Abstract
Rationale: Mucosal-associated invariant T (MAIT) cells are a recently described abundant, proinflammatory T-cell subset with unknown roles in pulmonary immunity. Nontypeable Haemophilus influenzae (NTHi) is the leading bacterial pathogen during chronic obstructive pulmonary disease (COPD) exacerbations and is a plausible target for MAIT cells.
Objectives: To investigate whether MAIT cells respond to NTHi and the effects of inhaled corticosteroids (ICS) on their frequency and function in COPD.
Methods: Eleven subjects with COPD receiving ICS, 8 steroid-naive subjects with COPD, and 21 healthy control subjects underwent phlebotomy, sputum induction, bronchoalveolar lavage, and endobronchial biopsy. Pulmonary and monocyte-derived macrophages were cultured in vitro with NTHi.
Measurements and Main Results: Frequencies of Vα7.2+CD161+ MAIT cells, surface expression of the major histocompatibility complex–related protein 1 (MR1), and intracellular IFN-γ expression were measured by flow cytometry. MAIT-cell frequencies were reduced in peripheral blood of ICS-treated subjects with COPD (median 0.38%; interquartile range [IQR], 0.25–0.96) compared with healthy control subjects (1.8%; IQR, 1.4–2.5; P = 0.001) or steroid-naive patients with COPD (1.8%; IQR, 1.2–2.3; P = 0.04). MAIT cells were reduced in bronchial biopsies from subjects with COPD treated with steroids (0.73%; IQR, 0.46–1.3) compared with healthy control subjects (4.0%; IQR, 1.6–5.0; P = 0.02). Coculture of live NTHi increased macrophage surface expression of MR1 and induced IFN-γ from CD4 cells and CD8 cells, but most potently from MAIT cells (median IFN-γ–positive frequencies, 2.9, 8.6, and 27.6%, respectively). In vitro fluticasone and budesonide reduced MR1 surface expression twofold and decreased NTHi-induced IFN-γ secretion eightfold.
Conclusions: MAIT cells are deficient in blood and bronchial tissue in steroid-treated, but not steroid-naive, COPD. NTHi constitutes a target for pulmonary MAIT-cell immune responses, which are significantly impaired by corticosteroids.
Keywords: MAIT cell, COPD, corticosteroids, NTHi
At a Glance Commentary
Scientific Knowledge on the Subject
Inhaled corticosteroids reduce exacerbations in chronic obstructive pulmonary disease (COPD) but increase the risk of developing pneumonia by unknown mechanisms. Nontypeable Haemophilus influenzae (NTHi) is the most common airway-colonizing bacterium in COPD, the leading bacterial pathogen during exacerbations and polymicrobial infections, with potential etiological roles in pneumonia. Mucosal-associated invariant T (MAIT) cells are a recently identified, abundant, proinflammatory T-cell subset with unknown roles in lung immunity.
What This Study Adds to the Field
MAIT cells can respond to macrophage-presented NTHi antigens by producing the T helper type 1 (Th1) cell cytokine IFN-γ. MAIT cells are deficient in the airways of steroid-treated patients with COPD, and in vitro MAIT-cell responses are impaired by steroids. Together, these findings demonstrate a new role for MAIT cells in human lung antimicrobial defense, with implications for a range of airway diseases.
Exacerbations are major drivers of morbidity, mortality, and health care use in chronic obstructive pulmonary disease (COPD) (1–3). Most exacerbations are triggered by bacterial or viral infections (4), or by acquisition of a new strain of a colonizing bacterium (1, 2). Nonencapsulated or nontypeable Haemophilus influenzae (NTHi) can be isolated in up to 80 to 87% of COPD exacerbations (5, 6) and is the most common bacteria colonizing the airways in COPD (6), with colonization correlated with exacerbations, severity of airway inflammation (5), and symptoms (2, 6). NTHi colonization is a major cause of tissue damage, and the bacteria can also invade the lung tissue, between epithelial cells and within macrophages (5, 6), potentially facilitating immune evasion and persistence (7, 8). NTHi colonization may induce airway inflammation through specific IgE-mediated hypersensitivity (9), through activation of innate immunity via Toll-like receptor (TLR)2/4, which leads to increased local production of IL-1β and IL-8 and reactive oxygen species, and through activation of adaptive immunity in which both B- and T-cell responses are implicated (6). Furthermore, NTHi can itself play an etiological role in pneumonia, either as a single pathogen (10) or in polymicrobial infection with other organisms such as Streptococcus pneumoniae (11).
The use of inhaled corticosteroids (ICS) has been shown to improve symptoms and health status, and to reduce the incidence of exacerbations in COPD (12–15). However, effects of ICS are modest and likely restricted to certain subgroups of this heterogeneous condition (3) whereas evidence is accumulating from well-designed clinical trials that ICS increase the incidence of community-acquired pneumonia (13–18). The mechanisms underlying this effect remain unknown, and have been highlighted as a research priority (3).
Mucosal-associated invariant (MAIT) T cells are a recently described subset of innate-like T lymphocytes that are abundant in humans, making up to 10% of T cells in blood and airway tissue (19–21). Like invariant natural killer T cells, MAIT cells express a semi-invariant T-cell receptor (TCR), which usually includes the TCR-α chain TRAV1–2–TRAJ33 (but also sometimes TRAV1–2–TRAJ12 or TRAV1–2–TRAJ20) and is associated with a limited repertoire of TCR-β chains (21, 22). High surface expression of CD161 allows MAIT cells to be identified as CD3+Vα7.2+CD161+ T cells (23, 24). When activated, MAIT cells can rapidly express a range of proinflammatory cytokines, including tumor necrosis factor (TNF)-α, IL-17, and IFN-γ (23). The remarkable evolutionary conservation of this system (25) implies an essential role in host defense, which has yet to be defined. It has recently been shown that MAIT cells recognize small molecule derivatives of highly conserved biosynthetic pathways of riboflavin and folic acid metabolism in bacteria, mycobacteria, and yeasts (26, 27) presented on the nonpolymorphic class 1b antigen presenting molecule major histocompatibility complex (MHC)-related protein 1 (MR1) (28, 29). Because of their propensity for intracellular invasion of epithelia and macrophages (6), and because they express this riboflavin pathway (30), NTHi is a likely target of MAIT-cell immunity.
We recently demonstrated a striking deficiency of MAIT cells in blood, sputum, and bronchial biopsies in asthma that was associated with use of ICS (20, 21). Therefore, we hypothesized that ICS use in COPD may also be associated with deficiency in MAIT cells in the airways, that NTHi may be a target for MAIT-cell activity, and that this activity may be modulated by steroids.
Methods
Participants
Bronchoscopy cohort
Forty participants (18–75 yr) were enrolled from the NIHR Southampton Respiratory Biomedical Research Unit and outpatient clinics at University Hospital Southampton, Southampton, United Kingdom; 19 participants had COPD, 11 of whom were receiving ICS and 8 were not, and 21 healthy nonatopic control subjects (Table 1).
Table 1.
Demographic and Clinical Characteristics of the Participants
| Healthy Control Subjects | COPD Subjects Not Receiving ICS | COPD Subjects Receiving ICS | P Value: COPD ICS vs. Health | P Value: COPD ICS vs. COPD No ICS | |
|---|---|---|---|---|---|
| N | 21 | 8 | 11 | ||
| Demographics | |||||
| Sex, M/F | 13/8 | 3/5 | 9/2 | 0.07 | 0.4 |
| Age, median (range), yr | 28 (24–35) | 66 (63–69) | 69 (65–73) | <0.0001 | 0.3 |
| Pulmonary function | |||||
| FEV1, % predicted | 108 (105–112) | 61 (55–64) | 60 (45–65) | <0.0001 | 0.4 |
| FEV1 reversibility, % | 2.9 (1.8–6.1) | 11 (4.5–19) | 10 (5–14) | 0.03 | 1 |
| FEV1/FVC ratio | 83 (79–88) | 58 (54–62) | 49 (40–59) | <0.0001 | 0.2 |
| DlCO, % predicted | ND | 74 (66–90) | 73 (55–83) | — | 0.7 |
| Va, % predicted | ND | 93 (86–96) | 85 (79–94) | — | 0.2 |
| Kco, % predicted | ND | 81 (74–94) | 82 (69–112) | — | 0.8 |
| Exhaled nitric oxide, ppb, at 50 L/s | 16 (11–21) | 31 (22–56) | 24 (13–30) | 0.2 | 0.09 |
| Clinical | |||||
| Duration of COPD | N/A | 4 (3–5) | 5 (3.5–7) | — | 0.8 |
| GOLD stage | N/A | 2 (2–2) | 2 (2–3) | — | 0.2 |
| Frequent exacerbator phenotype, ≥2/yr | N/A | 1/7 | 3/8 | — | 0.6 |
| MRC dyspnea score | 0 (0–0) | 2 (1.8–2) | 1 (1–3) | 0.001 | 0.8 |
| Atopy, skin test positive, Y/N | 0/21 | 2/6 | 5/6 | 0.002 | 0.6 |
| No. of skin test allergens positive | 0 (0–0) | 2 (1–2) | 4 (2–7) | — | 0.06 |
| Peripheral eosinophil count, 109/L | 0.1 (0.1–0.2) | 0.2 (0.1–0.2) | 0.2 (0.1–0.3) | 0.3 | 0.5 |
| Body mass index, kg/m2 | 25.3 (23.3–28.1) | 28.6 (25.7–32.2) | 28.3 (28.0–31.9) | 0.01 | 0.6 |
| Smoking status | 0/4/17 | 0/8/0 | 0/11/0 | ||
| Never smoker, n (%) | 17 (81) | 0 (—) | 0 (—) | — | — |
| Former smoker, n (% [mean pack-years]) | 4 (19 [3.5]) | 8 (100 [36]) | 11 (100 [61]) | 0.0001 | 1 |
| Current smoker, n (% [mean pack-years]) | 0 (—) | 0 (—) | 0 (—) | — | — |
| Treatment | |||||
| Inhaled steroids | No | No | Yes | — | — |
| Dose, equivalent μg BDP | N/A | N/A | 2,000 (800–2,000) | — | — |
| Maintenance oral corticosteroids, Y/N | No | No | No | — | — |
| Short-acting β-agonist, Y/N, n (%) | No | 5 (62)/3 (38) | 2 (18)/9 (82) | — | 0.07 |
| Long-acting β-agonist, Y/N, n (%) | No | 2 (25)/6 (75) | 11 (100)/0 (0) | — | 0.001 |
| Long-acting muscarinic agonist, Y/N, n (%) | No | 1 (13)/7 (87) | 7 (64)/4 (36) | — | 0.06 |
| Relevant comorbidities, n (%) | |||||
| Hypertension | 1 (5) | 1 (13) | 3 (27) | — | — |
| Cardiac disease | 0 (0) | 2 (25) | 3 (27) | — | — |
| Vascular disease | 0 (0) | 0 (0) | 3 (27) | — | — |
| Inflammatory bowel disease | 0 (0) | 1 (13) | 1 (9) | — | — |
| Other (n = 1 each) | Eczema | Hepatitis B | Spinal muscular atrophy, pernicious anemia, pleural plaques | — | — |
Definition of abbreviations: BDP = beclometasone dipropionate; COPD = chronic obstructive pulmonary disease; DlCO = diffusing capacity of the lung for carbon monoxide; GOLD = Global Initiative for Chronic Obstructive Lung Disease; ICS = inhaled corticosteroids; MRC = Medical Research Council; N/A = not applicable; ND = not determined.
Values are medians with interquartile ranges, unless stated otherwise. Percentages are of those with valid data.
Surgical cohort
For the isolation of pulmonary macrophages, lung parenchyma was obtained from additional participants who underwent resection of lung tumors (Table E1 in the online supplement).
Blood cohort
For the isolation of T cells and monocytes for in vitro experiments, blood was obtained from additional healthy volunteers.
The study was approved by the South Central–Hampshire Research Ethics Committees (13/SC/0416, 10/H0504/2, 08/H0502/32). All participants provided written informed consent.
Study Procedures
Bronchoscopy participants were assessed by history, examination, skin-prick testing with common aero-allergens, spirometry, carbon monoxide transfer testing, and exhaled nitric oxide. Lung samples were obtained by sputum induction (31), bronchoalveolar lavage (BAL), and endobronchial biopsy (32, 33). Using flow cytometry, MAIT-cell subsets were characterized by surface expression of TCR-Vα7.2 and CD161 (23, 34, 35) as previously described (20).
Blood T-Cell and Monocyte Isolation and Differentiation
CD3+ T cells and monocytes were isolated from human peripheral blood mononuclear cells using MACS technology (Miltenyi Biotec, Bisley, UK) and monocytes differentiated into macrophages (MDMs) using 2 ng/ml granulocyte-macrophage colony–stimulating factor as previously described (36, 37).
Lung Macrophage and MDM Infection
A clinical isolate of NTHi (ST201) was cultured on chocolate agar plates and grown to mid-log phase in brain–heart infusion medium culture media with 44 ml/L glycerol, 30 mg/L hemin, 10 mg/L nicotinamide adenine dinucleotide, and 20% heat inactivated fetal calf serum, and then stored in aliquots at −80°C until required, as previously described (38). Macrophages were cultured for 2 hours at 37°C with either live or paraformaldehyde (PFA)-fixed NTHi at multiplicity of infection (MOI) 1 or 10 in antibiotic-free medium. Macrophages were washed with complete medium and cultured for a further 22 hours before cells were harvested using nonenzymatic cell-dissociation solution (Sigma, Poole, UK). For T-cell activation assays, 106 autologous CD3+ cells were added per well to MDMs after the 2-hour infection, with monensin (eBioscience, Hatfield, UK) for the final 5 hours. MDM infection with H3N2 X31 strain of influenza was performed as previously described (36).
MDM stimulation with cytokines and LPS was performed by adding either TNF-α, IL-6, and IFN-γ (10 ng/ml) or LPS (100 ng/ml) to culture wells and incubated for 24 hour. To model the effects of steroids in vitro, MDMs from healthy volunteers were infected with NTHi as previously described, with the addition of 100-nM fluticasone propionate, 200-nM budesonide, or dimethyl sulfoxide (DMSO). Steroid or DMSO was present for the 2-hour infection of MDMs, and re-added after washing for the 22-hour coculture of NTHi-infected MDMs and T cells. For MR1 blocking, the 5-hour infected MDM–T-cell coculture was performed as described previously, but with the addition of 5 μg/ml IgG2a isotype control or anti-MR1 (26.5, Biolegend, San Diego, CA) as previously described (39).
Flow Cytometry Analysis
Samples were resuspended in fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline, 0.5% weight/volume bovine serum albumin, 2-mM ethylenediaminetetraacetic acid) containing 2 mg/ml human IgG before being incubated on ice in the dark for 30 min in the presence of fluorescently labeled antibodies as previously described (20, 36). Specific mean fluorescence intensity (SMFI) was derived by subtracting isotype fluorescence values from MFI. Flow cytometric analysis was performed on a FACSAria using FACSDiva software v5.0.3 (BD Biosciences, Oxford, UK) and FlowJo version 10.1 (FlowJo LLC, Ashland, OR).
RNA Isolation and RT-PCR
RNA was extracted from MDMs using TriFast (PeqLab, VWR, Erlangen, Germany). Reverse transcription was performed using a High Capacity cDNA Reverse Transcription Kit (Life Technologies, Loughborough, UK) with random hexamers performed according to the manufacturer’s protocols. MR1 gene expression was analyzed using TaqMan universal polymerase chain reaction (PCR) master mix, No AmpErase UNG in a 7900HT fast real-time PCR system instrument (all Life Technologies). Gene expression was normalized to β2-microglobulin gene expression and quantified using the ΔΔCt method.
Statistics
Statistical analyses were performed using a Mann-Whitney U test or Wilcoxon’s test as appropriate with Bonferroni’s correction (GraphPad Prism v6.0, GraphPad Software Inc., San Diego, CA). Data are expressed as medians with interquartile ranges (IQR), unless stated otherwise. For clinical characteristics, proportions were compared using Fisher’s exact text where appropriate. Results were considered significant if P < 0.05. We prospectively estimated 65% power to detect a 70% difference in log-transformed biopsy MAIT-cell frequencies with a sample size of 8 per group based on our previous findings in asthma (20).
For additional information on methods, please see the online supplement.
Results
Participant Demographics
The clinical characteristics of participants in the bronchoscopy study are presented in Table 1. Participants with COPD were all ex-smokers and were predominantly in Global Initiative for COPD (GOLD) stage 2, with a median FEV1 of 60 and 61% predicted in the steroid-naive and steroid-treated groups, respectively. The steroid-treated group received a median ICS dose of 2,000-μg beclometasone dipropionate equivalent/day as budesonide (n = 4) or fluticasone (n = 7). The median age of participants with COPD was 66 years (steroid-naive) and 69 years (steroid-treated), although healthy control participants were significantly younger (median age, 28 yr). Other clinical and demographic characteristics did not differ significantly between steroid-naive and steroid-treated groups, except for the proportion using long-acting β-agonists (25% vs. 100%, respectively; P = 0.001). We observed no significant effect of age on MAIT-cell frequencies (see Figure E1).
MAIT-Cell Frequencies in Health and COPD
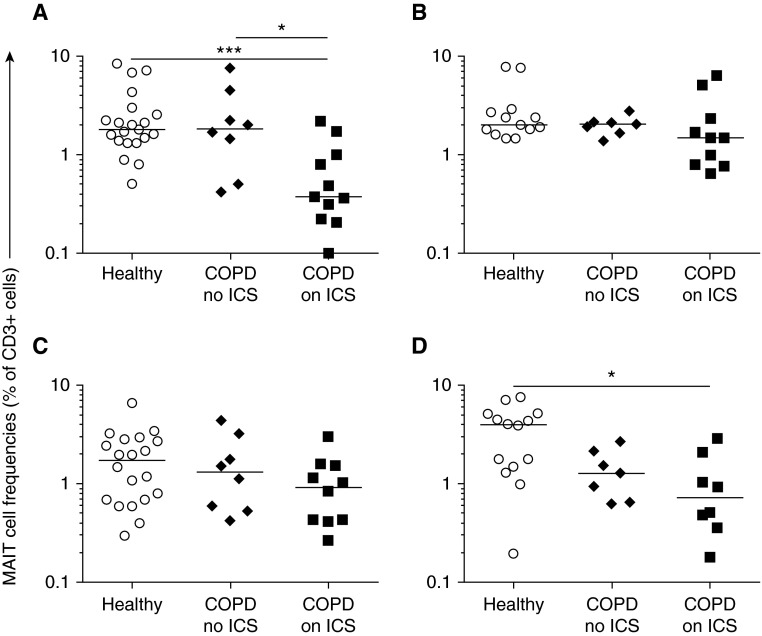
MAIT-cell frequencies were reduced in the peripheral blood in COPD among participants who received ICS (median, 0.38%; interquartile range [IQR], 0.25–0.96) compared with healthy participants (1.8%; IQR, 1.4–2.5; P = 0.001) and compared with participants with COPD not taking ICS (1.8%; IQR, 1.2–2.3; P = 0.04) (Figure 1). MAIT frequencies were also significantly reduced in bronchial biopsies in participants with COPD taking ICS (0.73%; IQR, 0.46–1.3) compared with healthy participants (4.0%; IQR, 1.6–5.0; P = 0.02), but these frequencies did not differ significantly in sputum or BAL. No significant differences were observed with conventional T-cell frequencies (see Figure E2).
Figure 1.
MAIT cells (Vα7.2+CD161+) as proportions of CD3+ T cells in (A) blood, (B) sputum, (C) bronchoalveolar lavage fluid, and (D) endobronchial biopsy specimens in healthy participants and participants with chronic obstructive pulmonary disease (COPD). Horizontal lines show medians. Mann-Whitney tests were performed on untransformed data with Bonferroni’s correction for multiple comparisons. *P < 0.05; ***P = 0.001. ICS = inhaled corticosteroids; MAIT = mucosal-associated invariant T.
NTHi Stimulates MR1 Expression on Macrophages
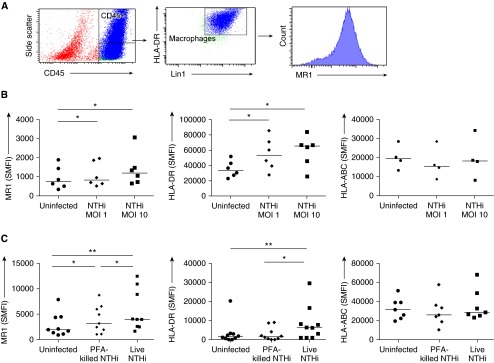
To investigate the potential effects of this MAIT-cell deficiency, we interrogated the interactions of MAIT cells and macrophages when stimulated with NTHi. Surface expression of the MAIT-cell restriction molecule MR1 and of class II MHC were up-regulated on human pulmonary macrophages by coculture with live NTHi at MOI 1 and MOI 10 (P < 0.05 for all comparisons) (Figures 2A and 2B). Likewise, on MDMs derived from the blood of healthy volunteers, expression of MR1 and class II MHC molecules were up-regulated by live NTHi at MOI 1 (P = 0.002 and P = 0.007, respectively) (Figure 2C). MHC class I was not affected by NTHi infection (Figures 2B and 2C). PFA-fixed NTHi induced up-regulation of MR1 (P = 0.02) but significantly less so than live bacteria (P = 0.05), and did not induce class II MHC.
Figure 2.
(A) Cytometric gating strategy. Lung macrophages were identified as positive for CD45-PE-CF594, HLA-DR-APC-Cy7, and Lin1-FITC. (B) Specific mean fluorescence intensity (SMFI) of surface major histocompatibility complex–related protein 1 (MR1), HLA-DR, and HLA-ABC on macrophages from resected human lung either uninfected or infected with live nontypeable Haemophilus influenzae (NTHi) at multiplicity of infection (MOI) 1 and MOI 10 (n = 6 or 4). (C) SMFI of surface MR1, HLA-DR, and HLA-ABC on monocytes differentiated into macrophages from peripheral blood of healthy volunteers either uninfected or infected with live NTHi at MOI 1 or with paraformaldehyde (PFA)-NTHi at MOI 1 (n = 9, 10, and 7, respectively). Bars indicate medians. P values represent Wilcoxon signed-rank tests. *P < 0.05; **P < 0.01. APC = allophycocyanin; Cy7 = cyanine 7; DR = D related; FITC = fluorescein isothiocyanate; HLA = human leukocyte antigen; Lin1 = lineage 1; PE = phycoerythrin.
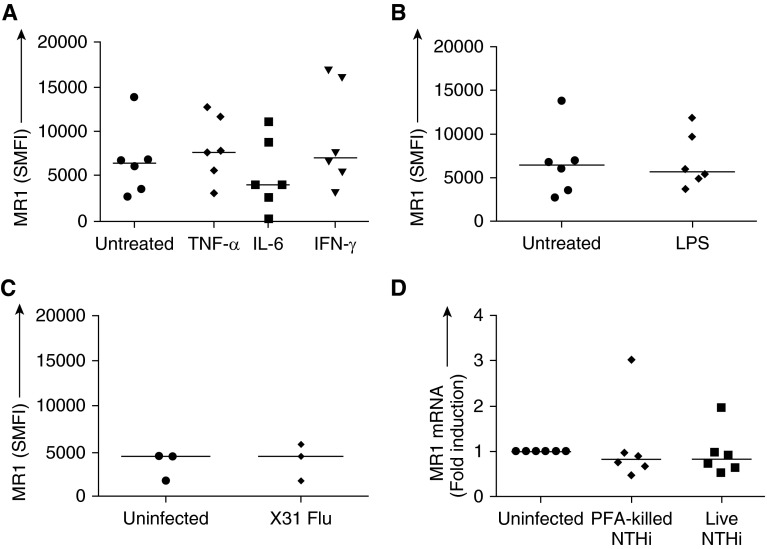
To investigate whether the MR1 up-regulation was mediated nonspecifically by autologous cytokine release or TLR activation, we measured surface expression of MR1 on MDMs in the presence of proinflammatory cytokines (TNF-α, IL-6, and IFN-γ), LPS, or live influenza A (X31), but we observed no significant changes in MR1 expression under any condition (Figures 3A–3C).
Figure 3.
Specific mean fluorescence intensity (SMFI) of major histocompatibility complex–related protein 1 (MR1) on monocyte-derived macrophages (MDMs) from peripheral blood either not treated or treated with (A) TNF-α, IL-6, or IFN-γ (10 ng/ml) (n = 6) or (B) LPS (100 ng/ml) (n = 6) or (C) infected with influenza virus X31 (n = 3). (D) MR1 mRNA from MDMs either uninfected or infected with live nontypeable Haemophilus influenzae (NTHi) at multiplicity of infection (MOI) 1 or with paraformaldehyde (PFA)-fixed NTHi at MOI 1. RNA expression is expressed as ΔΔCt normalized to β2M. Bars indicate medians. TNF = tumor necrosis factor.
Moreover, although NTHi induced MR1 surface expression on MDMs, there was no change in MR1 expression at the mRNA level in response to either live or dead NTHi (Figure 3D), implying that surface up-regulation occurred at a post-translational level. To ensure that this lack of effect on MR1 gene expression was not due to unresponsive MDMs, other genes were also measured in the same samples. HLA-DR gene expression was also unchanged, whereas TLR4 gene expression was decreased. In contrast, the expression of the intracellular pattern-recognition receptors, TLR7 and RIG-1, were found to be significantly up-regulated in response to live NTHi (see Figure E3).
In Vitro Modeling of MAIT Responses to NTHi
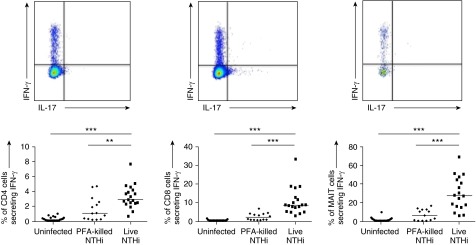
Next, we investigated whether NTHi-infected MDMs could activate autologous T-cell subsets from the peripheral blood of healthy volunteers. Coculture with NTHi-infected MDMs induced secretion of IFN-γ from autologous CD4 cells and CD8 cells, but most potently from MAIT cells (median IFN-γ–positive frequencies 2.9%, 8.6%, and 27.6% respectively; P < 0.0001 for all comparisons vs. unstimulated controls) (Figure 4). This effect was most marked with live bacteria, although significant IFN-γ induction was also induced by PFA-fixed NTHi (P < 0.01 for all comparisons vs. unstimulated controls). There was a significant correlation between the responsiveness of CD4 and CD8 T cells (r = 0.622; P = 0.006) and CD8 and MAIT (r = 0.695; P = 0.002) from the same donors, but this did not occur between CD4 and MAIT (r = 0.247; P = 0.2).
Figure 4.
Representative cytometry plots and graphs showing percentage of peripheral, healthy blood CD4+ T cells, CD8+ T cells, and Vα7.2+CD161+ MAIT cells expressing IFN-γ in response to monocyte-derived macrophages infected with live nontypeable Haemophilus influenzae (NTHi) or paraformaldehyde (PFA)-fixed NTHi at multiplicity of infection 10. Representative of at least 12 independent experiments. Bars indicate medians. P values represent Wilcoxon signed-rank tests. **P < 0.01; ***P < 0.001. MAIT = mucosal-associated invariant T.
IL-17A production was also measured by flow cytometry. No significant increase in MAIT IL-17A expression was observed after coculture with autologous NTHi-infected MDMs (data not shown). Stimulation of MAIT cells with phorbol myristate acetate/ionomycin gave only a minor increase of IL-17A (median 0.7% at baseline, increasing to 1.2% after phorbol myristate acetate/ionomycin; data not shown).
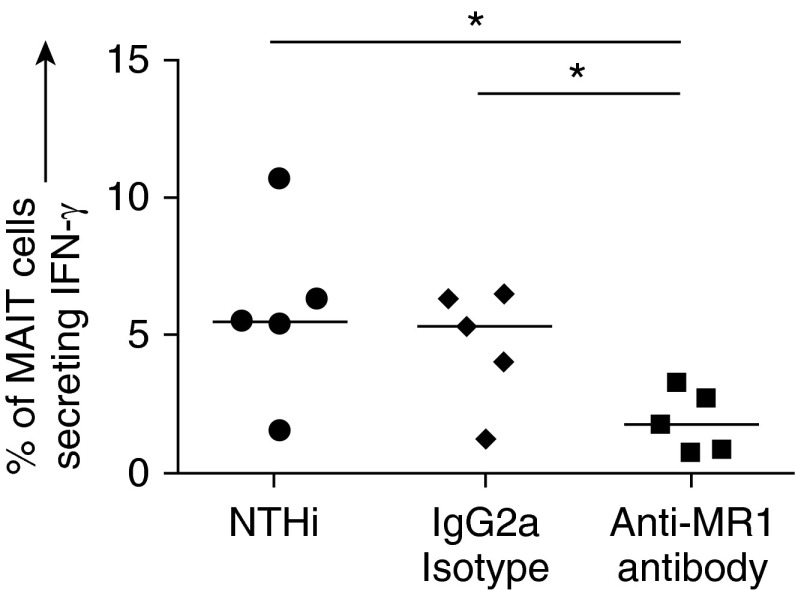
MR1 Is Required for MAIT Activation
To confirm that the MAIT-cell response to NTHi-infected MDMs was driven by MR1, cocultures of NTHi-infected MDMs with autologous T cells were repeated in the presence of an MR1 blocking antibody. Previously, Ussher and colleagues showed a time-dependent difference in the effect of MR1 blocking, so we opted to follow a similar protocol (39). When NTHi-infected MDMs were cocultured with T cells and anti-MR1 for 5 hours, IFN-γ production by MAIT cells was significantly inhibited (P = 0.03) (Figure 5). No IFN-γ production was observed in the conventional CD4 or CD8 subsets at this time point, so blocking with anti–HLA-DR and anti–HLA-ABC, respectively, was not attempted (data not shown). However, when T cells were cocultured with NTHi-infected MDMs for 22 hours, blocking of CD4 and CD8 cells by anti–HLA-DR and anti–HLA-ABC, respectively, had a significant effect (P = 0.004) (see Figure E4). Blocking of MR1 at 22 hours did not significantly affect IFN-γ production by CD4 or CD8 cells (data not shown).
Figure 5.
Blocking of major histocompatibility complex–related protein 1 (MR1). Percentage of peripheral blood mucosal-associated invariant T (MAIT) cells producing IFN-γ in response to autologous monocyte-derived macrophages (MDMs) infected with nontypeable Haemophilus influenzae (NTHi) at multiplicity of infection 10, in the presence of 5 μg/ml anti-MR1 (clone 26.5) or IgG2a isotype control (n = 5). Infected MDMs and autologous T cells were cocultured for 5 hours. MDMs and MAIT cells were obtained from the blood of healthy volunteers. Bars indicate medians. P values represent Wilcoxon signed-rank tests. *P < 0.05.
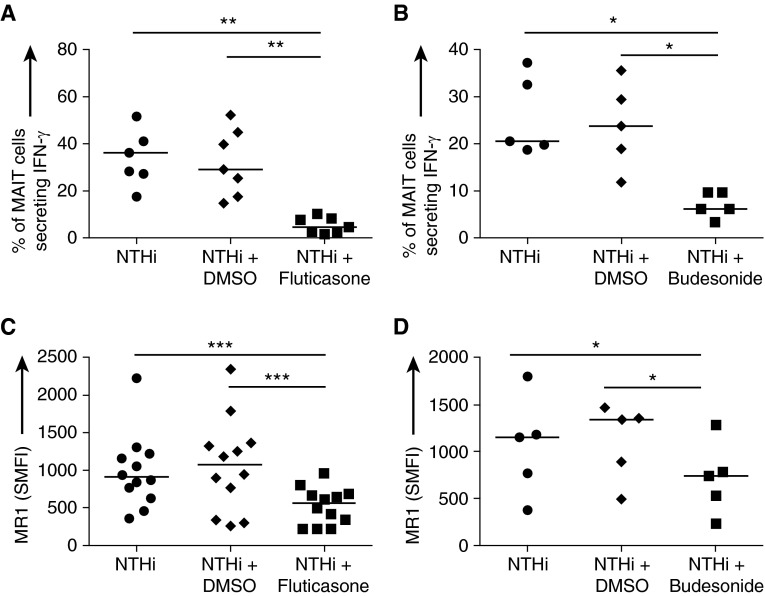
Effect of Steroids on NTHi-induced MAIT Activation
Finally, we investigated the effect of corticosteroids on healthy volunteer blood MAIT-cell activation. The presence of 100-nM fluticasone propionate or 200-nM budesonide significantly impaired surface up-regulation of MR1 on NTHi-infected MDMs, causing a twofold fall in SMFI (Figures 6C and 6D). Moreover, both steroids caused a significant decrease in NTHi-induced IFN-γ expression from autologous MAIT cells (Figures 6A and 6B).
Figure 6.
(A and B) Percentage of mucosal-associated invariant T (MAIT) cells producing IFN-γ in response to autologous monocyte-derived macrophages (MDMs) infected with live nontypeable Haemophilus influenzae (NTHi) at multiplicity of infection 10 (n = 7 and 5, respectively). (C and D) Specific mean fluorescence intensity (SMFI) of MR1 on MDMs infected with live NTHi at multiplicity of infection 1 (n = 12 and 5, respectively). MDMs and MAIT cells were obtained from the blood of healthy volunteers. In both experiments, dimethyl sulfoxide (DMSO) and either 100-nM fluticasone propionate (A and C) or 200-nM budesonide (B and D) were present throughout the experiment. Bars indicate medians. P values represent Wilcoxon signed-rank tests. *P < 0.05; **P < 0.01; ***P < 0.001. MR1 = major histocompatibility complex–related protein 1.
Discussion
We described a deficiency of TCR-Vα7.2+ CD161+ MAIT cells in blood and bronchial tissue in steroid-treated COPD. Furthermore, we showed, for the first time, that live NTHi infection could induce surface expression of the MAIT-cell restriction molecule MR1 on pulmonary macrophages and a potent IFN-γ response from MAIT cells, which provided strong evidence that H. influenzae is a target of MAIT-cell immunity. Significantly, both NTHi-induced surface expression of MR1 and IFN-γ responses were significantly impaired by the presence of steroids. Thus, ICS may impair both the frequency and the function of MAIT cells. The consequences of this in the airways may be complex. ICS improve symptoms and reduce the incidence of exacerbations in COPD (12–15), which is likely due to their efficacy in decreasing the inflammatory response to bacteria and viruses (6). Steroid suppression of inflammatory responses also likely explains the reduction in time to clinical stability achieved with oral prednisolone in community-acquired pneumonia (40). Because of their abundance (20) and proinflammatory phenotype (23), MAIT cells are likely to be significant contributors to immune pathology in both of these situations.
To our knowledge, this is the first description of MAIT cells and their deficiency in the COPD lung. We previously described a similar deficiency in blood, sputum, and bronchial biopsies in severe, steroid-treated, but not mild, steroid-naive asthma (20, 21). In that study, we observed a strong relationship between ICS dose and MAIT-cell frequencies in peripheral blood and induced sputum (20). Furthermore, in two open-label studies with inhaled and oral corticosteroids, we found a specific decrease in peripheral blood MAIT-cell frequencies after a short course of oral corticosteroids, but not of low-dose ICS (20). Our findings extend the recent report of reduced MAIT cells in peripheral blood in COPD (41). The effect of steroid use was not examined in that study, but 76% of their participants received ICS and 20% received oral corticosteroids. Furthermore, MAIT-cell deficiency was observed in GOLD stages 2 to 4, but not GOLD stage 1, which is the group likely to have the least steroid exposure (41). Our data suggest the explanation for these findings is that long-term use of higher doses of ICS can reduce MAIT-cell frequency and inflammatory cytokine production in patients with COPD.
A deficiency of MAIT cells in bronchial biopsy tissue may arise from corticosteroids suppressing the local expansion of pulmonary MAIT cells, which has been shown to occur in response to microbial or ligand-induced MAIT-cell stimulation (42; T. Hinks, unpublished observations). In addition, MAIT cells are likely to be sensitive to corticosteroid-induced apoptosis due to their high expression of caspase 3 (43). Furthermore, we showed previously that MAIT-cell frequencies in peripheral blood are very sensitive to serum concentrations of vitamin D3, which is a structurally related steroid. Because of these properties of MAIT cells, and the significant systemic bioavailability of ICS (44), the observed deficiencies of MAIT cells in peripheral blood might be expected. An alternative interpretation of our data is that corticosteroids may exert their effect in vivo through effects on anatomic localization. However, we did not observe a significant deficiency of MAIT cells in lavage and sputum, and it is possible that this lack of effect may result from phenotypic differences between MAIT cells in tissue and those that migrate into the lumen, where their survival may be reduced (43).
Although the nature of the ligands recognized by MAIT cells has been recently described as microbially derived riboflavin intermediates that trigger rapid secretion of effector cytokines from MAIT cells, but not conventional T cells (21, 23), the function of MAIT cells in health and disease is still unknown. It has been shown that MAIT cells can be activated by and kill intestinal epithelial cells infected with the invasive bacteria Shigella flexneri, but MAIT cells are equally abundant in the human airways; the average mucosal MAIT-cell frequencies are 4.0% in healthy human bronchi (20) compared with 1.5 to 4.9% in human intestines (23, 45, 46). To date, it is not known which respiratory pathogens induce a strong MAIT-cell response. MAIT cells are unlikely to be important for immunity to S. pneumonia because they do not induce MAIT-cell accumulation in murine S. pneumoniae infection (Zhenjun Chen, personal communication). In critically ill patients with severe bacterial infections, changes in peripheral MAIT-cell frequency were observed only in non-streptococcal infections (47). However, several factors implicate H. influenzae as a likely target of MAIT-cell responses. H. influenzae expresses the riboflavin biosynthetic pathway from which the MAIT-cell ligands are derived (30). The costimulatory molecule CD161 is a key expression marker for MAIT cells, which can recognize the ligand lectin-like transcript 1 (LLT1) expressed by respiratory epithelial cells in response to infection and proinflammatory cytokines (48). H. influenzae can invade the lung tissue (49, 50) and enter respiratory epithelial cells and macrophages in chronic bronchitis (6–8, 51). Exacerbations of chronic bronchitis are associated with an increase in the presence of NTHi bacteria inside epithelial cells as seen in 33 to 87% of biopsies (6, 8). Because H. influenzae is a facultative anaerobe, this may allow the bacterium to survive and persist intracellularly and evade humoral immunity (6, 7). T-cell immunity would be essential for eradication of such bacilli. The importance of T cells is underlined by the association of HIV with an increased risk of pneumonia, bronchiectasis, and acute lower respiratory tract infections, particularly in children (6), in which an important causative pathogen is H. influenzae (5). Furthermore, we recently described a defect in CD8+ T-cell immunity in COPD (52), and an additional MAIT deficiency could compound this impaired cellular immune response. Severe or recurrent infections with H. influenzae are also a feature of XLP-2, a rare genetic immunodeficiency with pleiotropic effects, including a 10-fold decrease in MAIT-cell frequencies (53), although hypogammaglobulinemia may also be a contributing factor.
Despite the potentially beneficial anti-inflammatory effects, the suppression of MAIT-cell responses is likely to occur at a cost. Although MAIT cells may not be required for defense against mono-microbial infections with S. pneumoniae, a deficiency of MAIT cells in non-streptococcal bacterial sepsis has been described in the intensive care setting, and is associated with increased acquisition of nosocomial infections (47). Thus, our findings may begin to provide an explanation for the increased risk of pneumonia associated with ICS use in COPD (13–18). In addition, although NTHi is a commensal of the upper respiratory tract, it is not present as a persistent, distinct microbial community in the lower respiratory tract in health. It is not known by what mechanisms it establishes a niche in the lower respiratory tract, but once colonization is established, NTHi can become pathogenic itself, or contribute to the pathology of other bacteria (10) or of viral infections that lead to increased bacterial biomass (54). It can also disrupt epithelial tight junctions, which allows bacterial invasion (55). It is possible that steroid-suppression of MAIT cells could also contribute to this initial colonization step in COPD and other airway diseases.
Glucocorticoids have long been known to inhibit T-cell proliferation and induce apoptosis (56), as well as inhibiting cytokine expression. Nuclear factor-κB is involved in the expression of IFN-γ (57), and fluticasone reduces activation of this transcription factor (58). The reduction of MAIT-cell frequency in response to steroids in COPD patients was not recapitulated in vitro (data not shown), although we observed reduced IFN-γ expression. Our in vitro investigations were limited to 24-hour cultures, and thus, culture times might not have been long enough to observe an effect of steroids on either T-cell proliferation or apoptosis. Regarding the action of steroids on the macrophages, our data suggest that MR1 may be regulated by post-transcriptional mechanisms, which is similar to a previous study that demonstrated post-transcriptional inhibition of MHC I expression by dexamethasone (59).
There are direct clinical implications to our findings. We demonstrated a dose–response relationship between MAIT-cell frequencies and ICS (20, 21) that underlines the importance of titrating ICS doses carefully to the indication and avoiding injudicious use of highly potent ICS at high doses unless supported by empiric data of efficacy. Because of the heterogeneous clinical spectrum of COPD (4), there should be a priority for careful clinical phenotyping and targeted use of inhaled or oral steroids only in subgroups likely to respond (60). The possibility of steroids facilitating initial lower airway colonization with NTHi should be further explored epidemiologically and in model systems.
Our study had several limitations. First, healthy control participants were younger than those with COPD. MAIT-cell frequencies may decline in older adults (61), although we observed no age-related difference between healthy individuals in their third and sixth decades (see Figure E1). Moreover, the two COPD cohorts were well matched with each other in age, but the MAIT-cell deficiencies were observed only in the steroid-treated groups, implying that this was related to steroids rather than age. Furthermore, even if increasing age were a contributory factor to MAIT-cell decline in COPD, this would only serve to further emphasize the importance of minimizing additional iatrogenic suppression in such individuals. A second limitation was that confounding by the indication for steroid treatment could not be excluded due to the cross-sectional design. A third limitation was that, due to low cell numbers from bronchoscopy samples, it was not possible to investigate whether airway MAIT cells differed in responsiveness to NTHi infection from participants either receiving or not receiving ICS, but this could be pursued in further studies using resected tissues. The study was also not powered to detect whether a previously reported sex difference in MAIT frequency (62) might have contributed to the observed deficiency in COPD participants who received ICS. Because the bronchoscopy data were exploratory in nature, findings will need further replication in a larger cohort.
In summary, we demonstrated a numerical and functional deficiency of MAIT cells in the airways in COPD that was related to therapeutic corticosteroids. We provided evidence of a role for this enigmatic new T-cell subset in a host defense against a leading respiratory pathogen. Our findings will have implications for a range of airways diseases in which acute and chronic NTHi infections contribute to immune pathology, including severe asthma, cystic fibrosis, bronchiectasis, and COPD.
Acknowledgments
Acknowledgment
The authors thank Richard Jewell and Dr. Carolann MacGuire of the University of Southampton Faculty of Medicine Flow Cytometry Unit. They also express their appreciation to the staff of the Southampton NIHR Respiratory Biomedical Research Unit. Furthermore, the authors express their gratitude to Dr. Yifang Gao for advice about MAIT-cell analysis and Clair Barber for assistance with cytospin analysis. They extend their gratitude to all the volunteers who gave of their time and enthusiasm to make this research possible.
Footnotes
Supported by a Wellcome Trust Clinical Research Fellowship (088365/z/09/z) and by the Academy of Medical Sciences (T.S.C.H.), by the British Medical Association H. C. Roscoe Award 2013 (K.J.S. and T.M.A.W.), and by GlaxoSmithKline Biologicals, Belgium via a Collaborative Research and Development Agreement (J.C.W. and K.J.S.).
Author Contributions: Conception and design: all authors. Data acquisition, analysis, and interpretation: T.S.C.H., J.C.W., and K.J.S. Drafting of manuscript for important intellectual content: T.S.C.H., J.C.W., A.P.W., K.J.S., and T.M.A.W.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201601-0002OC on April 26, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347:465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 2.Sethi S, Wrona C, Eschberger K, Lobbins P, Cai X, Murphy TF. Inflammatory profile of new bacterial strain exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:491–497. doi: 10.1164/rccm.200708-1234OC. [DOI] [PubMed] [Google Scholar]
- 3.Celli BR, Decramer M, Wedzicha JA, Wilson KC, Agustí A, Criner GJ, MacNee W, Make BJ, Rennard SI, Stockley RA, et al. ATS/ERS Task Force for COPD Research. An Official American Thoracic Society/European Respiratory Society Statement: research questions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191:e4–e27. doi: 10.1164/rccm.201501-0044ST. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Campos JL, Agustí A. Heterogeneity of chronic obstructive pulmonary disease exacerbations: a two-axes classification proposal. Lancet Respir Med. 2015;3:729–734. doi: 10.1016/S2213-2600(15)00242-8. [DOI] [PubMed] [Google Scholar]
- 5.Van Eldere J, Slack MP, Ladhani S, Cripps AW. Non-typeable Haemophilus influenzae, an under-recognised pathogen. Lancet Infect Dis. 2014;14:1281–1292. doi: 10.1016/S1473-3099(14)70734-0. [DOI] [PubMed] [Google Scholar]
- 6.King PT, Sharma R. The lung immune response to nontypeable Haemophilus influenzae (lung immunity to NTHi) J Immunol Res. 2015;(2015):706376. doi: 10.1155/2015/706376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clementi CF, Murphy TF. Non-typeable Haemophilus influenzae invasion and persistence in the human respiratory tract. Front Cell Infect Microbiol. 2011;1:1. doi: 10.3389/fcimb.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandi V, Apicella MA, Mason E, Murphy TF, Siddiqi A, Atmar RL, Greenberg SB. Nontypeable Haemophilus influenzae in the lower respiratory tract of patients with chronic bronchitis. Am J Respir Crit Care Med. 2001;164:2114–2119. doi: 10.1164/ajrccm.164.11.2104093. [DOI] [PubMed] [Google Scholar]
- 9.Otczyk DC, Clancy RL, Cripps AW. Haemophilus influenzae and smoking-related obstructive airways disease. Int J Chron Obstruct Pulmon Dis. 2011;6:345–351. doi: 10.2147/COPD.S19359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berndsen MR, Erlendsdóttir H, Gottfredsson M. Evolving epidemiology of invasive Haemophilus infections in the post-vaccination era: results from a long-term population-based study. Clin Microbiol Infect. 2012;18:918–923. doi: 10.1111/j.1469-0691.2011.03700.x. [DOI] [PubMed] [Google Scholar]
- 11.Kumagai S, Ishida T, Tachibana H, Ito A, Ito Y, Hashimoto T. Polybacterial aetiology and outcomes in patients with community-acquired pneumonia. Int J Tuberc Lung Dis. 2016;20:129–135. doi: 10.5588/ijtld.15.0353. [DOI] [PubMed] [Google Scholar]
- 12.Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320:1297–1303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 14.Sharafkhaneh A, Southard JG, Goldman M, Uryniak T, Martin UJ. Effect of budesonide/formoterol pMDI on COPD exacerbations: a double-blind, randomized study. Respir Med. 2012;106:257–268. doi: 10.1016/j.rmed.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Festic E, Scanlon PD. Incident pneumonia and mortality in patients with chronic obstructive pulmonary disease. A double effect of inhaled corticosteroids? Am J Respir Crit Care Med. 2015;191:141–148. doi: 10.1164/rccm.201409-1654PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson GT, Anzueto A, Fei R, Emmett A, Knobil K, Kalberg C. Effect of fluticasone propionate/salmeterol (250/50 microg) or salmeterol (50 microg) on COPD exacerbations. Respir Med. 2008;102:1099–1108. doi: 10.1016/j.rmed.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Anzueto A, Ferguson GT, Feldman G, Chinsky K, Seibert A, Emmett A, Knobil K, O’Dell D, Kalberg C, Crater G. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD. 2009;6:320–329. doi: 10.1080/15412550903140881. [DOI] [PubMed] [Google Scholar]
- 18.Calverley PM, Stockley RA, Seemungal TA, Hagan G, Willits LR, Riley JH, Wedzicha JA Investigating New Standards for Prophylaxis in Reduction of Exacerbations (INSPIRE) Investigators. Reported pneumonia in patients with COPD: findings from the INSPIRE study. Chest. 2011;139:505–512. doi: 10.1378/chest.09-2992. [DOI] [PubMed] [Google Scholar]
- 19.Hinks T, Zhou X, Staples K, Dimitrov B, Manta A, Petrossian T, Lum P, Smith C, Ward J, Howarth P, et al. Multidimensional endotypes of asthma: topological data analysis of cross-sectional clinical, pathological, and immunological data. Lancet. 2015;385:S42. doi: 10.1016/S0140-6736(15)60357-9. [DOI] [PubMed] [Google Scholar]
- 20.Hinks TS, Zhou X, Staples KJ, Dimitrov BD, Manta A, Petrossian T, Lum PY, Smith CG, Ward JA, Howarth PH, et al. Innate and adaptive T cells in asthmatic patients: relationship to severity and disease mechanisms. J Allergy Clin Immunol. 2015;136:323–333. doi: 10.1016/j.jaci.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinks TS. Mucosal-associated invariant T cells in autoimmunity, immune-mediated diseases and airways disease. Immunology. 2016;148:1–12. doi: 10.1111/imm.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, Bendelac A, Bonneville M, Lantz O. An invariant T cell receptor α chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted α/β T cell subpopulation in mammals. J Exp Med. 1999;189:1907–1921. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dusseaux M, Martin E, Serriari N, Péguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 24.Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C, Premel V, Devys A, Moura IC, Tilloy F, et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7:e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsukamoto K, Deakin JE, Graves JA, Hashimoto K. Exceptionally high conservation of the MHC class I-related gene, MR1, among mammals. Immunogenetics. 2013;65:115–124. doi: 10.1007/s00251-012-0666-5. [DOI] [PubMed] [Google Scholar]
- 26.Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 27.Patel O, Kjer-Nielsen L, Le Nours J, Eckle SB, Birkinshaw R, Beddoe T, Corbett AJ, Liu L, Miles JJ, Meehan B, et al. Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat Commun. 2013;4:2142. doi: 10.1038/ncomms3142. [DOI] [PubMed] [Google Scholar]
- 28.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 29.Hinks TS.MR1 (in mouse and man) Ratcliffe M.editor. Encyclopedia of Immunobiology. Oxford, UK: Elsevier; 2016. pp. 263–270 [Google Scholar]
- 30.Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Djukanović R, Sterk PJ, Fahy JV, Hargreave FE. Standardised methodology of sputum induction and processing. Eur Respir J Suppl. 2002;37:1s–2s. doi: 10.1183/09031936.02.00000102. [DOI] [PubMed] [Google Scholar]
- 32.Djukanović R, Wilson JW, Britten KM, Wilson SJ, Walls AF, Roche WR, Howarth PH, Holgate ST. Quantitation of mast cells and eosinophils in the bronchial mucosa of symptomatic atopic asthmatics and healthy control subjects using immunohistochemistry. Am Rev Respir Dis. 1990;142:863–871. doi: 10.1164/ajrccm/142.4.863. [DOI] [PubMed] [Google Scholar]
- 33.Vijayanand P, Seumois G, Pickard C, Powell RM, Angco G, Sammut D, Gadola SD, Friedmann PS, Djukanovic R. Invariant natural killer T cells in asthma and chronic obstructive pulmonary disease. N Engl J Med. 2007;356:1410–1422. doi: 10.1056/NEJMoa064691. [DOI] [PubMed] [Google Scholar]
- 34.Le Bourhis L, Martin E, Péguillet I, Guihot A, Froux N, Coré M, Lévy E, Dusseaux M, Meyssonnier V, Premel V, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11:701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 35.Walker LJ, Kang YH, Smith MO, Tharmalingham H, Ramamurthy N, Fleming VM, Sahgal N, Leslie A, Oo Y, Geremia A, et al. Human MAIT and CD8αα cells develop from a pool of type-17 precommitted CD8+ T cells. Blood. 2012;119:422–433. doi: 10.1182/blood-2011-05-353789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staples KJ, Nicholas B, McKendry RT, Spalluto CM, Wallington JC, Bragg CW, Robinson EC, Martin K, Djukanović R, Wilkinson TM. Viral infection of human lung macrophages increases PDL1 expression via IFNβ. PLoS One. 2015;10:e0121527. doi: 10.1371/journal.pone.0121527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staples KJ, Hinks TS, Ward JA, Gunn V, Smith C, Djukanovic R. Phenotypic characterization of lung macrophages in asthmatic patients: overexpression of CCL17. J Allergy Clin Immunol. 2012;130:1404–1412.e17. doi: 10.1016/j.jaci.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkham LA, Corscadden KJ, Wiertsema SP, Currie AJ, Richmond PC. A practical method for preparation of pneumococcal and nontypeable Haemophilus influenzae inocula that preserves viability and immunostimulatory activity. BMC Res Notes. 2013;6:522. doi: 10.1186/1756-0500-6-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ussher JE, Bilton M, Attwod E, Shadwell J, Richardson R, de Lara C, Mettke E, Kurioka A, Hansen TH, Klenerman P, et al. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol. 2014;44:195–203. doi: 10.1002/eji.201343509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blum CA, Nigro N, Briel M, Schuetz P, Ullmer E, Suter-Widmer I, Winzeler B, Bingisser R, Elsaesser H, Drozdov D, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015;385:1511–1518. doi: 10.1016/S0140-6736(14)62447-8. [DOI] [PubMed] [Google Scholar]
- 41.Kwon YS, Jin HM, Cho YN, Kim MJ, Kang JH, Jung HJ, Park KJ, Kee HJ, Kee SJ, Park YW. Mucosal-associated invariant T cell deficiency in chronic obstructive pulmonary disease. COPD. 2016;13:196–202. doi: 10.3109/15412555.2015.1069806. [DOI] [PubMed] [Google Scholar]
- 42.Meierovics A, Yankelevich WJ, Cowley SC. MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc Natl Acad Sci USA. 2013;110:E3119–E3128. doi: 10.1073/pnas.1302799110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gérart S, Sibéril S, Martin E, Lenoir C, Aguilar C, Picard C, Lantz O, Fischer A, Latour S. Human iNKT and MAIT cells exhibit a PLZF-dependent proapoptotic propensity that is counterbalanced by XIAP. Blood. 2013;121:614–623. doi: 10.1182/blood-2012-09-456095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baptist AP, Reddy RC. Inhaled corticosteroids for asthma: are they all the same? J Clin Pharm Ther. 2009;34:1–12. doi: 10.1111/j.1365-2710.2008.00970.x. [DOI] [PubMed] [Google Scholar]
- 45.Hiejima E, Kawai T, Nakase H, Tsuruyama T, Morimoto T, Yasumi T, Taga T, Kanegane H, Hori M, Ohmori K, et al. Reduced numbers and proapoptotic features of mucosal-associated invariant T cells as a characteristic finding in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1529–1540. doi: 10.1097/MIB.0000000000000397. [DOI] [PubMed] [Google Scholar]
- 46.Serriari NE, Eoche M, Lamotte L, Lion J, Fumery M, Marcelo P, Chatelain D, Barre A, Nguyen-Khac E, Lantz O, et al. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol. 2014;176:266–274. doi: 10.1111/cei.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grimaldi D, Le Bourhis L, Sauneuf B, Dechartres A, Rousseau C, Ouaaz F, Milder M, Louis D, Chiche JD, Mira JP, et al. Specific MAIT cell behaviour among innate-like T lymphocytes in critically ill patients with severe infections. Intensive Care Med. 2014;40:192–201. doi: 10.1007/s00134-013-3163-x. [DOI] [PubMed] [Google Scholar]
- 48.Satkunanathan S, Kumar N, Bajorek M, Purbhoo MA, Culley FJ. Respiratory syncytial virus infection, TLR3 ligands, and proinflammatory cytokines induce CD161 ligand LLT1 expression on the respiratory epithelium. J Virol. 2014;88:2366–2373. doi: 10.1128/JVI.02789-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Möller LV, Timens W, van der Bij W, Kooi K, de Wever B, Dankert J, van Alphen L. Haemophilus influenzae in lung explants of patients with end-stage pulmonary disease. Am J Respir Crit Care Med. 1998;157:950–956. doi: 10.1164/ajrccm.157.3.9707010. [DOI] [PubMed] [Google Scholar]
- 50.Drömann D, Rupp J, Rohmann K, Osbahr S, Ulmer AJ, Marwitz S, Röschmann K, Abdullah M, Schultz H, Vollmer E, et al. The TGF-β-pseudoreceptor BAMBI is strongly expressed in COPD lungs and regulated by nontypeable Haemophilus influenzae. Respir Res. 2010;11:67. doi: 10.1186/1465-9921-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel IS, Seemungal TA, Wilks M, Lloyd-Owen SJ, Donaldson GC, Wedzicha JA. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax. 2002;57:759–764. doi: 10.1136/thorax.57.9.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKendry RT, Spalluto CM, Burke H, Nicholas B, Cellura D, Al-Shamkhani A, Staples KJ, Wilkinson TM. Dysregulation of anti-viral function of CD8+ T cells in the COPD lung: role of the PD1/PDL1 axis. Am J Respir Crit Care Med. 2016;193:642–621. doi: 10.1164/rccm.201504-0782OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pachlopnik Schmid J, Canioni D, Moshous D, Touzot F, Mahlaoui N, Hauck F, Kanegane H, Lopez-Granados E, Mejstrikova E, Pellier I, et al. Clinical similarities and differences of patients with X-linked lymphoproliferative syndrome type 1 (XLP-1/SAP deficiency) versus type 2 (XLP-2/XIAP deficiency) Blood. 2011;117:1522–1529. doi: 10.1182/blood-2010-07-298372. [DOI] [PubMed] [Google Scholar]
- 54.Molyneaux PL, Mallia P, Cox MJ, Footitt J, Willis-Owen SA, Homola D, Trujillo-Torralbo MB, Elkin S, Kon OM, Cookson WO, et al. Outgrowth of the bacterial airway microbiome after rhinovirus exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:1224–1231. doi: 10.1164/rccm.201302-0341OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sajjan U, Wang Q, Zhao Y, Gruenert DC, Hershenson MB. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am J Respir Crit Care Med. 2008;178:1271–1281. doi: 10.1164/rccm.200801-136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tuosto L, Cundari E, Gilardini Montani MS, Piccolella E. Analysis of susceptibility of mature human T lymphocytes to dexamethasone-induced apoptosis. Eur J Immunol. 1994;24:1061–1065. doi: 10.1002/eji.1830240508. [DOI] [PubMed] [Google Scholar]
- 57.Blanco B, Pérez-Simón JA, Sánchez-Abarca LI, Carvajal-Vergara X, Mateos J, Vidriales B, López-Holgado N, Maiso P, Alberca M, Villarón E, et al. Bortezomib induces selective depletion of alloreactive T lymphocytes and decreases the production of Th1 cytokines. Blood. 2006;107:3575–3583. doi: 10.1182/blood-2005-05-2118. [DOI] [PubMed] [Google Scholar]
- 58.Escotte S, Tabary O, Dusser D, Majer-Teboul C, Puchelle E, Jacquot J. Fluticasone reduces IL-6 and IL-8 production of cystic fibrosis bronchial epithelial cells via IKK-β kinase pathway. Eur Respir J. 2003;21:574–581. doi: 10.1183/09031936.03.00031803. [DOI] [PubMed] [Google Scholar]
- 59.Lohmann S, Wollscheid U, Huber C, Seliger B. Multiple levels of MHC class I down-regulation by ras oncogenes. Scand J Immunol. 1996;43:537–544. doi: 10.1046/j.1365-3083.1996.d01-73.x. [DOI] [PubMed] [Google Scholar]
- 60.Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Venge P, Lomas DA, Barer MR, Johnston SL, Pavord ID, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186:48–55. doi: 10.1164/rccm.201108-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee OJ, Cho YN, Kee SJ, Kim MJ, Jin HM, Lee SJ, Park KJ, Kim TJ, Lee SS, Kwon YS, et al. Circulating mucosal-associated invariant T cell levels and their cytokine levels in healthy adults. Exp Gerontol. 2014;49:47–54. doi: 10.1016/j.exger.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Novak J, Dobrovolny J, Novakova L, Kozak T. The decrease in number and change in phenotype of mucosal-associated invariant T cells in the elderly and differences in men and women of reproductive age. Scand J Immunol. 2014;80:271–275. doi: 10.1111/sji.12193. [DOI] [PubMed] [Google Scholar]