presence of cycloheximide for 16 h. Endogenous CRAF was pulled down from total cell lysate using anti-CRAF antibody. Associated Hsp90 was detected using anti-Hsp90 antibody. Actin was used as a loading control. neg, IP negative control.
Reduction in CRAF level down-regulates MAPK signaling (61). We therefore sought to estimate MEK phosphorylation after treating the cells with GA alone and in addition with cycloheximide for 16 h. Interestingly, MEK phosphorylation remained unaltered after co-treatment with cycloheximide and GA, whereas GA alone reduced MAPK signaling (Fig. 1I). In addition, intrinsic kinase activity of CRAF was significantly reduced after GA treatment (at the 16th h) (Fig. 1J), although alteration in kinase activity was not noticed when cycloheximide was added (Fig. 1K). Thus, Hsp90 inhibition affects the stability and activity of CRAF as reported previously (12, 61); however, our data suggest that folded CRAF remains insensitive toward Hsp90 inhibition, which is rarely observed for bona fide Hsp90 clients (62–64).
Reduced stability of client in the presence of Hsp90 inhibitor occurs due to the dissociation of Hsp90 from the client complex (65, 66). To determine whether GA treatment also disrupts Hsp90-CRAF interaction as observed for other strong clients, cells were treated with GA alone and in combination with cycloheximide for 16 h. Endogenous CRAF was then immunoprecipitated followed by immunoblotting with anti-Hsp90 antibody. Strikingly, we found that GA treatment alone enhances the association of Hsp90 with CRAF (Fig. 1L) as observed previously with herbimycin A (67); however, the interaction between Hsp90 and CRAF remained unaltered when protein synthesis was blocked (Fig. 1L). Co-IP experiment suggests that pharmacologic inhibition of Hsp90 has no influence upon CRAF-Hsp90 complex dissociation, which is commonly observed for other clients. Thus, stable complex formation between Hsp90 and folded CRAF does not rely on the ATPase activity of Hsp90, and postfolding association of Hsp90 has no causative role toward CRAF stability. However, continuous chaperone dependence even after CRAF folding signifies an additional role of Hsp90 apart from kinase stability in CRAF functioning.
Perturbation of Hsp90 and Cdc37 Functions Affects de Novo Maturation of CRAF
Basal phosphorylation at the Ser-621 residue is crucial for CRAF kinase activity (68, 69). Intramolecular autophosphorylation at this site stabilizes CRAF and maintains its kinase activity (70, 71). Hence, the phosphorylation-deficient CRAFS621A mutant is unstable (70) and kinetically inactive (69, 72) even in the yeast system (data not shown). Instability of this mutant has led us to speculate a correlation between inefficient Ser-621 phosphorylation and reduced CRAF level upon prolonged GA treatment. Thus, the phospho-Ser-621 level was analyzed after expressing CRAF in hsp90 ts yeast strains (see “Experimental Procedures”). We found significant reduction in Ser-621 phosphorylation of CRAF from hsp90 ts mutant strains (Fig. 2A). In contrast, no effect was observed for other basal phosphorylation, phospho-Ser-259, located in the regulatory domain. We also noticed reduced CRAF basal kinase activity in hsp90 impaired strains (Fig. 2B). To ascertain the role of Hsp90 in CRAF maturation, GA was added to both yeast and mammalian cells. In both systems, prolonged GA treatment reduced Ser-621 phosphorylation (Fig. 2, C and D); however, the Ser(P)-621 level remained unaltered when cycloheximide was added in HEK293T cells (Fig. 2E). Notably, substitution of Ser-621 to either Ala (CRAFS621A) or phosphomimetic Glu (CRAFS621E) was found insensitive to Hsp90 inhibition (Fig. 2F), further confirming that Hsp90 impairment reduces the CRAF level by affecting Ser-621 phosphorylation. Hence, the dependence on Hsp90 for CRAF stabilization is uniquely directed by the purpose to phosphorylate the Ser-621 residue of the kinase. Altogether, our data provide an explanation for the reduced CRAF stability and activity upon Hsp90 inhibition.
FIGURE 2.
Hsp90 promotes Ser-621 phosphorylation of CRAF. A and B, impairment in Hsp90 function affects CRAF maturation in yeast. p416GPD-CRAF was transformed into yeast cells. Phospho-Ser-621 was monitored in hsp90 ts yeast strains (G313N and G170D) after incubation at 34 °C for 4 h (A). B, in vitro kinase assay was performed by immunoprecipitating CRAF from hsp90 ts yeast strains (G170D and G313N). The percentage of MEK1 phosphorylation was estimated by normalizing with total CRAF pulled down. Each error bar shows significant differences (p < 0.05). Pgk1 was used as a loading control. C, phospho-Ser-621 was checked in erg6Δ knock-out (erg6::KanMx) yeast cells after treatment with GA (50 μm) for 6 h. The CRAF level was normalized by comparing with either p82 yeast strain or non-treated (indicated by −) and immunoblotted using Ser(P)-621 and Ser(P)-259 antibodies. The band intensity was quantified by densitometry analysis. The error bars represent S.D. from four independent experiments (p < 0.05). D and E, Hsp90 governs Ser-621 phosphorylation of de novo made CRAF kinase. HEK293T cells were treated with GA (2 μm) or GA in combination with cycloheximide (CHX) (100 μm) for the indicated times. Ser(P)-259 and Ser(P)-621 were analyzed by loading an equal amount of CRAF from each sample. Each blot is a representative of three independent experiments. The quantification of the reduced Ser(P)-621 level in the presence of GA is given in the adjacent graph (p < 0.01, n = 3). F, inhibition of Hsp90 had no effect on the stability of either CRAFS621A or CRAFS621E mutant. HEK293T cells were transfected with CRAFWT, CRAFS621A, and CRAFS621E, respectively. Cells were treated with GA (2 μm) for the indicated time intervals. The CRAF level was checked with anti-FLAG antibody. Here, GAPDH serves as a loading control. NT, non-transfected.
Co-chaperones assist Hsp90 to facilitate kinase folding and maturation. Being the stable client loader of Hsp90, co-chaperone Cdc37 actively participates in kinase activation (73). Phosphorylation at Ser-13 (mammal)/Ser-14 (yeast) by CK2 kinase maintains client stability by forming a stable kinase-Cdc37 complex for different clients including CRAF, AKT, and Src (29, 32, 74–76). To elucidate the role of Cdc37 in CRAF maturation, we expressed CRAF cloned under the constitutive GPD promoter (yeast) in WT and cdc37S14A mutant yeast strains (76). The CRAF level was largely diminished in the cdc37S14A strain in both steady state (data not shown) and under pulsed condition, implying the rapid degradation of CRAF kinase (Fig. 3A), similar to yeast kinases (76). Strikingly, Cdc37 impairment has a negligible effect over folded CRAF as depicted from cycloheximide chase (Fig. 3B). We then examined the reason behind the reduced stability of CRAF in the cdc37S14A yeast strain. We observed significant reduction in phospho-Ser-621 level of CRAF kinase (Fig. 3C), thus affecting its basal kinase activity (Fig. 3D). Similarly, overexpression of hCdc37S13A in HEK293T cells reduced Ser-621 phosphorylation of CRAF kinase (Fig. 3E). Silencing of Cdc37 in MCF7 cells further confirmed the involvement of Cdc37 in CRAF maturation. As reported previously (77), silencing of Cdc37 significantly reduced CRAF accumulation without affecting the Hsp90 level (Fig. 3F). Additionally, we observed reduced CRAF Ser-621 phosphorylation from the remaining CRAF pool under Cdc37-silenced condition (Fig. 3F). Impairing the function of Cdc37 by either mutating the Ser-14 residue (cdc37S14A strain) (Fig. 3G) or silencing Cdc37 (77) particularly disrupts CRAF-Hsp90 interaction. Therefore, combining previous observations (78, 79) and our findings, we suggest that Cdc37, by acting as a kinase sorting factor, assists Hsp90 in conformational maturation of CRAF.
FIGURE 3.
Cdc37 maintains the stability and activity of CRAF by assisting its Ser-621 phosphorylation. A, pulse-chase analysis of CRAF in WT and cdc37S14A yeast strains at 37 °C. B, cycloheximide chase of CRAF from the same strain at 37 °C. C, Ser-621 phosphorylation was evaluated from equally loaded CRAF from WT and cdc37S14A yeast strains. Quantification of Ser(P)-621 level is represented in the adjacent graph. Bars represent mean Ser(P)-621 level with error bars showing ±S.D. from three independent experiments (p < 0.0003). D, in vitro kinase assay was done from the same strain by immunoprecipitating an equal amount of CRAF kinase. Purified CRAF supplied with the assay kit was used as a positive control. E, similar to C, the Ser(P)-621 level was analyzed from GFP-tagged hCdc37- and hcdc37S13A- overexpressing HEK293T cells. EV, pEGFP empty vector. GAPDH serves as a loading control. Normalized quantification of Ser-621 phosphorylation obtained from three independent experiments is shown in the adjacent graph (p < 0.05). F, Cdc37 was silenced in MCF7 cells using SMARTpool siRNA (Santa Cruz Biotechnology). NT, non-transfected; Mock, non-targeted siRNA (scrambled). Cdc37 silencing significantly reduced the CRAF level 9 days (d) post-transfection. Ser-621 phosphorylation was checked by loading an equal amount of CRAF from the silenced sample. GAPDH serves as a loading control. G, CRAF-Hsp90 association was deprived in the cdc37S14A strain. The co-IP assay of CRAF-Hsp90 was done from the cdc37S14A strain.
Hsp90 Recruits Actin to CRAF during Activation of MAPK Pathway
In cancer cells, chaperones are often up-regulated to maintain stability and activation of oncogenic clients (43, 80–82). Overexpression of Cdc37 enhances CDK4 stability following increased binding to Hsp90 (78). Similarly, we found that Cdc37 upon overexpression activates the MAPK pathway by enhancing binding of Hsp90 to CRAF (Fig. 4, A and B), consistent with its role as a client loader that renders increased CRAF activity (both basal and stimulatory) (59). We then questioned whether up-regulation of Hsp90 alone could also activate CRAF kinase in vivo. Elevation of cellular Hsp90 significantly induced both p-MEK and Ser-338 phosphorylation, a known CRAF activation marker (Fig. 4,C and D). This observation was further supported by increased CRAF kinase activity upon Hsp90 overexpression (Fig. 4E). Imaging data showed the localization of GFP-tagged CRAF predominantly in the cytoplasm, whereas overexpression of Hsp90 induced clustering of CRAF at the peripheral region of the cell (Fig. 4F). This finding signifies that Hsp90 up-regulation within the cell alters CRAF subcellular distribution, thereby promoting efficient MAPK pathway activation.
FIGURE 4.
Hsp90 overexpression leads to the activation of MAPK pathway via CRAF kinase. A and B, Cdc37 activates MEK phosphorylation by recruiting Hsp90 to CRAF. Human GFP-Cdc37 construct and corresponding empty vector (EV) were transfected in HEK293T cells. Cell lysates were probed with respective antibodies. MEK activation was detected with phospho-MEK1/2 antibody (A). B, IP of endogenous CRAF was done using anti-CRAF antibody from the same cell lysate. Associated Hsp90 was detected using anti-Hsp90 antibody. The corresponding graph represents percentage of bound Hsp90. Error bars represent S.D. (p < 0.05, n = 3). C, Western blotting analysis of HEK293T cell lysates after transfecting different amounts of HA-tagged Hsp90β. CRAF activation and enhanced MEK phosphorylation were detected using anti-Ser(P)-338 and anti-p-MEK1/2 antibodies, respectively. Densitometry analysis of Ser(P)-338 and p-MEK1/2 levels is represented in the adjacent graph. Error bars represent S.D. of three independent experiments (p < 0.001). D, up-regulation of MAPK signal was detected using anti-Ser(P)-338 CRAF and anti-p-MEK1/2 antibodies after transfecting 3 μg of HA-Hsp90β for 24 and 48 h, respectively. Quantification is given in the adjacent graph. Error bars represent S.D. of three independent experiments (p < 0.01). GAPDH (C) and actin (D) serve as loading controls. E, overexpression of Hsp90 activates CRAF. In vitro kinase activity of CRAF was measured using a kinase assay kit after transfection of Hsp90β (3 μg) for 24 h. The adjacent graph represents the percentage of CRAF intrinsic activity. F, cellular distribution of GFP-tagged CRAF upon Hsp90 overexpression. pEGFP-CRAF and HA-Hsp90β constructs were co-transfected in HEK293T cells. After 48 h, cells were fixed with 3.7% formaldehyde and mounted on a coverslip. Increased peripheral localization of CRAF was detected in the presence of overexpressed Hsp90. The boxed region is shown in the inset, keeping the scale bar identical (20 μm). N represents nucleus. The adjacent blot shows the expression of the respective proteins. G, overexpression of Hsp90 increases Ras-CRAF association. Enhanced Ras-CRAF binding was identified by immunoprecipitating endogenous CRAF in the presence of overexpressed Hsp90. Activation of EGFR and ERBB2 was checked in cell lysate using anti-p-EGFR and anti-p-ERBB2 antibodies. GAPDH was used as a loading control. NT, non-transfected; endo, endogenous.
Receptor tyrosine kinases (Her-2/ERBB2 and EGFR), which are upstream of CRAF in the MAPK pathway, are known clients of Hsp90. To clarify whether overexpression of Hsp90 activates CRAF independently of Her-2/ERBB2 and EGFR kinases, we checked their activatory phosphorylation. p-EGFR (Tyr-845) and p-ERBB2 (Tyr-1221/1222) remained unaltered upon Hsp90 overexpression, confirming selective activation of CRAF by Hsp90 (Fig. 4G). Furthermore, enhanced Ras-CRAF association in the presence of up-regulated cellular Hsp90 (Fig. 4G) establishes CRAF activation, concomitantly inducing the MAPK pathway.
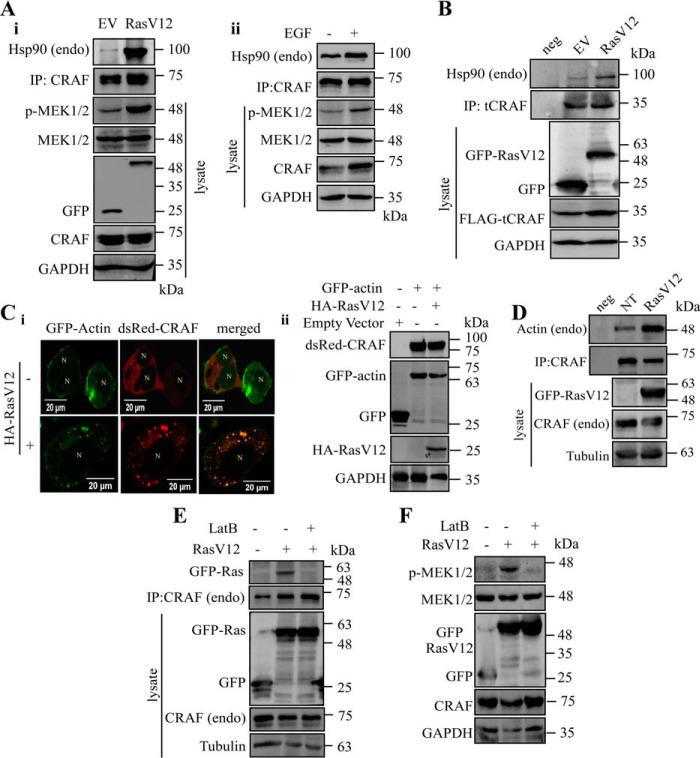
As proposed earlier, CRAF-Hsp90 complex might act as a signalosome during CRAF activation (12) promoted by signal molecules such as growth factors and oncogenic Ras (RasV12) (53). Interestingly, our co-IP data revealed increased CRAF-Hsp90 interaction when the MAPK pathway was activated by either RasV12 or EGF (Fig. 5A, panels i and ii). These findings indicate that the recruitment of Hsp90 into the CRAF complex is a consistent factor during CRAF activation and raised the question whether this enhanced interaction between CRAF and Hsp90 occurs prior to the Ras-CRAF association. To investigate this, we used the catalytic domain of CRAF kinase (tCRAFΔ1–342) in which the Ras binding domain was deleted and found increased recruitment of Hsp90 to the truncated CRAF, similar to full-length kinase, in the presence of RasV12 (Fig. 5B). This observation suggests that during RAF activation stable complex formation between CRAF and Hsp90 occurs prior to Ras interaction.
FIGURE 5.
Interaction of both Hsp90 and actin with CRAF is enhanced during MAPK pathway activation. A, panels i and ii, Hsp90 is recruited to CRAF during stimulation of the MAPK pathway. The MAPK pathway was activated by expressing mEGFP-RasV12 (A, panel i) or treatment with EGF (20 ng/ml) (A, panel ii) in HEK293T cells. EV denotes pEGFP empty vector. Cells were harvested after 48 h of transfection (A, panel i) or 15 min of EGF treatment (A, panel ii). Endogenous CRAF was immunoprecipitated using anti-CRAF antibody, and associated Hsp90 was detected using anti-Hsp90 antibody. B, increased recruitment of Hsp90 to CRAF is independent of Ras-CRAF interaction during MAPK signaling. truncated CRAF (tCRAF) construct with regulatory domain deleted (CRAFΔ1–342) was transfected in the presence of RasV12. FLAG-tCRAF was immunoprecipitated, and associated Hsp90 was immunoblotted using anti-Hsp90 antibody. C and D, RasV12 overexpression enhances CRAF-actin interaction. C, panel i, CRAF and actin co-localize in the presence of RasV12. GFP-actin and dsRed-CRAF were co-expressed in the presence of HA-RasV12. Co-localization is indicated by the yellow color in the confocal image. N represents nucleus. Both pcDNA3 and pEGFP were used as empty vectors. Expressed proteins are represented in the adjacent blot (C, panel ii). GAPDH serves as a loading control. D, co-IP analysis of actin-CRAF in the presence of up-regulated RasV12. GFP-RasV12 was expressed in HEK293T cells. Endogenous CRAF was immunoprecipitated, and associated actin was detected using anti-actin antibody. Tubulin serves as a loading control. E and F, CRAF activation by RasV12 requires an intact actin network. RasV12-transfected cells were treated with 0.1 μm LatB for 24 h, and MAPK activation was analyzed by MEK1/2 phosphorylation (F). CRAF was pulled down from RasV12-overexpressing cells in the presence of LatB. Associated Ras was identified by using anti-GFP antibody (E). neg, IP negative control; NT, non-transfected; EV, empty vector; endo, endogenous.
Inactive CRAF resides at the cytosol, and upon stimulation it moves to the plasma membrane. Being a cytosolic kinase, CRAF movement across the cytoplasm must require assistance of the cytoskeletal network. Interestingly, RasV12 overexpression significantly enhanced the interaction between CRAF and actin (Fig. 5,C and D). Depolymerization of actin by the addition of latrunculin B (LatB) reduced CRAF-Ras interaction and consequently RasV12 signaling (Fig. 5, E and F) (83). Hence, we thought that Hsp90-driven CRAF activation might also involve the actin network. Consistent with our assumption, LatB treatment (Fig. 6Ai) significantly reduced both Hsp90-mediated CRAF activation and efficient Ras-CRAF interaction (Fig. 6B, panels i and ii) without affecting CRAF basal activity (Fig. 6A, panel ii). However, tubulin disruption with nocodazole left CRAF signaling intact (Fig. 6C, panels i and ii). Furthermore, CRAF kinase selectively forms a complex with actin (Fig. 6D). Among the chaperones, only Hsp90 and Hsp100 are found to be associated with actin (84). In addition, CRAF directly interacts with Hsp90 as described previously (59). Therefore, we examined the complex formation ability of CRAF with actin in the presence of overexpressed Hsp90. We found increased association of both actin and Hsp90 with CRAF under the aforementioned condition (Fig. 6E). Confocal data further confirmed the increased interaction between actin and CRAF as depicted in Fig. 6F, panel B. In contrast, actin overexpression kept the CRAF-Hsp90-actin interaction intact (Fig. 6G). Overall, our data suggest that Hsp90 drives a stable assemblage between CRAF and the actin network during MAPK signaling.
FIGURE 6.
Hsp90 modulates CRAF-actin interaction during MAPK signaling. A, LatB does not affect basal kinase activity of CRAF. A, panel i, disruption of actin cytoskeleton upon LatB treatment is shown by immunostaining with rhodamine-tagged phalloidin. A, panel ii, in vitro kinase assay was done from LatB (100 nm)-treated HEK293T cells. Activity of CRAF kinase was measured using purified MEK1, and phospho-MEK was detected using anti-p-MEK1 antibody. B, panels i and ii, actin depolymerization restricts Hsp90-mediated CRAF activation. HEK293T cells were treated with LatB as mentioned earlier after overexpressing HA-Hsp90β for 24 h. Cell lysates were immunoblotted with respective antibodies as indicated. Endogenous CRAF was pulled down using anti-CRAF antibody. CRAF-Ras interaction was interrupted upon LatB treatment in Hsp90β overexpression condition. GAPDH serves as a loading control. C, tubulin is not involved in Hsp90-mediated CRAF activation. C, panel i, Hsp90-mediated activation of MEK and CRAF was checked in HEK293T cell lysates after overexpression of Hsp90 for 24 h in the presence of nocodazole (0.1 μm) for the indicated times. C, panel ii, the effect of nocodazole on disruption of tubulin. D, CRAF interacts with actin. CRAF was immunoprecipitated with anti-FLAG antibody after transfection in HEK293T cells. Actin association was detected by Western blotting with actin antibody. E, Hsp90 overexpression increases actin recruitment to CRAF. Enhancement in CRAF-actin association was detected in HA-Hsp90β-overexpressing cell lysate by immunoprecipitating endogenous CRAF followed by immunoblotting with actin antibody. F, enhanced co-localization between actin and CRAF was observed in the presence of overexpressed Hsp90. GFP-actin and dsRed-CRAF were co-expressed in the presence and absence of ectopically expressed Hsp90β. The merged image represents the co-localized actin and CRAF, indicated by yellow color. N represents nucleus. Expression of each protein is shown in the adjacent blot. GAPDH serves as a loading control in the blot. G, actin is not involved in recruiting Hsp90 to the CRAF complex. GFP-actin was overexpressed in HEK293T cells at different amounts (1.5 and 3 μg, respectively). CRAF was immunoprecipitated using anti-CRAF antibody. Bound Hsp90 and actin were detected with respective antibodies. Tubulin serves as a loading control. H and I, inhibition of the ATPase activity of Hsp90 does not affect actin recruitment to CRAF. CRAF-actin association was analyzed either from GA (2 μm)-treated HEK293T cells in the absence and presence of HA-Hsp90β overexpression (H) or in the presence of HA-Hsp90DN mutant (I). CRAF was immunoprecipitated, and bound actin was detected by the indicated antibody. GAPDH serves as a loading control for all the experiments. NT, non-treated; EV, empty vector; endo, endogenous; neg, IP negative control.
Hsp90 functioning is primarily regulated by its intrinsic ATPase cycle (21, 85, 86). The requirement of ATPase activity in maintaining CRAF-actin complex formation was examined by treating the cells with GA for 5.5 h or overexpressing ATPase-deficient mutant Hsp90DN. The duration of GA treatment was selected on the basis of CRAF stability. We observed that neither GA treatment nor Hsp90DN overexpression altered the CRAF-actin association (Fig. 6,H and I). A similar result was obtained in the presence of GA for 16 h (data not shown). Notably, we did not observe any significant effect of GA treatment for 5.5 h on CRAF activation in the presence of either EGF or RasV12 (data not shown). Altogether, our findings from Fig. 6 suggest that Hsp90 facilitates stable Ras-CRAF association by enhancing CRAF-actin interaction independently of its ATPase activity.
RAF activation requires intact dimeric structure (7). This mechanism is conserved among RAF isoforms and is critical for Ras-dependent RAF activation (87–89). Thus, we questioned whether the dimeric structure of CRAF is also required for Hsp90-mediated CRAF activation. Dimer-deficient CRAFR401Hmutant was ectopically expressed, and its activation was assessed in the presence of overexpressed Hsp90. In comparison with CRAFWT, up-regulation of Hsp90 or EGF treatment failed to activate CRAFR401H (Fig. 7A). Having observed that enhancement in Hsp90 binding potentially activates MAPK pathway, we checked Hsp90 interaction with CRAFR401H by co-IP analysis in the presence of up-regulated Hsp90. Consistent with our observation, Hsp90 binding to exogenously expressed CRAFWT was enhanced (Fig. 7B); in contrast, negligible recruitment of Hsp90 was observed for CRAFR401H (Fig. 7B). Surprisingly, CRAFR401H also failed to interact with endogenous Hsp90 (Fig. 7C) even in the yeast system (Fig. 7D). However, we noticed that CRAFR401A, which is capable of forming a CRAF dimer (Fig. 7E) (90, 91), binds to Hsp90 (Fig. 7C). Intact Ser-621 phosphorylation (Fig. 7C) and sensitivity to GA (Fig. 7, F and G) suggest that maturation of CRAFR401H is regulated by Hsp90. Interestingly, the loss of physical association with Hsp90 was reflected in the association between CRAF dimer-deficient mutant and actin (Fig. 7H), although the stability of CRAFR401H remained unaltered (Fig. 7I). Thus, comparing the regulatory behavior of Hsp90 toward CRAF dimer and monomer, we suggest that the assemblage of Hsp90 during CRAF folding is essential to mature the kinase irrespective of dimerization. However, movement of CRAF across the cell requires continuous assistance of Hsp90, acting as a recruiter of actin during MAPK signaling.
FIGURE 7.
Physical assemblage of Hsp90 with CRAF is essential to maintain actin-CRAF interaction. A, up-regulation of Hsp90 failed to activate CRAF dimer-deficient mutant. MEK phosphorylation was analyzed from Hsp90-overexpressing and EGF-treated samples. Phosphorylation was monitored for both CRAFWT and CRAFR401H, respectively. CRAFR401H was not activated by either Hsp90 overexpression or EGF treatment. B, CRAFR401H showed impaired interaction with overexpressed Hsp90. IP was done for N-terminally FLAG-tagged CRAFWT and CRAFR401H, respectively, in the presence of exogenously expressed HA-Hsp90β. Associated Hsp90 was detected using anti-HA antibody. Total lysates for IP samples are identical to those represented in A. GAPDH serves as a loading control. C and D, CRAF dimer-deficient mutant CRAFR401H shows compromised binding with endogenous Hsp90 in both mammalian and yeast systems. A binding assay was performed from HEK293T cells transfected with either CRAFWT or CRAFR401H and CRAFR401A, respectively. IP was done using anti-FLAG antibody, and endogenous Hsp90 was detected. Ser-621 and Ser-259 phosphorylation remained unaltered for both CRAF mutants. CRAFR401H showed negligible binding with cellular Hsp90, whereas CRAFR401A showed significant Hsp90 binding. Quantification of binding interaction is given in the adjacent graph (C). Error bars represent S.D. NT represents non-transfected. D, wild-type yeast cells (BY4741) were transformed with CRAFWT and CRAFR401H constructs, respectively. Expressed CRAF was pulled down using anti-FLAG antibody, and associated endogenous yeast Hsp90 was detected by immunoblotting with yeast Hsp90 antibody. Pgk1 serves as a loading control. NT represents non-transformed. E, CRAF homodimerization is severely interrupted in CRAFR401H, whereas CRAFR401A shows intact homodimer. For homodimerization, FLAG-tagged CRAFWT was transfected in combination with GFP-tagged CRAFWT, CRAFR401H, and CRAFR401A, respectively, into HEK293T cells. Additionally, FLAG-tagged CRAFR401A and GFP-tagged CRAFR401A were co-transfected into HEK293T cells. CRAF dimers were isolated by anti-FLAG immunoprecipitation. IP samples were immunoblotted with anti-GFP antibody. GAPDH serves as a loading control. F and G, CRAFR401H is sensitive to GA treatment. F, HEK293T cells were transfected with CRAFWT and CRAFR401H, respectively. Transfected cells were treated with 2 μm GA for the indicated time intervals. The CRAF level was analyzed using anti-FLAG antibody. Actin was used as a loading control. G, reduced Ser-621 phosphorylation in the presence of GA was observed for CRAFR401H. HEK293T cells were transfected with both CRAFWT and CRAFR401H, respectively. Each cell set was treated with 2 μm GA for 16 h. Ser-621 phosphorylation was analyzed from equally loaded CRAF pool from each set. Normalization was done using an equal amount of CRAF as a control. H, CRAF dimer-deficient mutant shows abrogated interaction with actin. Immunoprecipitation of FLAG-tagged CRAF was done from HEK293T cells transfected with CRAFWT, CRAFR401H, and CRAFR401A, respectively. IP samples were separated by SDS-PAGE and immunoblotted with the indicated antibodies. GAPDH serves as a loading control. I, CRAFWT and CRAFR401H have similar half-lives under the physiological condition. HEK293T cells were transfected with CRAFWT and CRAFR401H, respectively. Protein synthesis was blocked by cycloheximide, and the chase reaction was carried for the indicated time intervals. NT represents non-transfected cells. EV, empty vector; W/O AB, without antibody; WB, Western blotting; endo, endogenous.
Thus, cumulatively our observations illustrated that Hsp90 together with Cdc37 facilitates the stabilizing phosphorylation of CRAF, thereby controlling client kinase activity. Our findings have also elucidated the importance of Hsp90 binding to CRAF that depends upon the client protomeric structure. Hsp90 provides the central stage for stable CRAF-Ras interaction during CRAF activation by recruiting actin to the CRAF complex. In brief, all the above mentioned results establish an important bidirectional role of Hsp90, which acts as a fundamental requirement to attune CRAF-mediated signaling accurately (Fig. 8).
FIGURE 8.
Proposed model for the role of Hsp90 in CRAF functioning. CRAF polypeptides after emerging from the ribosome interact with the early chaperone complex Hsp40-Hsp70 and finally associate with late complex chaperones Hsp90 and Cdc37. CRAF maturation via Ser-621 phosphorylation is prompted by Hsp90 and Cdc37 (indicated by 1). Impairment in either Hsp90 (90) or Cdc37 (37) function perturbs CRAF autophosphorylation at the Ser-621 position, resulting in kinase degradation (indicated by 2). Matured CRAF remains insensitive to Hsp90 and Cdc37 inhibition (indicated by 3). During stimulation, more Hsp90 is bound to CRAF (A), which facilitates actin engagement (B) and CRAF activation (indicated by 4) for stable signal output (indicated by 5). Note that the enhanced assemblage between Hsp90 and CRAF (the mechanism is currently unknown) occurs prior to Ras-CRAF association, and Hsp90-mediated actin recruitment to CRAF is independent of the ATPase activity of Hsp90. RTK, receptor tyrosine kinase.
Discussion
Ser-621 Phosphorylation of CRAF Kinase Is a Chaperone-dependent Event
Intramolecular phosphorylation at the Ser-621 residue keeps CRAF kinase stable and active (70, 71). Similar autophosphorylation has also been reported for other kinases such as dual specificity tyrosine-phosphorylation-regulated kinase (DYRK) and GSK3β (92–94). It is noteworthy that autophosphorylation of GSK3β is Hsp90-dependent. In this study, we observed that only prolonged GA treatment accelerates CRAF degradation, thereby reducing CRAF signaling (Fig. 1I) without dissociating CRAF from Hsp90 (Fig. 1L). This observation contradicts the conventional notion of geldanamycin-mediated kinase degradation (62–64, 94, 95). The dilemma was solved when we investigated the role of Hsp90 in CRAF autophosphorylation. We found that inhibition of Hsp90 particularly hinders Ser-621 phosphorylation of immature CRAF, thereby reducing CRAF intrinsic kinase activity. Insensitivity of mature CRAF toward Hsp90 impairment suggests that Hsp90 inhibition promotes rapid clearance of immature CRAF by disrupting its stabilizing Ser-621 phosphorylation.
Client protein interaction of Hsp90 depends on nucleotide binding. Thus, inhibition of ATP binding reduces client-Hsp90 interaction, thereby accelerating client clearance by the ubiquitin proteasome system (96). Being a bona fide client of Hsp90, CRAF degrades in presence of the Hsp90 inhibitor geldanamycin (12). Interestingly, we found that Hsp90 impairment, either genetic or pharmacologic, specifically destabilizes newly made CRAF kinase and enhances CRAF-Hsp90 association. Such increased binding of Hsp90 was observed previously for both AKT (40) and CRAF kinase (67). Oscillation in client conformations influences the affinity toward Hsp90 (35, 97–99). Thus, enhanced recruitment of Hsp90 was observed for unstable CRAF mutant (CRAFD486A) (70) or CRAFS621A (data not shown) and in the presence of GA/herbimycin A, which induce protein misfolding. A common link among these observations is reduced Ser-621 phosphorylation of CRAF that is essential for CRAF stability and activity. CRAF kinase lacking Ser-621 phosphorylation is suggested to be a misfolded protein and susceptible to degradation (70). Thus, GA application might accelerate the formation of a new CRAF structure different from its native conformation, thereby creating two distinct affinity ranges for both mature and immature CRAF toward Hsp90, although a detailed study is required to unravel the mechanism by which Hsp90 interacts differentially with CRAF depending upon the kinase maturation state.
Molecular chaperone via the early and late chaperone complex facilitates proper folding of newly synthesized polypeptide after it emerges from the ribosome (100). Hsp90, being the part of the late chaperone complex, assists CRAF kinase maturation; however, other co-chaperones, except Cdc37, have no effect (data not shown). Genetic impairment (Fig. 3G) or silencing of Cdc37 (77) affects its client transfer ability to Hsp90 and impedes CRAF Ser-621 phosphorylation (Fig. 3). Therefore, the kinase becomes unstable. This finding suggests that substrate binding and efficient client transfer ability of Cdc37 are essential to promote autophosphorylation at the Ser-621 residue. Altogether, we conclude that Ser-621 phosphorylation of CRAF is a chaperone-dependent event governed by the late complex components Hsp90 and its co-chaperone Cdc37.
Being a client of Hsp90, CRAF attains its native structure aided by Hsp90 and Cdc37. In agreement with our data, this suggests a possible mechanism by which binding of chaperones might stabilize an intermediate step during CRAF folding where the C-terminal tail region comprising the Ser-621 residue of CRAF kinase flips back in close proximity to the ATP binding region of the kinase for efficient autophosphorylation. Phosphorylation at the equivalent position (Ser-729) on BRAF kinase is chaperone-independent and is carried by AMP-activated protein kinase (AMPK) (101). Thus, the requirement of chaperones Hsp90 and Cdc37 during CRAF autophosphorylation establishes a sharp demarcation in the purpose of chaperone dependence for closely related RAF homologs BRAF and CRAF kinase.
Hsp90 Forms Efficient Transport Machinery to Keep CRAF Dimer Poised for Activation
Cellular abundance of Hsp90 facilitates activation of several clients primarily involved in the signaling pathway. Enhanced MAPK signaling via active CRAF when Cdc37 was up-regulated was reported previously (59). In this study, we have shown that ectopic expression of Hsp90 induces the MAPK pathway by active CRAF kinase. Surprisingly, elevation of Hsp90 activates CRAF without affecting its upstream receptor tyrosine kinases (Fig. 4G). During activation of the MAPK pathway, CRAF translocates to the membrane and interacts with Ras molecules (56). From our data, it is evident that CRAF activation by Hsp90 is Ras-dependent. Interestingly, binding of Hsp90 increases with CRAF activated by either growth factor (EGF) or oncogenic Ras (RasV12). This result indicates a strong correlation between enhanced recruitment of Hsp90 and efficient CRAF-Ras interaction. A study with truncated (regulatory domain-deleted) CRAF establishes that Hsp90 recruitment to CRAF during stimulation occurs prior to the Ras-CRAF interaction. Hsp90 is involved in intracellular translocation of client molecules such as steroid receptors and pp60src (102). Moreover, Hsp90 can interact with both actin and tubulin (84, 103). A earlier study (83) and down-regulation of MAPK signaling in the presence of LatB (Fig. 5F) validate the involvement of the actin network during CRAF activation. Co-IP analysis and confocal imaging confirmed that Hsp90 facilitates CRAF activation by increasing association with actin (Fig. 6, E and F). Thus, our findings establish the function of Hsp90 as a recruiter of actin to CRAF, producing an efficient CRAF transportosome during MAPK signaling, as suggested previously (12). This function of Hsp90 is independent of its ATPase activity. The participation of Hsp90 in CRAF movement across the cell during activation justifies the purpose of forming a heterocomplex comprising CRAF-Hsp90 as observed for other clients such as steroid receptors and p53 (104, 105).
Interestingly, kinetically inactive R401H mutant (7) remains insensitive to Hsp90-mediated activation as Hsp90 binding is significantly abolished in the dimer-deficient CRAF mutant. However, the disruption of Ser-621 phosphorylation of CRAF monomer in the presence of GA suggests that Hsp90 recognizes different sites of CRAF depending upon the mature state of the kinase. This speculation is justifiable as the client binding site recognized by Hsp90 during maturation might differ from that identified for mature protein (106). However, loss of physical association of Hsp90 with CRAFR401H significantly affected the interaction with the cytoskeletal network (Fig. 7H), keeping the stability of the monomeric kinase intact, as observed in Fig. 7I. Therefore, combining our observations, we suggest that CRAF maturation and activation are two independent events, and efficient CRAF-Hsp90 interaction attunes both processes.
Hsp90 inhibition prevents re-entry of clients into the folding cycle, thereby triggering client-Hsp90 dissociation (107). In contrast, our co-IP data in the presence of cycloheximide and GA (Fig. 1L) showed that assemblage of Hsp90 with mature CRAF is not driven by the chaperoning activity of Hsp90. In contrast to Hsp70, a client binding site of Hsp90 remained difficult to identify. Several studies have shown that hydrophobic surface charge in the αC-β4 loop of the client governs Hsp90 binding (108). We observed that introduction of a mutation (Arg to His) in the αC-β4 loop of CRAF restricts side-to-side dimerization and abrogates Hsp90 binding (Fig. 7, E and C). Interestingly, Arg to Ala mutation shows intact CRAF dimerization and moderately reduced but significant binding with Hsp90 (Fig. 7, E and C). This observation, however, cannot exclude the involvement of other regions of the kinase domain for Hsp90 binding (109, 110). Structural analysis revealed that the αC helix in monomeric BRAF is positioned outward (111), generating a kinase off-state conformation where proper positioning of the αC helix is disrupted due to impaired dimerization. Enhanced recruitment of Hsp90 during CRAF activation is possibly driven by the balanced cross-talk between suitable positioning of the αC helix and favorable alignment of two CRAF protomers, which might be absent for the CRAFR401H dimer mutant. In contrast, recruitment of Hsp90 requires client on-pathway conformations as evident from previous studies (35, 112). Thus, our data strongly emphasize the requirement of CRAF on-state conformation for efficient Hsp90 interaction. However, further study is required to identify the structural determinants of the kinase essential to hold Hsp90 in close vicinity of the client CRAF kinase.
In summary, our study provides mechanistic detail of the regulation of CRAF kinase functioning by Hsp90. CRAF activation is regulated at different layers to maintain proper MAPK signaling. Experimental evidence suggests that Hsp90 keeps CRAF kinase maturation and its intracellular translocation in balance to sustain accurate cellular growth and proliferation. The dual role of Hsp90 establishes a new framework of CRAF kinase regulation in understanding the importance behind the stringent assistance of Hsp90.
Experimental Procedures
Plasmids
FLAG-tagged pcDNA3-CRAF plasmid was a kind gift from Bruce Gelb, Mount Sinai School of Medicine, NY. For expression in yeast, CRAF gene was excised with BamHI and cloned into yeast centromeric vector p416GPD under the constitutive GPD promoter. Plasmids for HA-Hsp90β, HA-Hsp90βDN, HA-RasV12, mEGFP-RasV12, and HA-RasWT were obtained from Addgene; pEGFP-actin and pheromone-responsive element (PRE)-LacZ were a kind gift; and pEGFP-CRAFWT, pdsRed-CRAFWT, and pEGFP-hCdc37 were generated. Point mutants of each gene used in this study were generated by site-directed mutagenesis.
Yeast Strains
Yeast strains BY4741 (MATa his3Δ0 leu2Δ0 met15Δ0 ura3Δ0) and W303 (MATa ade2–1 ura3–1 his3–11,15 trp1–1 leu2–3,112 can1–100) were used in this study. For inhibitor treatment, Erg6-deleted (erg6::nat) strain was used. Hsp90 yeast strains used were p82 (W3031A hsc82::LEU2 hsp82::LEU2 HSP82::HIS3), G170D (W3031A hsc82::LEU2 hsp82::LEU2 hsp82, G170D::HIS3), and G313N (W303 1A hsc82::LEU2 hsp82::LEU2 hsp82, G313S::HIS3) (113).
Yeast Cell Growth, Cell Culture, Transient Transfection, Gene Silencing, and Inhibitors Used
Yeast cells were grown in selection media overnight at 30 °C. Cells were harvested at A600 by centrifuging at 3000 rpm for 7 min. Harvested pellets were processed according to the protocol requirement. MCF7 and HEK293T cells were maintained in Dulbecco's modified Eagles medium (DMEM; Himedia, India). All media were supplemented with 10% FBS (Gibco, ThermoFisher Scientific), 150 μg/ml penicillin/streptomycin, and 50 mg/ml gentamicin. Plasmids were transfected at 80% confluence using Lipofectamine-LTX (Invitrogen) following the manufacturer's protocol. Silencing was done at 60% confluence using Lipofectamine 2000, and validation of siRNA transfection was done by Western blotting.
Reagents Used
The following antibodies are used. Anti-CRAF (610151, 1:5000, GAM) and anti-BRAF (612375, 1:5000, GAM) were from BD Biosciences. Anti-Hsp90 (PA1-14210, 1:5000, GAR), anti-Hsp70 (MA3-008, 1:5000, GAM), anti-total Ras (NA1-012, 1:5000, GAM), anti-phospho-Ser-338-CRAF (MA5-15176, 1:5000, GAR), anti-phospho-Ser-259-CRAF (PA1-14363, 1:5000, GAR), and anti-phospho-Ser-621-CRAF (PA1-26655, 1:5000, GAR) were from Thermo Pierce. Anti-CDK2 (78B2, 1:5000, GAR), anti-pan-actin (D18C11, 1:5000, GAR), anti-GFP (2956, 1:5000, GAR), anti-Cdc37 (D11A3, 1:5000, GAR), anti-phospho-ERBB2 (2243S, 1:1000, GAR), anti-ERBB2 (2165S, 1:1000, GAR), and anti-phospho-MEK1/2 (41G9, 1:5000, GAR) were from Cell Signaling Technology. Anti-HA (H3663, 1:5000, GAM), anti-FLAG (F1804, 1:2500, GAM), and anti-FLAG (F7425, 1:2500, GAR) were from Sigma. Anti-phospho-EGFR (SC23420R, 1:1000, GAR) and anti-EGFR (SC373746, 1:1000, GAR) were from Santa Cruz Biotechnology. Anti-GAPDH (BB-AB0060, 1:5000, GAR) was obtained from BioBharati Lifescience, Kolkata, India. Anti-Pgk1 (E1161, 1:2500, GAM) was from Invitrogen. Anti-total MEK (PKPS-222, 1:5000, GAR) was from Neo BioLab. Anti-tubulin (CP06, 1:100, GAM) was from EMD Millipore. Anti-rhodamine-tagged phalloidin (R415, 1:100) and anti-Alexa Fluor 594 (A11062, 1:500) were from Molecular Probes. Geldanamycin was from Invivogen, San Diego, CA, and cycloheximide was from Sisco Research Laboratories Pvt. Ltd. (SRL), India.
Immunoblotting and Immunoprecipitation
CRAF expression from both yeast and cell lines was checked by using radioimmune precipitation assay buffer (G-Bioscience, St. Louis, MO) supplemented with a mixture of protease inhibitors (ThermoFisher Scientific), phosphatase inhibitor (ThermoFisher Scientific), and 10 mm PMSF (Sigma). Lysates were cleared by centrifugation at 13,500 rpm for 15 min at 4 °C. For immunoprecipitation, cell lysis was performed in IP buffer (20 mm Tris, pH 7.5, 150 mm NaCl, 0.l % Nonidet P-40, protease and phosphatase inhibitors, PMSF). For the IP reaction, 800–1000 μg of lysate was incubated with either anti-FLAG or anti-CRAF antibody overnight at 4 °C. 25 μl of protein A/G-Sepharose beads (Calbiochem) was added and incubated for 2 h at 4 °C. The immunocomplex was washed with IP dilution buffer (20 mm Tris, pH 7.5, 150 mm NaCl, 0.l % Nonidet P-40, protease and phosphatase inhibitors, PMSF) three times, and 20 μl of 2× sample buffer (0.1 m Tris-HCl, pH 6.8, 20% glycerol, 4% SDS, 0.2 m β-mercaptoethanol, 0.1% bromphenol blue) was added and boiled for 5 min at 95 °C. The cell lysate or immunocomplex was separated by 10% SDS-PAGE, transferred to membrane, and immunoblotted with respective antibodies.
Pheromone Assay
Yeast cells were grown at an A600 of 0.2 in selective media with or without α-factor (5 μm). Cells from 1.5 ml of culture were harvested and lysed by resuspending in 200 μl of extract buffer (HEPES-KOH, pH 7.4, 150 mm NaCl, 1 mm EDTA, 10% glycerol, 5 mm DTT, 1 mm PMSF, 1× protease inhibitor EDTA-free mixture) in a bead beater. β-Galactosidase activity was measured by 4-methylumbelliferone β-d-galactopyranoside (MUG) assay using 20 μl of lysate, 80 μl of Z buffer (60 mm Na2HPO4, 40 mm NaH2PO4·7H2O, 10 mm KCl, 1 mm MgSO4·7H2O, 50 mm β-mercaptoethanol) and 5 μl of 0.1% MUG solution. The absorbance was measured by luminometer (λ360 = excitation, λ460 = emission).
Pulse-Chase Analysis
Pulse-chase analysis in yeast was done using the protocol described previously (114).
Kinase Assay
The kinase assay was performed using the protocol described previously (115).
Confocal Imaging
Cells were seeded onto a 6-well Petri plate (BD Falcon) containing 22-mm-diameter glass coverslips, and DNA transfection was done using the Lipofectamine-LTX transfection protocol (Invitrogen). Cells were fixed with 3.7% formaldehyde after 48 h of DNA transfection. Sample washing was done with 1× PBS (Himedia). Coverslips were mounted onto slides with DPX mountant (mixture of distyrene, a plasticizer, and xylene; Himedia). The slides were allowed to dry at room temperature and sealed with nail polish. Cells were visualized under a Leica TCS SP8 confocal microscope using a 63× oil objective.
For actin staining, HEK293T cells were treated with latrunculin B (0.1 μm) for 24 h. Cells were washed with PBS and fixed in 3.7% formaldehyde solution for 20 min. The fixative was removed by washing with PBS, and cells were treated with 0.1% Triton X-100. After washing with PBS, blocking was done with 3% BSA, and cells were incubated with 50 μg/ml phalloidin tagged with rhodamine for 1 h in the dark. The staining agents were removed with PBS, and cells were viewed under a Leica TCS SP8 confocal microscope.
Statistical Analysis
Statistical data were scored using Prism 5 (GraphPad Software).
Author Contributions
A. K. M. conceived the idea. S. M. and A. K. M. designed the experiments. S. M. performed majority of the experiments. B. G., N. G., and J. R. contributed in some part of experiments. S. M. and A. K. M. analyzed the results and wrote the manuscript. All the authors reviewed the manuscript.
Acknowledgments
We thank Subhodeep Das for generating FLAG-tagged CRAF clone in yeast vector. We are also thankful to Tanya Saha, Barun Mahata, and Gopa Dhar for helpful discussions during the study. We thank Dr. Aparajita Ghosh for providing ERBB2, EGFR, and the corresponding phosphoantibodies; Dr. Jose M Lizcano for kindly providing human Cdc37 construct; and Dr. Bhaswati Pandit and Pramit Bhattacharya for valuable scientific input during manuscript preparation.
This work was supported by Department of Biotechnology, Ministry of Science and Technology, Government of India Grant BT/PR6544/BRB/10/1151/2012 (to A. K. M.). The authors declare that they have no conflicts of interest with the contents of this article.
- CRAF
- Raf1 kinase
- Hsp
- heat shock protein
- GA
- geldanamycin
- LatB
- latrunculin B
- GAM
- goat anti-mouse
- GAR
- goat anti-rabbit
- ts
- temperature-sensitive
- IP
- immunoprecipitation
- GPD
- glyceraldehyde-3-phosphate dehydrogenase
- hCdc37
- human Cdc37
- p-MEK
- phospho-MEK
- EGFR
- EGF receptor
- mEGFP
- monomeric enhanced GFP
- p-ERBB2
- phospho-ERBB2
- p-EGFR
- phospho-EGFR
- MUG
- 4-methylumbelliferone β-d-galactopyranoside
- PRE
- pheromone-responsive element
- tCRAF
- truncated CRAF.
References
- 1. Avruch J., Zhang X. F., and Kyriakis J. M. (1994) Raf meets Ras: completing the framework of a signal transduction pathway. Trends Biochem. Sci. 19, 279–283 [DOI] [PubMed] [Google Scholar]
- 2. Leicht D. T., Balan V., Kaplun A., Singh-Gupta V., Kaplun L., Dobson M., and Tzivion G. (2007) Raf kinases: function, regulation and role in human cancer. Biochim. Biophys. Acta 1773, 1196–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tartaglia M., Gelb B. D., and Zenker M. (2011) Noonan syndrome and clinically related disorders. Best Pract. Res. Clin. Endocrinol. Metab. 25, 161–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allanson J. E., Annerén G., Aoki Y., Armour C. M., Bondeson M. L., Cave H., Gripp K. W., Kerr B., Nystrom A. M., Sol-Church K., Verloes A., and Zenker M. (2011) Cardio-facio-cutaneous syndrome: does genotype predict phenotype? Am. J. Med. Genet. C Semin. Med. Genet. 157C, 129–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Röring M., and Brummer T. (2012) Aberrant B-Raf signaling in human cancer—10 years from bench to bedside. Crit. Rev. Oncog. 17, 97–121 [DOI] [PubMed] [Google Scholar]
- 6. Flaherty K. T., Puzanov I., Kim K. B., Ribas A., McArthur G. A., Sosman J. A., O'Dwyer P. J., Lee R. J., Grippo J. F., Nolop K., and Chapman P. B. (2010) Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 363, 809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rushworth L. K., Hindley A. D., O'Neill E., and Kolch W. (2006) Regulation and role of Raf-1/B-Raf heterodimerization. Mol. Cell. Biol. 26, 2262–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tzivion G., Luo Z., and Avruch J. (1998) A dimeric 14-3-3 protein is an essential cofactor for Raf kinase activity. Nature 394, 88–92 [DOI] [PubMed] [Google Scholar]
- 9. Luo Z., Tzivion G., Belshaw P. J., Vavvas D., Marshall M., and Avruch J. (1996) Oligomerization activates c-Raf-1 through a Ras-dependent mechanism. Nature 383, 181–185 [DOI] [PubMed] [Google Scholar]
- 10. Xing H., Kornfeld K., and Muslin A. J. (1997) The protein kinase KSR interacts with 14-3-3 protein and Raf. Curr. Biol. 7, 294–300 [DOI] [PubMed] [Google Scholar]
- 11. Yeung K., Seitz T., Li S., Janosch P., McFerran B., Kaiser C., Fee F., Katsanakis K. D., Rose D. W., Mischak H., Sedivy J. M., and Kolch W. (1999) Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature 401, 173–177 [DOI] [PubMed] [Google Scholar]
- 12. Schulte T. W., Blagosklonny M. V., Ingui C., and Neckers L. (1995) Disruption of the Raf-1-Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. J. Biol. Chem. 270, 24585–24588 [DOI] [PubMed] [Google Scholar]
- 13. Grbovic O. M., Basso A. D., Sawai A., Ye Q., Friedlander P., Solit D., and Rosen N. (2006) V600E B-Raf requires the Hsp90 chaperone for stability and is degraded in response to Hsp90 inhibitors. Proc. Natl. Acad. Sci. U.S.A. 103, 57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pratt W. B., and Toft D. O. (1997) Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr. Rev. 18, 306–360 [DOI] [PubMed] [Google Scholar]
- 15. Richter K., Soroka J., Skalniak L., Leskovar A., Hessling M., Reinstein J., and Buchner J. (2008) Conserved conformational changes in the ATPase cycle of human Hsp90. J. Biol. Chem. 283, 17757–17765 [DOI] [PubMed] [Google Scholar]
- 16. Zhao R., Davey M., Hsu Y. C., Kaplanek P., Tong A., Parsons A. B., Krogan N., Cagney G., Mai D., Greenblatt J., Boone C., Emili A., and Houry W. A. (2005) Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell 120, 715–727 [DOI] [PubMed] [Google Scholar]
- 17. Borkovich K. A., Farrelly F. W., Finkelstein D. B., Taulien J., and Lindquist S. (1989) hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol. Cell. Biol. 9, 3919–3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Young J. C., Moarefi I., and Hartl F. U. (2001) Hsp90: a specialized but essential protein-folding tool. J. Cell Biol. 154, 267–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Richter K., Haslbeck M., and Buchner J. (2010) The heat shock response: life on the verge of death. Mol. Cell 40, 253–266 [DOI] [PubMed] [Google Scholar]
- 20. Pullen L., and Bolon D. N. (2011) Enforced N-domain proximity stimulates Hsp90 ATPase activity and is compatible with function in vivo. J. Biol. Chem. 286, 11091–11098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Panaretou B., Prodromou C., Roe S. M., O'Brien R., Ladbury J. E., Piper P. W., and Pearl L. H. (1998) ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 17, 4829–4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roe S. M., Ali M. M., Meyer P., Vaughan C. K., Panaretou B., Piper P. W., Prodromou C., and Pearl L. H. (2004) The Mechanism of Hsp90 regulation by the protein kinase-specific cochaperone p50(cdc37). Cell 116, 87–98 [DOI] [PubMed] [Google Scholar]
- 23. Panaretou B., Siligardi G., Meyer P., Maloney A., Sullivan J. K., Singh S., Millson S. H., Clarke P. A., Naaby-Hansen S., Stein R., Cramer R., Mollapour M., Workman P., Piper P. W., Pearl L. H., et al. (2002) Activation of the ATPase activity of hsp90 by the stress-regulated cochaperone aha1. Mol. Cell 10, 1307–1318 [DOI] [PubMed] [Google Scholar]
- 24. Richter K., Walter S., and Buchner J. (2004) The co-chaperone Sba1 connects the ATPase reaction of Hsp90 to the progression of the chaperone cycle. J. Mol. Biol. 342, 1403–1413 [DOI] [PubMed] [Google Scholar]
- 25. Jakob U., and Buchner J. (1994) Assisting spontaneity: the role of Hsp90 and small Hsps as molecular chaperones. Trends Biochem. Sci. 19, 205–211 [DOI] [PubMed] [Google Scholar]
- 26. Nathan D. F., Vos M. H., and Lindquist S. (1997) In vivo functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc. Natl. Acad. Sci. U.S.A. 94, 12949–12956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buchner J. (1999) Hsp90 & Co.—a holding for folding. Trends Biochem. Sci. 24, 136–141 [DOI] [PubMed] [Google Scholar]
- 28. Caplan A. J., Mandal A. K., and Theodoraki M. A. (2007) Molecular chaperones and protein kinase quality control. Trends Cell Biol. 17, 87–92 [DOI] [PubMed] [Google Scholar]
- 29. Shao J., Prince T., Hartson S. D., and Matts R. L. (2003) Phosphorylation of serine 13 is required for the proper function of the Hsp90 co-chaperone, Cdc37. J. Biol. Chem. 278, 38117–38120 [DOI] [PubMed] [Google Scholar]
- 30. Dey B., Lightbody J. J., and Boschelli F. (1996) CDC37 is required for p60v-src activity in yeast. Mol. Biol. Cell 7, 1405–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stepanova L., Leng X., Parker S. B., and Harper J. W. (1996) Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 10, 1491–1502 [DOI] [PubMed] [Google Scholar]
- 32. Bandhakavi S., McCann R. O., Hanna D. E., and Glover C. V. (2003) A positive feedback loop between protein kinase CKII and Cdc37 promotes the activity of multiple protein kinases. J. Biol. Chem. 278, 2829–2836 [DOI] [PubMed] [Google Scholar]
- 33. Siligardi G., Panaretou B., Meyer P., Singh S., Woolfson D. N., Piper P. W., Pearl L. H., and Prodromou C. (2002) Regulation of Hsp90 ATPase activity by the co-chaperone Cdc37p/p50cdc37. J. Biol. Chem. 277, 20151–20159 [DOI] [PubMed] [Google Scholar]
- 34. Citri A., Alroy I., Lavi S., Rubin C., Xu W., Grammatikakis N., Patterson C., Neckers L., Fry D. W., and Yarden Y. (2002) Drug-induced ubiquitylation and degradation of ErbB receptor tyrosine kinases: implications for cancer therapy. EMBO J. 21, 2407–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boczek E. E., Reefschläger L. G., Dehling M., Struller T. J., Häusler E., Seidl A., Kaila V. R., and Buchner J. (2015) Conformational processing of oncogenic v-Src kinase by the molecular chaperone Hsp90. Proc. Natl. Acad. Sci. U.S.A. 112, E3189–E3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Loo M. A., Jensen T. J., Cui L., Hou Y., Chang X. B., and Riordan J. R. (1998) Perturbation of Hsp90 interaction with nascent CFTR prevents its maturation and accelerates its degradation by the proteasome. EMBO J. 17, 6879–6887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu W., Marcu M., Yuan X., Mimnaugh E., Patterson C., and Neckers L. (2002) Chaperone-dependent E3 ubiquitin ligase CHIP mediates a degradative pathway for c-ErbB2/Neu. Proc. Natl. Acad. Sci. U.S.A. 99, 12847–12852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu Y., Singer M. A., and Lindquist S. (1999) Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc. Natl. Acad. Sci. U.S.A. 96, 109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bijlmakers M. J., and Marsh M. (2000) Hsp90 is essential for the synthesis and subsequent membrane association, but not the maintenance, of the Src-kinase p56(lck). Mol. Biol. Cell 11, 1585–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sato S., Fujita N., and Tsuruo T. (2000) Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl. Acad. Sci. U.S.A. 97, 10832–10837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blagosklonny M. V., Toretsky J., Bohen S., and Neckers L. (1996) Mutant conformation of p53 translated in vitro or in vivo requires functional HSP90. Proc. Natl. Acad. Sci. U.S.A. 93, 8379–8383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shi Y., Mosser D. D., and Morimoto R. I. (1998) Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 12, 654–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Whitesell L., and Lindquist S. L. (2005) HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 5, 761–772 [DOI] [PubMed] [Google Scholar]
- 44. Donzé O., Abbas-Terki T., and Picard D. (2001) The Hsp90 chaperone complex is both a facilitator and a repressor of the dsRNA-dependent kinase PKR. EMBO J. 20, 3771–3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pratt W. B., and Toft D. O. (2003) Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. 228, 111–133 [DOI] [PubMed] [Google Scholar]
- 46. Morrison D. K., and Cutler R. E. (1997) The complexity of Raf-1 regulation. Curr. Opin. Cell Biol. 9, 174–179 [DOI] [PubMed] [Google Scholar]
- 47. Wartmann M., and Davis R. J. (1994) The native structure of the activated Raf protein kinase is a membrane-bound multi-subunit complex. J. Biol. Chem. 269, 6695–6701 [PubMed] [Google Scholar]
- 48. Marais R., and Marshall C. J. (1996) Control of the ERK MAP kinase cascade by Ras and Raf. Cancer Surv. 27, 101–125 [PubMed] [Google Scholar]
- 49. Morrison D. (1994) 14-3-3: modulators of signaling proteins? Science 266, 56–57 [DOI] [PubMed] [Google Scholar]
- 50. Morrison D. K. (1995) Mechanisms regulating Raf-1 activity in signal transduction pathways. Mol. Reprod. Dev. 42, 507–514 [DOI] [PubMed] [Google Scholar]
- 51. Williams J. G., Drugan J. K., Yi G. S., Clark G. J., Der C. J., and Campbell S. L. (2000) Elucidation of binding determinants and functional consequences of Ras/Raf-cysteine-rich domain interactions. J. Biol. Chem. 275, 22172–22179 [DOI] [PubMed] [Google Scholar]
- 52. Luo Z., Diaz B., Marshall M. S., and Avruch J. (1997) An intact Raf zinc finger is required for optimal binding to processed Ras and for ras-dependent Raf activation in situ. Mol. Cell. Biol. 17, 46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Diaz B., Barnard D., Filson A., MacDonald S., King A., and Marshall M. (1997) Phosphorylation of Raf-1 serine 338-serine 339 is an essential regulatory event for Ras-dependent activation and biological signaling. Mol. Cell. Biol. 17, 4509–4516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. King A. J., Sun H., Diaz B., Barnard D., Miao W., Bagrodia S., and Marshall M. S. (1998) The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature 396, 180–183 [DOI] [PubMed] [Google Scholar]
- 55. Fabian J. R., Daar I. O., and Morrison D. K. (1993) Critical tyrosine residues regulate the enzymatic and biological activity of Raf-1 kinase. Mol. Cell. Biol. 13, 7170–7179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Marais R., Light Y., Paterson H. F., and Marshall C. J. (1995) Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 14, 3136–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mason C. S., Springer C. J., Cooper R. G., Superti-Furga G., Marshall C. J., and Marais R. (1999) Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J. 18, 2137–2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chong H., Lee J., and Guan K. L. (2001) Positive and negative regulation of Raf kinase activity and function by phosphorylation. EMBO J. 20, 3716–3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Grammatikakis N., Lin J. H., Grammatikakis A., Tsichlis P. N., and Cochran B. H. (1999) p50(cdc37) acting in concert with Hsp90 is required for Raf-1 function. Mol. Cell. Biol. 19, 1661–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Miao R. Q., Fontana J., Fulton D., Lin M. I., Harrison K. D., and Sessa W. C. (2008) Dominant-negative Hsp90 reduces VEGF-stimulated nitric oxide release and migration in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 28, 105–111 [DOI] [PubMed] [Google Scholar]
- 61. Schulte T. W., Blagosklonny M. V., Romanova L., Mushinski J. F., Monia B. P., Johnston J. F., Nguyen P., Trepel J., and Neckers L. M. (1996) Destabilization of Raf-1 by geldanamycin leads to disruption of the Raf-1-MEK-mitogen-activated protein kinase signalling pathway. Mol. Cell. Biol. 16, 5839–5845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chavany C., Mimnaugh E., Miller P., Bitton R., Nguyen P., Trepel J., Whitesell L., Schnur R., Moyer J., and Neckers L. (1996) p185erbB2 binds to GRP94 in vivo. Dissociation of the p185erbB2/GRP94 heterocomplex by benzoquinone ansamycins precedes depletion of p185erbB2. J. Biol. Chem. 271, 4974–4977 [DOI] [PubMed] [Google Scholar]
- 63. Xu W., Mimnaugh E., Rosser M. F., Nicchitta C., Marcu M., Yarden Y., and Neckers L. (2001) Sensitivity of mature Erbb2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J. Biol. Chem. 276, 3702–3708 [DOI] [PubMed] [Google Scholar]
- 64. Blagosklonny M. V., Toretsky J., and Neckers L. (1995) Geldanamycin selectively destabilizes and conformationally alters mutated p53. Oncogene 11, 933–939 [PubMed] [Google Scholar]
- 65. Whitesell L., Mimnaugh E. G., De Costa B., Myers C. E., and Neckers L. M. (1994) Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. U.S.A. 91, 8324–8328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Basso A. D., Solit D. B., Chiosis G., Giri B., Tsichlis P., and Rosen N. (2002) Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J. Biol. Chem. 277, 39858–39866 [DOI] [PubMed] [Google Scholar]
- 67. Schneider C., Sepp-Lorenzino L., Nimmesgern E., Ouerfelli O., Danishefsky S., Rosen N., and Hartl F. U. (1996) Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proc. Natl. Acad. Sci. U.S.A. 93, 14536–14541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dhillon A. S., Meikle S., Peyssonnaux C., Grindlay J., Kaiser C., Steen H., Shaw P. E., Mischak H., Eychène A., and Kolch W. (2003) A Raf-1 mutant that dissociates MEK/extracellular signal-regulated kinase activation from malignant transformation and differentiation but not proliferation. Mol. Cell. Biol. 23, 1983–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Morrison D. K., Heidecker G., Rapp U. R., and Copeland T. D. (1993) Identification of the major phosphorylation sites of the Raf-1 kinase. J. Biol. Chem. 268, 17309–17316 [PubMed] [Google Scholar]
- 70. Noble C., Mercer K., Hussain J., Carragher L., Giblett S., Hayward R., Patterson C., Marais R., and Pritchard C. A. (2008) CRAF autophosphorylation of serine 621 is required to prevent its proteasome-mediated degradation. Mol. Cell 31, 862–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dhillon A. S., Yip Y. Y., Grindlay G. J., Pakay J. L., Dangers M., Hillmann M., Clark W., Pitt A., Mischak H., and Kolch W. (2009) The C-terminus of Raf-1 acts as a 14-3-3-dependent activation switch. Cell. Signal. 21, 1645–1651 [DOI] [PubMed] [Google Scholar]
- 72. Fabian J. R., Morrison D. K., and Daar I. O. (1993) Requirement for Raf and MAP kinase function during the meiotic maturation of Xenopus oocytes. J. Cell Biol. 122, 645–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kimura Y., Rutherford S. L., Miyata Y., Yahara I., Freeman B. C., Yue L., Morimoto R. I., and Lindquist S. (1997) Cdc37 is a molecular chaperone with specific functions in signal transduction. Genes Dev. 11, 1775–1785 [DOI] [PubMed] [Google Scholar]
- 74. Miyata Y., and Nishida E. (2004) CK2 controls multiple protein kinases by phosphorylating a kinase-targeting molecular chaperone, Cdc37. Mol. Cell. Biol. 24, 4065–4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Vaughan C. K., Gohlke U., Sobott F., Good V. M., Ali M. M., Prodromou C., Robinson C. V., Saibil H. R., and Pearl L. H. (2006) Structure of an Hsp90-Cdc37-Cdk4 complex. Mol. Cell 23, 697–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mandal A. K., Lee P., Chen J. A., Nillegoda N., Heller A., DiStasio S., Oen H., Victor J., Nair D. M., Brodsky J. L., and Caplan A. J. (2007) Cdc37 has distinct roles in protein kinase quality control that protect nascent chains from degradation and promote posttranslational maturation. J. Cell Biol. 176, 319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Smith J. R., Clarke P. A., de Billy E., and Workman P. (2009) Silencing the cochaperone CDC37 destabilizes kinase clients and sensitizes cancer cells to HSP90 inhibitors. Oncogene 28, 157–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Smith J. R., de Billy E., Hobbs S., Powers M., Prodromou C., Pearl L., Clarke P. A., and Workman P. (2015) Restricting direct interaction of CDC37 with HSP90 does not compromise chaperoning of client proteins. Oncogene 34, 15–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Keramisanou D., Aboalroub A., Zhang Z., Liu W., Marshall D., Diviney A., Larsen R. W., Landgraf R., and Gelis I. (2016) Molecular mechanism of protein kinase recognition and sorting by the Hsp90 kinome-specific cochaperone Cdc37. Mol. Cell 62, 260–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Karnitz L. M., and Felts S. J. (2007) Cdc37 regulation of the kinome: when to hold 'em and when to fold 'em. Sci. STKE 2007, pe22. [DOI] [PubMed] [Google Scholar]
- 81. Stepanova L., Yang G., DeMayo F., Wheeler T. M., Finegold M., Thompson T. C., and Harper J. W. (2000) Induction of human Cdc37 in prostate cancer correlates with the ability of targeted Cdc37 expression to promote prostatic hyperplasia. Oncogene 19, 2186–2193 [DOI] [PubMed] [Google Scholar]
- 82. Schwarze S. R., Fu V. X., and Jarrard D. F. (2003) Cdc37 enhances proliferation and is necessary for normal human prostate epithelial cell survival. Cancer Res. 63, 4614–4619 [PubMed] [Google Scholar]
- 83. Wang R., Mercaitis O. P., Jia L., Panettieri R. A., and Tang D. D. (2013) Raf-1, actin dynamics, and Abelson tyrosine kinase in human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 48, 172–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Koyasu S., Nishida E., Kadowaki T., Matsuzaki F., Iida K., Harada F., Kasuga M., Sakai H., and Yahara I. (1986) Two mammalian heat shock proteins, HSP90 and HSP100, are actin-binding proteins. Proc. Natl. Acad. Sci. U.S.A. 83, 8054–8058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Prodromou C., and Pearl L. H. (2003) Structure and functional relationships of Hsp90. Curr. Cancer Drug Targets 3, 301–323 [DOI] [PubMed] [Google Scholar]
- 86. Obermann W. M., Sondermann H., Russo A. A., Pavletich N. P., and Hartl F. U. (1998) In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J. Cell Biol. 143, 901–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Freeman A. K., Ritt D. A., and Morrison D. K. (2013) Effects of Raf dimerization and its inhibition on normal and disease-associated Raf signaling. Mol. Cell 49, 751–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Farrar M. A., Alberol-Ila J., and Perlmutter R. M. (1996) Activation of the Raf-1 kinase cascade by coumermycin-induced dimerization. Nature 383, 178–181 [DOI] [PubMed] [Google Scholar]
- 89. Weber C. K., Slupsky J. R., Kalmes H. A., and Rapp U. R. (2001) Active Ras induces heterodimerization of cRaf and BRaf. Cancer Res. 61, 3595–3598 [PubMed] [Google Scholar]
- 90. Poulikakos P. I., Zhang C., Bollag G., Shokat K. M., and Rosen N. (2010) RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 464, 427–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Baljuls A., Mahr R., Schwarzenau I., Müller T., Polzien L., Hekman M., and Rapp U. R. (2011) Single substitution within the RKTR motif impairs kinase activity but promotes dimerization of RAF kinase. J. Biol. Chem. 286, 16491–16503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lochhead P. A., Kinstrie R., Sibbet G., Rawjee T., Morrice N., and Cleghon V. (2006) A chaperone-dependent GSK3β transitional intermediate mediates activation-loop autophosphorylation. Mol. Cell 24, 627–633 [DOI] [PubMed] [Google Scholar]
- 93. Lochhead P. A., Sibbet G., Morrice N., and Cleghon V. (2005) Activation-loop autophosphorylation is mediated by a novel transitional intermediate form of DYRKs. Cell 121, 925–936 [DOI] [PubMed] [Google Scholar]
- 94. Schulte T. W., Akinaga S., Soga S., Sullivan W., Stensgard B., Toft D., and Neckers L. M. (1998) Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones 3, 100–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Soga S., Kozawa T., Narumi H., Akinaga S., Irie K., Matsumoto K., Sharma S. V., Nakano H., Mizukami T., and Hara M. (1998) Radicicol leads to selective depletion of Raf kinase and disrupts K-Ras-activated aberrant signaling pathway. J. Biol. Chem. 273, 822–828 [DOI] [PubMed] [Google Scholar]
- 96. Csermely P., Schnaider T., Soti C., Prohászka Z., and Nardai G. (1998) The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol. Ther. 79, 129–168 [DOI] [PubMed] [Google Scholar]
- 97. Falsone S. F., Leptihn S., Osterauer A., Haslbeck M., and Buchner J. (2004) Oncogenic mutations reduce the stability of SRC kinase. J. Mol. Biol. 344, 281–291 [DOI] [PubMed] [Google Scholar]
- 98. Müller P., Ceskova P., and Vojtesek B. (2005) Hsp90 is essential for restoring cellular functions of temperature-sensitive p53 mutant protein but not for stabilization and activation of wild-type p53: implications for cancer therapy. J. Biol. Chem. 280, 6682–6691 [DOI] [PubMed] [Google Scholar]
- 99. Walerych D., Kudla G., Gutkowska M., Wawrzynow B., Muller L., King F. W., Helwak A., Boros J., Zylicz A., and Zylicz M. (2004) Hsp90 chaperones wild-type p53 tumor suppressor protein. J. Biol. Chem. 279, 48836–48845 [DOI] [PubMed] [Google Scholar]
- 100. Young J. C., Agashe V. R., Siegers K., and Hartl F. U. (2004) Pathways of chaperone-mediated protein folding in the cytosol. Nat. Rev. Mol. Cell Biol. 5, 781–791 [DOI] [PubMed] [Google Scholar]
- 101. Shen C. H., Yuan P., Perez-Lorenzo R., Zhang Y., Lee S. X., Ou Y., Asara J. M., Cantley L. C., and Zheng B. (2013) Phosphorylation of BRAF by AMPK impairs BRAF-KSR1 association and cell proliferation. Mol. Cell 52, 161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Frydman J. (2001) Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu. Rev. Biochem. 70, 603–647 [DOI] [PubMed] [Google Scholar]
- 103. Garnier C., Barbier P., Gilli R., Lopez C., Peyrot V., and Briand C. (1998) Heat-shock protein 90 (hsp90) binds in vitro to tubulin dimer and inhibits microtubule formation. Biochem. Biophys. Res. Commun. 250, 414–419 [DOI] [PubMed] [Google Scholar]
- 104. Dasgupta G., and Momand J. (1997) Geldanamycin prevents nuclear translocation of mutant p53. Exp. Cell Res. 237, 29–37 [DOI] [PubMed] [Google Scholar]
- 105. Galigniana M. D., Scruggs J. L., Herrington J., Welsh M. J., Carter-Su C., Housley P. R., and Pratt W. B. (1998) Heat shock protein 90-dependent (geldanamycin-inhibited) movement of the glucocorticoid receptor through the cytoplasm to the nucleus requires intact cytoskeleton. Mol. Endocrinol. 12, 1903–1913 [DOI] [PubMed] [Google Scholar]
- 106. Xu W., Yuan X., Xiang Z., Mimnaugh E., Marcu M., and Neckers L. (2005) Surface charge and hydrophobicity determine ErbB2 binding to the Hsp90 chaperone complex. Nat. Struct. Mol. Biol. 12, 120–126 [DOI] [PubMed] [Google Scholar]
- 107. Krukenberg K. A., Street T. O., Lavery L. A., and Agard D. A. (2011) Conformational dynamics of the molecular chaperone Hsp90. Q. Rev. Biophys. 44, 229–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Citri A, Harari D., Shohat G., Ramakrishnan P., Gan J., Lavi S., Eisenstein M., Kimchi A., Wallach D., Pietrokovski S., and Yarden Y. (2006) Hsp90 recognizes a common surface on client kinases. J. Biol. Chem. 281, 14361–14369 [DOI] [PubMed] [Google Scholar]
- 109. Gould C. M., Kannan N., Taylor S. S., and Newton A. C. (2009) The chaperones Hsp90 and Cdc37 mediate the maturation and stabilization of protein kinase C through a conserved PXXP motif in the C-terminal tail. J. Biol. Chem. 284, 4921–4935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Prince T., and Matts R. L. (2005) Exposure of protein kinase motifs that trigger binding of Hsp90 and Cdc37. Biochem. Biophys. Res. Commun. 338, 1447–1454 [DOI] [PubMed] [Google Scholar]
- 111. Thevakumaran N., Lavoie H., Critton D. A., Tebben A., Marinier A., Sicheri F., and Therrien M. (2015) Crystal structure of a BRAF kinase domain monomer explains basis for allosteric regulation. Nat. Struct. Mol. Biol. 22, 37–43 [DOI] [PubMed] [Google Scholar]
- 112. Taipale M., Krykbaeva I., Koeva M., Kayatekin C., Westover K. D., Karras G. I., and Lindquist S. (2012) Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell 150, 987–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Nathan D. F., and Lindquist S. (1995) Mutational analysis of Hsp90 function: interactions with a steroid receptor and a protein kinase. Mol. Cell. Biol. 15, 3917–3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Roy J., Mitra S., Sengupta K., and Mandal A. K. (2015) Hsp70 clears misfolded kinases that partitioned into distinct quality-control compartments. Mol. Biol. Cell 26, 1583–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Pandit B., Sarkozy A., Pennacchio L. A., Carta C., Oishi K., Martinelli S., Pogna E. A., Schackwitz W., Ustaszewska A., Landstrom A., Bos J. M., Ommen S. R., Esposito G., Lepri F., Faul C., et al. (2007) Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat. Genet. 39, 1007–1012 [DOI] [PubMed] [Google Scholar]