Abstract
Rationale: Transforming growth factor-β (TGF-β) ligands signal via type I and type II serine-threonine kinase receptors to regulate broad transcriptional programs. Excessive TGF-β–mediated signaling is implicated in the pathogenesis of pulmonary arterial hypertension, based in part on the ability of broad inhibition of activin-like kinase (ALK) receptors 4/5/7 recognizing TGF-β, activin, growth and differentiation factor, and nodal ligands to attenuate experimental pulmonary hypertension (PH). These broad inhibition strategies do not delineate the specific contribution of TGF-β versus a multitude of other ligands, and their translation is limited by cardiovascular and systemic toxicity.
Objectives: We tested the impact of a soluble TGF-β type II receptor extracellular domain expressed as an immunoglobulin–Fc fusion protein (TGFBRII-Fc), serving as a selective TGF-β1/3 ligand trap, in several experimental PH models.
Methods: Signaling studies used cultured human pulmonary artery smooth muscle cells. PH was studied in monocrotaline-treated Sprague-Dawley rats, SU5416/hypoxia–treated Sprague-Dawley rats, and SU5416/hypoxia–treated C57BL/6 mice. PH, cardiac function, vascular remodeling, and valve structure were assessed by ultrasound, invasive hemodynamic measurements, and histomorphometry.
Measurements and Main Results: TGFBRII-Fc is an inhibitor of TGF-β1 and TGF-β3, but not TGF-β2, signaling. In vivo treatment with TGFBRII-Fc attenuated Smad2 phosphorylation, normalized expression of plasminogen activator inhibitor-1, and mitigated PH and pulmonary vascular remodeling in monocrotaline-treated rats, SU5416/hypoxia–treated rats, and SU5416/hypoxia–treated mice. Administration of TGFBRII-Fc to monocrotaline-treated or SU5416/hypoxia–treated rats with established PH improved right ventricular systolic pressures, right ventricular function, and survival. No cardiac structural or valvular abnormalities were observed after treatment with TGFBRII-Fc.
Conclusions: Our findings are consistent with a pathogenetic role of TGF-β1/3, demonstrating the efficacy and tolerability of selective TGF-β ligand blockade for improving hemodynamics, remodeling, and survival in multiple experimental PH models.
Keywords: transforming growth factor-β, pulmonary artery, vascular smooth muscle cells, vascular remodeling, pulmonary hypertension
At a Glance Commentary
Scientific Knowledge on the Subject
Human and experimental pulmonary hypertension are characterized by an excess of transforming growth factor-β (TGF-β)–mediated signaling, and in particular, an imbalance between TGF-β and bone morphogenetic protein signaling. Previous work suggests that broad inhibition of TGF-β family signaling may be beneficial in pulmonary hypertension, but it is unclear if this strategy is generalizable to different etiologies of disease or if this strategy will be tolerable. We tested whether or not more selective inhibition of TGF-β signaling might be tolerable and effective in several distinct animal models of pulmonary hypertension.
What This Study Adds to the Field
We found that selective blockade of TGF-β1 and -β3 was helpful in reducing pulmonary hypertension and pulmonary vascular remodeling, and in improving survival, using three mechanistically distinct animal models, all of which exhibited signatures of excessive TGF-β signaling. These findings implicate TGF-β 1/3 ligands as drivers of disease, and further investigation of this strategy is warranted.
Pulmonary arterial hypertension (PAH) is a highly morbid condition characterized by elevated pulmonary vascular resistance and arterial pressures, driven by a progressive pulmonary vasculopathy that leads to right ventricular hypertrophy (RVH), and ultimately, RV failure and death (1, 2). In PAH, arteriolar remodeling is characterized by medial hypertrophy, neointimal and obstructive lesions consisting of proliferating myofibroblast and endothelial lineages, and multichanneled plexiform lesions that are pathognomonic of this disease. Idiopathic and hereditary forms of PAH are associated with heterozygous mutations in BMPR2 encoding the bone morphogenetic protein (BMP) type II receptor (BMPRII) (3, 4), a member of the transforming growth factor-β (TGF-β) signaling family that has essential functions in the physiologic homeostasis of the vascular endothelium and smooth muscle, as well as other tissues (5–8). The TGF-β family includes a structurally diverse set of more than 33 cytokines that regulate the differentiation, proliferation, migration, and survival of diverse cell types, and include among their members BMPs, activins, inhibins, growth and differentiation factors (GDFs), lefty, nodal, and anti-Mullerian hormone, as well as TGF-β isoforms 1, 2, and 3 (9–11).
Although PAH-associated mutations of BMPR2 result in loss of BMP signaling function, it has long been observed that lung tissues from human idiopathic PAH are marked by enhanced activity of the TGF-β pathway (12). Multiple animal models of pulmonary hypertension (PH), including those induced by hypoxia, monocrotaline (MCT)-induced injury, or infection with schistosomiasis, have shown similar evidence of elevated TGF-β ligand expression and downstream transcriptional activity (13–16). Enhanced TGF-β signaling in these models has been associated with PH accompanied by smooth muscle hypertrophy, perivascular fibrosis, and extracellular matrix remodeling, all of which can be ameliorated with pharmacologic inhibitors of the TGF-β type I receptor kinases ALK5 (13, 17); these are inhibitors that also inhibit the highly homologous receptors ALK4 and ALK7 and their cognate ligands. However, the therapeutic potential of this strategy has been limited by the observation that ALK4/5/7 inhibitors have caused cardiovascular toxicity in the form of hemorrhagic valve necrosis, as well as physeal hypertrophy and dysplasia in the femoral tibial joints of adolescent animals (18). It is unclear which of the individual ligands of the TGF-β family may be primarily responsible for these therapeutic or toxic effects, because ALK4, ALK5, and ALK7 collectively transduce the signals of nearly 20 ligands, including activins, GDFs, and nodal, as well as TGF-β1, 2, and 3. In the present study, we sought to determine the effects of selective TGF-β ligand blockade in pulmonary vascular remodeling using a recombinant TGFBRII-Fc extracellular domain fusion protein. This ligand trap binds TGF-β1 and β3, but does not bind TGF-β2, whose signaling requires coreceptor TGFBRIII, also known as betaglycan (19). We propose that this ligand trap strategy might ameliorate several aspects of pulmonary vascular remodeling and PH that are specifically caused by the activities of TGF-β1 and TGF-β3, without incurring the toxic liabilities due to the inhibition of TGF-β2. TGF-β2 is known to be unique among TGF-β ligands for its essential roles in cardiac valve morphogenesis, and regulating endothelial and epithelial-to-mesenchymal transition in a variety of tissues (20–28). Part of the present study has been previously reported in abstract form (29).
Methods
Reagents
Recombinant TGFBRII was expressed as a fusion protein with an IgG Fc domain (TGFBRII-Fc) in CHO cells and purified with two rounds of affinity column chromatography, similar to that previously described in another study (30). Additional information on reagents is provided in the online supplement.
Experimental PH Models
Adult male Sprague-Dawley rats (150–170 g) and male C57BL/6J mice (20–25 g) were purchased from Charles River Laboratory (Wilmington, MA). Experimental protocols were approved by Harvard Institutional Animal Care and Use Committee. Animals were housed at 24°C during a 12-hour light–dark cycle in which food and water were accessible ad libitum. In rats, PH was induced either by a single subcutaneous injection of MCT (40 mg/kg) followed by 3 to 5 weeks of normoxia, or a single subcutaneous injection of vascular endothelial growth factor receptor antagonist (SU5416, also known as SUGEN; 20 mg/kg) followed by 3 weeks of normobaric hypoxia (FiO2 = 10%), then followed by 3 weeks of normoxia. Mice received weekly subcutaneous injections of SU5416 (20 mg/kg) and were exposed to normobaric hypoxia (FiO2 = 10%) for 3 weeks. Mortality and the total number of animals studied are listed in Table E1 in the online supplement.
Prophylaxis in MCT-treated Rats
Twenty-four hours after administration of MCT, rats were randomized to receive TGFBRII-Fc or vehicle for 21 days. At Day 14, ventricular function and RV hypertrophy (RVH) were examined by echocardiography. At Day 21, rats were subjected to invasive hemodynamic measurements, and tissues were harvested.
Rescue in MCT-treated Rats
To test the ability of TGFBRII-Fc to reverse established PH, starting 18 days after MCT exposure, rats were randomized to receive TGFBRII-Fc or vehicle. Invasive hemodynamic measurements and tissue harvest were performed on Day 35.
Prophylaxis in SU5416/Hypoxia–treated Mice
Twenty-four hours after treatment with SU5416/hypoxia, mice were randomized to receive TGFBRII-Fc or vehicle. Invasive hemodynamic measurements and tissue harvest were performed on Day 21.
Rescue in SU5416/Hypoxia–treated Rats
Rats were treated with SU5416/hypoxia for 3 weeks. Rats were returned to normoxia and randomized to receive TGFBRII-Fc or vehicle for 3 weeks. Echocardiographic, invasive hemodynamic measurements, and tissue harvest were performed on Day 42. Additional details are provided in the online supplement.
Invasive Hemodynamic Measurements
Rats were anesthetized with pentobarbital (50 μg/kg i.p.) and intubated intratracheally for mechanical ventilation (tidal volume = 8 ml/kg, frequency = 80/min). Invasive hemodynamic measurements of RV pressures were obtained by cannulation via the RV apex for MCT experiments as described previously (31), or alternatively for SU5416/hypoxia experiments, by a minimally invasive closed chest approach using a curved tip 2F pressure transducer catheter (Millar, Houston, TX; SPR-513) inserted into the RV through the right internal jugular vein. Invasive hemodynamic measurements of RV pressure in anesthetized mice were performed using a 1.2-F pressure catheter (Transonic Scisense Inc., Ithaca, NY) via the internal jugular vein.
Statistical Analysis
Measurements and analysis of physiological parameters and vascular remodeling were performed in blinded fashion. Data are presented as mean ± SEM and compared between groups using the Student’s t test, with the Bonferroni correction for multiple tests, or analysis of variance as appropriate; P < 0.05 was considered statistically significant.
Results
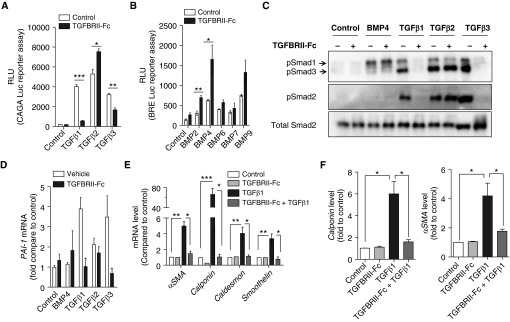
Recombinant TGFBRII-Fc selectively inhibited TGF-β1– and TGF-β3–mediated transcriptional activity, measured in HEK293 cells stably transfected with the CAGA-luciferase reporter (Figure 1A). TGFBRII-Fc failed to suppress, and in fact modestly enhanced, the activity of TGF-β2. TGFBRII-Fc did not inhibit BMP-mediated transcriptional activity, measured in C2C12 cells stably transfected with the BMP response element (BRE)-luciferase reporter, but enhanced the activity of several ligands in this assay (Figure 1B). These results were consistent with selective activity of this ligand trap against a subset of TGF-β ligands, and suggested a feedback mechanism in which suppression of TGF-β signaling resulted in enhanced BMP and TGF-β2 signaling. TGFBRII-Fc inhibited TGF-β1– and TGF-β3–induced phosphorylation of Smads 2/3 (Figure 1C and Figure E1 in the online supplement) and inhibited the increase in Pai-1 expression induced by TGF-β1 or TGF-β3 (Figure 1D) in human pulmonary artery smooth muscle cells, without affecting the activity of TGF-β2. TGF-β1 potently elicited the expression of smooth muscle contractility genes Caldesmon, Smoothelin, and Calponin in human pulmonary artery smooth muscle cells, all of which were inhibited by TGFBRII-Fc (Figures 1E and 1F). Taken together, TGFBRII-Fc acts as a potent antagonist of TGF-β1 and TGF-β3 signaling and abrogates the impact of these ligands on vascular smooth muscle cell plasticity in vitro.
Figure 1.
TGFBRII-Fc selectively inhibits transforming growth factor-β1 (TGF-β1)- and TGF-β3–induced signaling and blocks TGF-β–induced phenotypic modulation of smooth muscle cells. (A) HEK293 cells stably expressing a TGF-β–responsive CAGA-luciferase (Luc) reporter transgene and (B) C2C12 cells stably expressing a bone morphogenetic protein (BMP) response element reporter (BRE)–Luc reporter transgene were incubated in the presence or absence of TGFBRII-Fc (2 μg/ml) for 30 minutes before stimulation with various BMP ligands (10 ng/ml) or TGF-β1, -2, or -3 (1 ng/ml) overnight, revealing selective inhibition by TGFBRII-Fc of TGF-β1– and TGF-β3– but not TGF-β2–induced activation of CAGA-luciferase activity, and slightly increased BRE-luciferase activity in response to BMP2 and BMP4 in the presence of TGFBRII-Fc. Human pulmonary artery smooth muscle cells were deprived of serum overnight, pretreated with TGFBRII-Fc (2 μg/ml), followed by incubation with BMP4 (10 ng/ml), TGF-β1, -2, or -3 (1 ng/ml of each for 30 min), and analyzed by immunoblot for (C) phosphorylated Smads (pSmad) 1, 2, and 3 and (D) for mRNA expression of the TGF-β transcriptional target PAI-1 by quantitative reverse transcriptase polymerase chain reaction. TGFBRII-Fc inhibited SMAD2 and SMAD3 activation, CAGA-Luc reporter activity, and Pai-1 mRNA expression via TGF-β1 and TGF-β3, but not TGF-β2. After stimulation with TGF-β1, the relative levels of (E) mRNA and (F) protein expression of smooth muscle cell contractile markers were examined at 24 and 72 hours, respectively. Data are expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 as indicated. αSMA = α-smooth muscle actin; PAI-1 = plasminogen activator inhibitor 1; RLU = relative light units.
Rats treated with MCT developed progressive elevation of RV systolic pressures (RVSPs) (Figure E2a) and Fulton's ratio, which was consistent with RVH (Figure E2b) and evolved in a time-dependent fashion over 3 weeks after MCT treatment. In the lung tissues of MCT rats, we observed that the development of PH over 3 weeks was associated with progressively diminished expression of Bmpr2, progressively diminished BMP-mediated signaling, and gene transcription based on the expression of Id1, accompanied with a marked increase in TGF-β–mediated signaling and transcription based on the expression of Pai-1 (Figures E2c–E2f) (32). Disease severity, measured by RVSP and Fulton’s ratio, were positively correlated with TGF-β signaling activity, based on Tgfb1 and Pai-1 expression, and negatively correlated with Bmpr2 expression and downstream transcriptional activity based on Id1 (Figure E3).
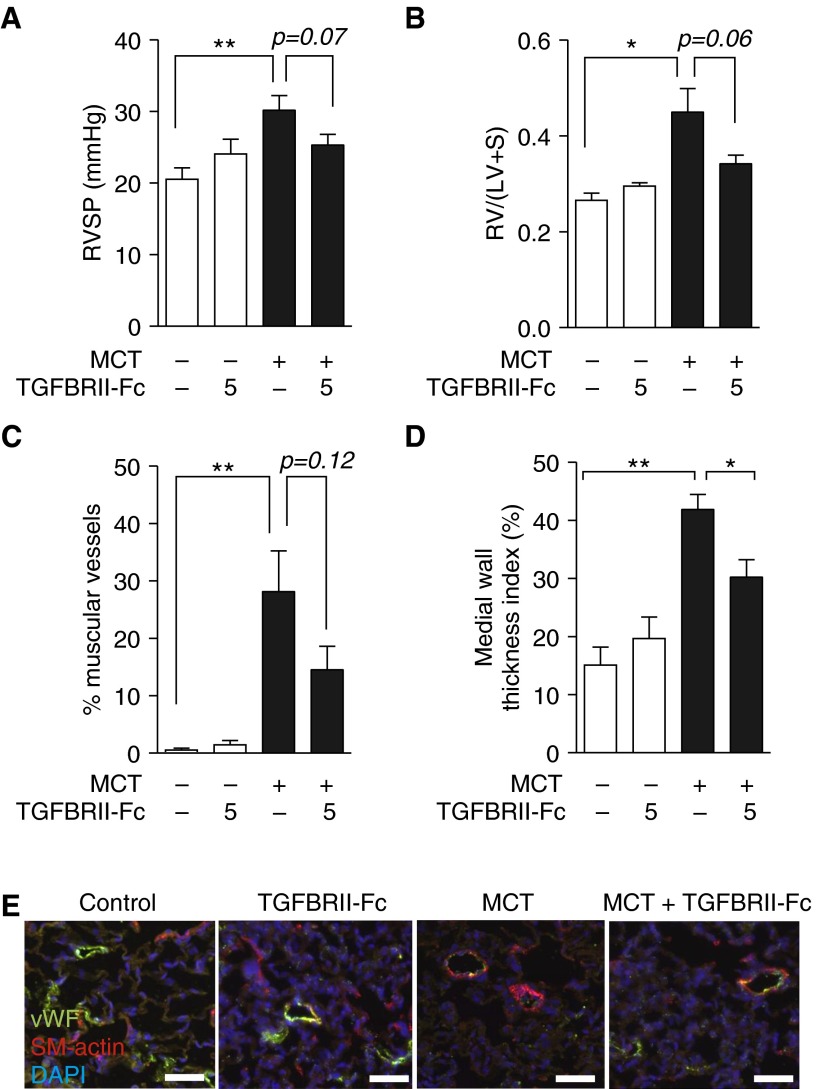
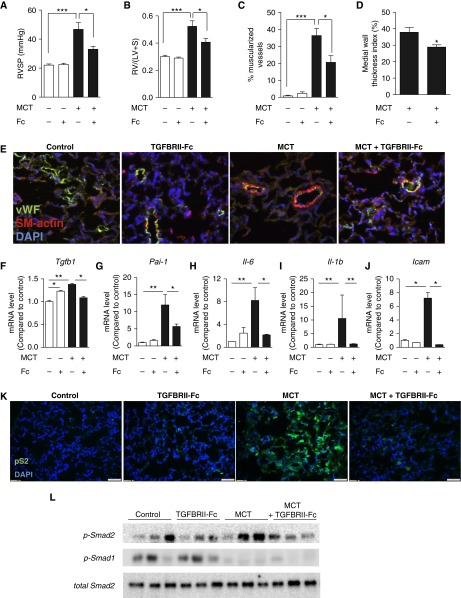
We tested the hypothesis that TGFBRII-Fc could inhibit TGF-β1/3 signaling activity in vivo to modulate disease progression in experimental PH. Treatment with a low dose of TGFBRII-Fc (5 mg/kg i.p., twice weekly, starting 1 day after MCT injection) resulted in a trend toward decreased RVSP, RVH, and muscularization of small pulmonary vessels, and significantly decreased medial wall thickness index after MCT treatment (Figure 2). Testing whether or not there might be a dose-dependent effect, treatment with higher doses of TGFBRII-Fc (15 mg/kg i.p., twice weekly, starting 1 day after MCT injection) was found to significantly alter RVSP (33.0 ± 2.1 vs. 46.7 ± 4.7 mm Hg in vehicle-treated control animals) (Figure 3A) and RVH (0.41 ± 0.02 vs. 0.52 ± 0.04 in vehicle-treated control animals) (Figure 3B). TGFBRII-Fc treatment under this regimen effectively prevented vascular remodeling in MCT rats (Figures 3C–3E). Whole-lung tissues of MCT rats that received TGFBRII-Fc exhibited decreased mRNA levels of Pai-1 and unexpectedly exhibited reduced levels of Tgfb1 (Figures 3F and 3G). The previously observed reduction in Bmpr2 and Id1 expression observed in MCT rats was unaffected by TGFBRII-Fc treatment (Figure E4). TGFBRII-Fc treatment also reduced the expression of Il6 (Figure 3H), Il1b (Figure 3I), and intercellular adhesion molecule (Icam) (Figure 3J) mRNA levels in the lungs of MCT-treated rats. Consistent with enhanced TGF-β signaling activity, MCT-induced PH was associated with increased expression of phosphorylated Smad2 as detected by immunohistochemistry, primarily in the nuclei of vascular and perivascular cells, whereas treatment with TGFBRII-Fc attenuated this increase in phosphorylated Smad2 (Figure 3K). These data were confirmed by Western blot (Figure 3L and Figure E4e). MCT treatment robustly decreased the expression of phosphorylated Smad1/5/8 in lung extracts. However, in contrast to the reflexive increases in BMP signaling observed in vitro, there were no gains in the phosphorylation of Smad1/5/8 in the lung tissues of MCT rats that received TGFBRII-Fc.
Figure 2.
Lower-dose treatment with TGFBRII-Fc elicits trends toward improved pulmonary hypertension, right ventricular hypertension (RVH), and pulmonary vascular remodeling. (A) Three weeks after treatment with monocrotaline (MCT) with or without TGFBRII-Fc (5 mg/kg, twice weekly i.p.), rats were analyzed in a blinded fashion to determine right ventricular systolic pressure (RVSP). (B) The degree of RVH was assessed based on measurement of Fulton’s ratio [RV/(LV + S)]. (C) Muscularization of distal intraacinar vessels (10–50 μm diameter) was quantified, and the percentages of fully (circumferentially) muscularized vessels were calculated. (D) Medial wall thickness was calculated for all fully muscularized intraacinar vessels (10–50 μm diameter). Wall thickness index was calculated as index = (external diameter – internal diameter)/external diameter × 100. TGFBRII-Fc treatment under this dosing regimen trended toward decreased frequency of muscularized vessels, and significantly reduced medial wall thickness, based on 100 to 150 vessels per treatment group from 6 to 8 rats each. *P < 0.05 or **P < 0.01 versus control animals or otherwise as shown. (E) Immunofluorescence of lung sections for von Willebrand’s factor (vWF) to mark vessels and smooth muscle actin (SM-actin) revealed qualitatively increased muscularization of small (<50 μm) arterioles following MCT treatment, which was reduced by the administration of TGFBRII-Fc (scale bars = 50 μm). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Data are shown as mean ± SEM (n = 6–8). *P < 0.05 and **P < 0.01 as indicated. LV = left ventricle; RV = right ventricle; S = septum.
Figure 3.
Higher-dose treatment with TGFBRII-Fc prevented pulmonary hypertension and vascular remodeling. Adult rats treated with monocrotaline (MCT) (40 mg/kg s.c. × 1) were administered TGFBRII-Fc (15 mg/kg i.p. twice weekly, starting 1 day after MCT injection) or vehicle for 3 weeks. Treatment with TGFBRII-Fc significantly attenuated (A) right ventricular systolic pressure (RVSP) and (B) right ventricular hypertension in comparison to vehicle (n = 6–8). (C and D) TGBRII-Fc decreased the percentage of fully muscularized vessels (10–50 μm diameter) (C) and medial wall thickness, calculated as (external diameter – internal diameter)/external diameter × 100 (D), based on 89 to 127 vessels per treatment group from 6 to 8 rats each. (E) TGFBRII-Fc treatment reduced muscularization evident by smooth muscle actin staining of von Willebrand (vWF)+ small vessels. (F–J) TGFBRII-Fc treatment normalized the expression of Tgfb1(F), Pai-1 (G), Il-6 (H), Il1b (I), and Icam (J) in the lung tissues of MCT-treated rats (n = 3–5). (K) Immunohistochemistry of lung sections and (L) immunoblotting of whole-lung lysates demonstrated qualitatively increased p-Smad2 (pS2) expression in the lungs of MCT-treated animals, which was normalized by treatment with TGFBRII-Fc. Conversely, treatment with MCT robustly decreased expression of p-Smad1/5/8, which was not significantly affected by treatment with TGFBRII-Fc. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Data are expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 as indicated. Scale bars = 50 μm. Icam = intercellular adhesion molecule; i.p. = intraperitoneal; LV = left ventricle; Pai-1 = plasminogen activator inhibitor 1; RV = right ventricle; S = septum; s.c. = subcutaneous; Tgfb1 = transforming growth factor-β1.
Using echocardiography to provide a complementary measurement of RVH, treatment with TGFBRII-Fc was found to attenuate the increase in RV free-wall thickness observed in MCT rats at Day 14 (0.77 ± 0.15 vs. 0.59 ± 0.11 mm; P < 0.05) (Table E2). However, the decreased pulmonary acceleration time (PAT) observed in MCT rats (19.8 ± 2.6 vs. 32.6 ± 2.2 ms; P < 0.05) (Table E2) was not significantly affected by TGFBRII-Fc treatment. Importantly, echocardiographic M-mode, pulsed-wave Doppler, and two-dimensional color Doppler assessment did not reveal evidence of valvular dysfunction or degeneration due to TGFBRII-Fc administration in normal or MCT rats (Table E2). Similarly, TGFBRII-Fc treatment was not associated with morphological changes in mitral valve structure, with no evidence of sclerotic or degenerative remodeling in sectioned valve tissues in rats (Figure E5). TGFBRII-Fc treatment did not significantly affect body weight in rats (Figure E6).
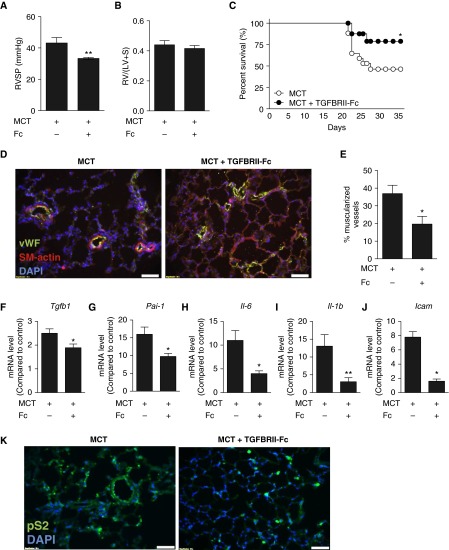
To assess whether or not modulation of TGF-β signaling might affect the progression of established PH, we tested the impact of TGFBRII-Fc (15 mg/kg i.p., three times per week) starting at 18 days after MCT exposure by assessing hemodynamics and pulmonary arteriolar remodeling at 35 days. When administered in this delayed fashion, TGFBRII-Fc significantly reduced RVSP (33.3 ± 0.8 vs. 43.2 ± 3.5 mm Hg in vehicle-treated control animals; P < 0.01) and attenuated arteriolar muscularization (P < 0.05) in MCT rats (Figure 4). Consistent with its impact on hemodynamics and remodeling, delayed treatment with TGFBRII-Fc improved survival compared with the vehicle (Figure 4C). In addition to reducing the mRNA expression of Tgfb1 and Pai-1 (Figures 4F–4J), delayed administration of TGFBRII-Fc significantly decreased the expression of Il6, Il1b, and Icam in the lungs of MCT rats, again suggesting an antiinflammatory effect of this treatment. As in previous experiments, delayed treatment with TGFBRII-Fc in MCT rats decreased levels of phosphorylated Smad2 (Figure 4K).
Figure 4.
Treatment with TGFBRII-Fc after establishment of pulmonary hypertension (PH) is associated with partial rescue of PH and mortality. After monocrotaline (MCT) treatment, rats were administered TGFBRII-Fc (15 mg/kg three times weekly) in a delayed fashion starting on Day 17, after the establishment of PH. Among surviving animals at 35 days, there was (A) significantly decreased right ventricular systolic pressure (RVSP) with TGFBRII-Fc treatment, but (B) no significant difference in right ventricular hypertension. Values are shown as mean ± SEM (n = 8–11 per group). (C) Kaplan-Meier analysis revealed improved survival in the TGFBRII-Fc–treated group (n = 18 per group). (D and E) TGFBRII-Fc treatment attenuated pulmonary vascular remodeling in rats with established PH. (F–J) Delayed treatment with TGFBRII-Fc reduced the expression of Tgfb1 (F), Pai-1 (G), Il6 (H), Il1b (I), and Icam (J) in the lung tissues of MCT-treated rats (n = 3–5). (K) TGFBRII-Fc treatment reduced elevated phospho-Smad2 (pS2) levels in MCT lungs. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Data are shown as mean ± SEM. *P < 0.05; **P < 0.01 as indicated. Scale bars = 50 μm. Icam = intercellular adhesion molecule; LV = left ventricle; Pai-1 = plasminogen activator inhibitor 1; RV = right ventricle; S = septum; SM-actin = smooth muscle actin; Tgfb1 = transforming growth factor-β1; vWF = von Willebrand factor.
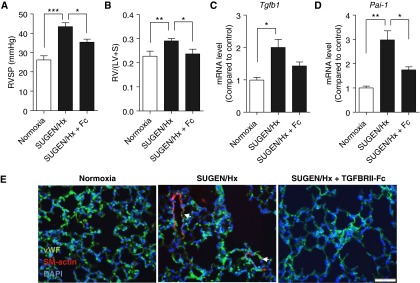
To explore the role of TGF-β–mediated signaling in a mechanistically distinct model of PH, we tested the effects of TGFBRII-Fc in the SU5416/hypoxia mouse model, in which mice were treated with vascular endothelial growth factor receptor inhibitor SU5416 and exposed to chronic hypoxia for 3 weeks. Combined treatment of SU5416 and hypoxia triggered more severe and consistent PH and RVH than that seen in mice exposed to hypoxia alone (see Figures E8a and E8b in the online supplement). Prophylactic treatment with TGFBRII-Fc significantly reduced RVSP (35 ± 6 mm Hg vs. 43 ± 6 mm Hg in vehicle-treated controls animals; P < 0.05) (Figure 5A) and RVH (0.24 ± 0.02 mm vs. 0.29 ± 0.01 mm in vehicle-treated control animals; P < 0.05) (Figure 5B) in SU5416/hypoxia mice. TGFBRII-Fc treatment decreased pulmonary arteriolar muscularization in the lungs of SU5416/hypoxia mice (Figure 5E). Administration of TGFBRII-Fc elicited a trend toward decreased expression of Tgfb1 (Figure 5C) and significantly reduced Pai-1 mRNA expression in the lungs of SU5416/hypoxia mice (Figure 5D). TGFBRII-Fc treatment qualitatively reduced the frequency of Ki67-positive cells in the lungs of SU5416/hypoxia mice, which was seen primarily in the adventitial, airway epithelial, and alveolar cells in vehicle-treated mice (see Figure E8c in the online supplement).
Figure 5.
Efficacy of TGFBRII-Fc in a murine model of pulmonary hypertension. Adult male mice were treated with SU5416 (SUGEN) and exposed to hypoxia for 3 weeks. (A and B) TGFBRII-Fc treatment (15 mg/kg, three times per week) reduced right ventricular systolic pressure (RVSP) (A) and prevented right ventricular hypertrophy (B) compared with vehicle-treated mice. (C and D) TGFBRII-Fc treatment trended toward reduced Tgfb1 (C) and prevented the up-regulation of Pai-1 (D) mRNA levels in lungs of SU5416/hypoxia–treated mice. (E) TGFBRII-Fc treatment ameliorated pulmonary vascular remodeling. Data are expressed as mean ± SEM (n = 6–8). *P < 0.05; **P < 0.01; ***P < 0.001 as indicated. Scale bars = 50 μm. DAPI = 4′,6-diamidino-2-phenylindole; Hx = hypoxia; LV = left ventricle; Pai-1 = plasminogen activator inhibitor 1; RV = right ventricle; S = septum; SM-actin = smooth muscle actin; Tgfb1 = transforming growth factor-β1; vWF = von Willebrand factor.
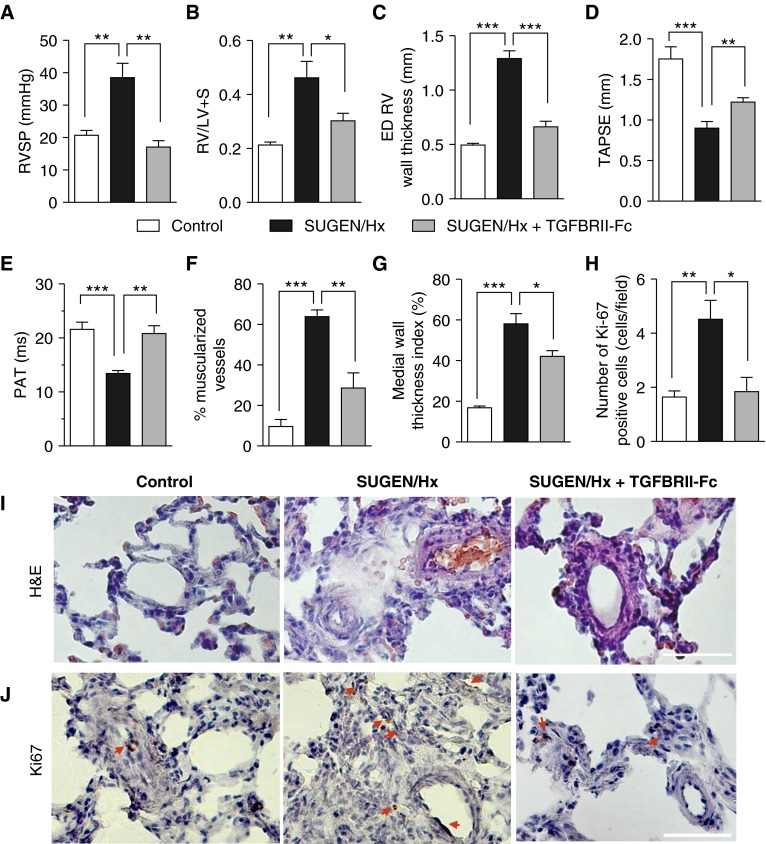
Finally, we tested the efficacy of TGFBRII-Fc in ameliorating PH in an SU5416/hypoxia–induced rat model of PH, which was shown previously to closely recapitulate vascular remodeling features observed in patients with severe PAH (33). Rats were treated with SU5416 during 3 weeks of chronic normobaric hypoxia (FiO2 = 10%), after which they were returned to normoxia for 3 weeks, and randomized to receive either TGFBRII-Fc or vehicle. At the end of the 6-week period, SU5416/hypoxia–treated rats that received vehicle had significantly higher RVSP (38.5 ± 4.4 mm Hg vs. 20.8 ± 1.4 mm Hg; P < 0.01) (Figure 6) and a significantly shorter PAT (13.4 ± 0.5 ms vs. 21.6 ± 1.3 ms in vehicle-treated control animals; P = 0.001; Figure 6 and Figure E9 in the online supplement). TGFBRII-Fc–treated animals exhibited significant improvement in PH demonstrated by a normalization of RVSP (17.2 ± 1.9 mm Hg; P < 0.01 vs. SU5416/hypoxia–treated animals) (Figure 6) and PAT (20.8 ± 1.4 ms; P < 0.01 vs. SU5416/hypoxia–treated animals) (Figure 6 and Figure E9 in the online supplement). SU5416/hypoxia–treated rats exhibited severe RVH based on an increased ratio of the weight of the right ventricle to that of the left ventricle and septum [RV/(LV + S)] (0.46 ± 0.1 vs. 0.21 ± 0.1; P < 0.01) and increased RV end-diastolic wall thickness by echocardiography (1.3 ± 0.1 vs. 0.5 ± 0.02 mm in vehicle-treated control animals; P < 0.001; Figure 6 and Figure E10). These rats developed RV dysfunction as demonstrated by decreased tricuspid annular plane systolic excursion, a noninvasive echocardiographic surrogate metric of RV function (0.9 ± 0.1 mm vs. 1.8 ± 0.2 mm in vehicle-treated control animals; P = 0.001; Figure 6D). TGFBRII-Fc–treated rats exhibited improved RVH on the basis of decreased RV/(LV + S) (0.30 ± 0.03; P < 0.05 vs. SU5416/hypoxia–treated rats; Figure 6) and decreased RV end-diastolic wall thickness by echocardiography (0.7 ± 0.1 mm; P < 0.001 vs. SU5416/hypoxia–treated rats; Figure 6 and Figure E10 in the online supplement), as well as improved RV function demonstrated by increased tricuspid annular plane systolic excursion (1.2 ± 0.1 mm; P = 0.01 vs. SU5416/hypoxia–treated rats; Figure 6D). In addition, TGFBRII-Fc treatment markedly attenuated vascular remodeling based on muscularization and medial wall thickness (Figures 6F, 6G, and 6I), reduced vascular cell proliferation based on the frequency of Ki67-positive cells in the adventitia and intima (Figures 6H and J), and attenuated RV fibrosis in SU5416/hypoxia–treated rats (Figure S11). Consistent with the observed improvements in PH, RVH, and PAT, there was an observed trend toward improved survival among TGFBRII-Fc–treated rats (Figure E12).
Figure 6.
Treatment with TGFBRII-Fc reverses established pulmonary hypertension in SU5416 (SUGEN)/hypoxia–treated rats. Male adult Sprague-Dawley rats received a single subcutaneous injection of SU5416 and were subjected to normobaric hypoxia (FiO2 = 0.10) for 3 weeks, followed by maintenance in normoxia for 3 weeks, during which rats were randomized to receive either vehicle or TGFBRII-Fc (15 mg/kg, three times per week). (A–C) TGFBRII-Fc treatment reduced right ventricular systolic pressure (RVSP) (A), attenuated right ventricular hypertension (B), and reduced echocardiographic end-diastolic (ED) right ventricular free wall thickness (C) compared with vehicle-treated rats. (D and E) TGFBRII-Fc treatment improved echocardiographic measures of right ventricular function via tricuspid annular plane systolic excursion (TAPSE) (D) and of pulmonary hypertension via pulmonary acceleration time (PAT) (E) in SU5416/hypoxia–treated rats. There were no differences in pulmonary ejection time among various experimental groups (data not shown). (F, G, and I) TGBRII-Fc decreased the percentage of fully muscularized vessels (10–50 μm diameter) and medial wall thickness, calculated as (external diameter – internal diameter)/external diameter × 100, based on 50 to 85 vessels per treatment group from 5 to 6 rats each. (H and J) TGFBRII-Fc also reduced the frequency of Ki67-positive cells in the intima and adventitia (indicated by red arrows) seen in SU5416/hypoxia–treated rats. Data are expressed as mean ± SEM (n = 5–6). *P < 0.05; **P < 0.01; ***P < 0.001 as indicated. Scale bars = 50 μm. H&E = hematoxylin and eosin; Hx = hypoxia; LV = left ventricle; RV = right ventricle; S = septum.
Discussion
Based on the known importance of TGF-β signaling in pulmonary vascular disease, and the ability of recombinant ligand traps to modulate the activity of selected ligands in vivo, we tested whether or not a selective TGF-β ligand trap could affect experimental PH and pulmonary vascular remodeling. We characterized recombinant TGFBRII-Fc fusion protein as a potent inhibitor of TGF-β1 and TGF-β3 signaling that did not affect the signaling of TGF-β2, which is an important regulator of endothelial-to-mesenchymal transition, extracellular matrix remodeling, and cardiac valve formation (24, 26). Consistent with this activity, TGFBRII-Fc blocked canonical Smad 2/3 signaling via TGF-β1 and TGF-β3, but did not block TGF-β2, and prevented TGF-β1–mediated switching of pulmonary artery smooth muscle cells from a synthetic to a contractile phenotype in vitro (34, 35). Hypothesizing that TGF-β1 and/or TGF-β3 contribute to pulmonary vascular remodeling seen in PH, we found that TGFBRII-Fc treatment corrected excessive TGF-β signaling activity and thereby attenuated pulmonary vascular remodeling, pulmonary hypertension, and RV hypertrophy in three distinct models of experimental PH. TGFBRII-Fc not only prevented the progression of established PH and RVH in rats treated with MCT or SU5416/hypoxia, but also improved their survival. These present data are the first demonstration that selective inhibition of specific TGF-β ligands may attenuate PH, using multiple, mechanistically distinct experimental models of PH, by applying a strategy that appears to be more tolerable than nonselective approaches.
TGF-β family ligands orchestrate numerous processes during embryogenesis and play critical roles in the pathophysiologic remodeling seen in vascular and fibrotic diseases, roles that have generated tremendous interest in their blockade for therapy (36–41). Broad blockade of TGF-β signaling via small molecules IN-1333, SD-208, and SB-525334 was shown to attenuate MCT-induced PH in rats, purportedly by inhibiting TGF-β signaling via the activity of its type I receptor ALK5 (13, 17, 32). However, these molecules demonstrated potent activity for closely related type I receptors ALK4 and ALK7 (42–44), and would thus affect the activity of not only TGF-β1 and TGF-β3 but nearly 20 other ligands, including activins, GDFs, and nodal, whose functions may or may not be concordant with TGF-β in the vasculature, and many of which may serve important roles in tissue homeostasis. These potent ALK4/5/7 inhibitors appear to have a class-wide effect of causing hemorrhagic valve necrosis in animals (18), possibly due to their lack of selectivity, a property that has limited their deployment for human therapy. It was recently shown that a neutralizing monoclonal antibody (1D11) that recognizes TGF-β ligands TGF-β1, -β2, and -β3 was also able to attenuate PH in a MCT rats (1, 31). The use of a similar pan–TGF-β neutralizing antibody in humans, fresolimumab, although generally well-tolerated, has been associated with dose-related side effects, including skin rashes and lesions, epistaxis, gingival bleeding, and fatigue (36). Moreover, TGF-β2 is essential in cardiac valve morphogenesis, which is consistent with its central role in regulating endothelial- and epithelial-to-mesenchymal transition in a variety of tissues (20–28), and raising the possibility that its inhibition might contribute to the valvular toxicity seen with small molecule inhibitors of ALK4/5/7. Consistent with the concept that the TGF-β type II receptor does not transduce TGF-β2 signals in the absence of the type III co-receptor betaglycan (19, 45–47), soluble TGFBRII-Fc did not inhibit TGF-β2 or cause any observable changes in cardiac valve structure or function. In contrast to the effects of ALK4/5/7 inhibitors seen in toxicology studies (18), TGFBRII-Fc was well tolerated, even when higher doses were used in rescue studies with established PH. Although it cannot be ruled out that toxicity might be observed at higher or longer exposures, the doses used in this study were sufficient to inhibit TGF-β–induced Smad2 activation and Pai-1 expression in lung tissues.
A limitation of previous studies investigating the role of TGF-β in PH has been the reliance on MCT-induced PH in rats, a preclinical model known to exhibit a molecular signature of exaggerated TGF-β signaling and profibrotic transcriptional activity, suppression of BMPR2 expression, and downstream BMP signaling activity (32), with evidence of diffuse inflammation and lung injury (48), as were observed in the present study. Although this phenotype might highlight potential effects of TGF-β inhibition, these characteristics may or may not reflect the spectrum of human PAH disease. Other animal models including hypoxia-induced PH in rats (49) and PH associated with the administration of Schistosoma mansoni (16) were found to exhibit signatures of enhanced TGF-β expression. Consistent with a role of TGF-β in human PAH, elevated levels of TGF-β1 have been reported in the transpulmonary flux of individuals with PAH (50), as well as in the circulation and lungs of individuals with idiopathic PAH (51), and increased Smad2 activity was found in the lung tissues of individuals with heritable PAH (52). Similarly, functional polymorphisms regulating the expression of TGFB1 affect the age at diagnosis and penetrance of PAH associated with BMPR2 mutations, consistent with the concept that BMP and TGF-β signaling imbalance contributes to disease (52). In the mechanistically distinct SU5416/hypoxia–induced mouse model (53), we found evidence of increased TGF-β signaling activity that was responsive to TGFBRII-Fc with improved RVSPs and RVH. In SU5416/hypoxia–treated rats, we found that TGFBRII-Fc treatment reversed RVH and fibrosis, improved RV function, and normalized hemodynamic and echocardiographic measures of PH. Taken together, our current and previous data indicate that the pathogenetic role of TGF-β may be broadly generalizable across several experimental PH models and human PAH.
TGFBRII-Fc inhibited TGF-β1–induced smooth muscle phenotypic modulation in vitro, although its administration in animal models of PH blocked TGF-β–mediated transcription and the expression of inflammatory signaling molecules previously implicated in PH (54, 55). The effects of TGF-β signaling in PH have been attributed to recruitment of myogenic and fibrogenic transcriptional programs in vascular medial hypertrophy and medial extension, but the present findings suggest TGF-β may also potentiate inflammation in affected lungs. Unexpectedly, we observed that administration of TGFBRII-Fc decreased the expression of Tgfb1 mRNA itself when analyzed in severe stages of disease (Figures 3–5). Dampening of ligand expression by a TGF-β ligand trap suggests TGFBRII-Fc may disrupt positive feedback mechanisms of TGF-β signaling that may be intrinsic to the TGF-β pathway or to profibrotic or proinflammatory cascades triggered by TGF-β1. Positive feedback or signal amplification is a motif frequently observed in BMP/TGF-β signaling, and the present observations are reminiscent of the recent report that administration of exogenous BMP9 ligand stimulates the BMP pathway while simultaneously up-regulating its cognate receptor BMPRII in animal models of PH (56).
In summary, these data demonstrate direct contributions of TGF-β1/3 to PH and remodeling, showing that the receptor fusion protein TGFBRII-Fc acts as a selective ligand trap to attenuate vascular remodeling and hemodynamics, and improve survival in established disease. Antagonism of specific TGF-β ligands may be an effective and tolerable approach to mitigating PAH and other disease processes that arise from the profibrotic and promyogenic effects of TGF-β ligands.
Acknowledgments
Acknowledgment
The authors thank Drs. Kenneth Bloch and Nicholas Morrell for their critical feedback and advice in these studies.
Footnotes
Supported by U.S. National Institutes of Health (NIH) grants HL079943 and AR057374 (P.B.Y.) and T32HL007604 (I.N.); the Pulmonary Hypertension Association (P.B.Y.); a Leducq Foundation Transatlantic Network of Excellence Award (P.B.Y.); a Howard Hughes Medical Institute Early Career Physician-Scientist Award (P.B.Y.); and the John S. LaDue Fellowship at Harvard Medical School (I.N.).
Author Contributions: Conception and design: L.-M.Y., I.N., S.D.P.-F., R.S.P., R.K., and P.B.Y. Performing experiments: L.-M.Y., I.N., and S.D.P.-F. Analysis and interpretation: L.-M.Y., I.N., S.D.P.-F., and P.B.Y. Drafting paper for intellectual content: L.-M.Y., I.N., S.D.P.-F., R.K., and P.B.Y.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201510-1955OC on April 26, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Megalou AJ, Glava C, Oikonomidis DL, Vilaeti A, Agelaki MG, Baltogiannis GG, Papalois A, Vlahos AP, Kolettis TM. Transforming growth factor-β inhibition attenuates pulmonary arterial hypertension in rats. Int J Clin Exp Med. 2010;3:332–340. [PMC free article] [PubMed] [Google Scholar]
- 2.Strelau J, Schober A, Sullivan A, Schilling L, Unsicker K. Growth/differentiation factor-15 (GDF-15), a novel member of the TGF-beta superfamily, promotes survival of lesioned mesencephalic dopaminergic neurons in vitro and in vivo and is induced in neurons following cortical lesioning. J Neural Transm Suppl. 2003;(65):197–203. doi: 10.1007/978-3-7091-0643-3_12. [DOI] [PubMed] [Google Scholar]
- 3.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67:737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, III, Loyd JE, Nichols WC, Trembath RC International PPH Consortium. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 5.Waite KA, Eng C. From developmental disorder to heritable cancer: it’s all in the BMP/TGF-beta family. Nat Rev Genet. 2003;4:763–773. doi: 10.1038/nrg1178. [DOI] [PubMed] [Google Scholar]
- 6.Yu PB, Deng DY, Beppu H, Hong CC, Lai C, Hoyng SA, Kawai N, Bloch KD. Bone morphogenetic protein (BMP) type II receptor is required for BMP-mediated growth arrest and differentiation in pulmonary artery smooth muscle cells. J Biol Chem. 2008;283:3877–3888. doi: 10.1074/jbc.M706797200. [DOI] [PubMed] [Google Scholar]
- 7.Rudarakanchana N, Flanagan JA, Chen H, Upton PD, Machado R, Patel D, Trembath RC, Morrell NW. Functional analysis of bone morphogenetic protein type II receptor mutations underlying primary pulmonary hypertension. Hum Mol Genet. 2002;11:1517–1525. doi: 10.1093/hmg/11.13.1517. [DOI] [PubMed] [Google Scholar]
- 8.Yu PB, Beppu H, Kawai N, Li E, Bloch KD. Bone morphogenetic protein (BMP) type II receptor deletion reveals BMP ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J Biol Chem. 2005;280:24443–24450. doi: 10.1074/jbc.M502825200. [DOI] [PubMed] [Google Scholar]
- 9.Moon JS, Kim SH, Oh SH, Jeong YW, Kang JH, Park JC, Son HJ, Bae S, Park BI, Kim MS, et al. Relaxin augments BMP-2-induced osteoblast differentiation and bone formation. J Bone Miner Res. 2014;29:1586–1596. doi: 10.1002/jbmr.2197. [DOI] [PubMed] [Google Scholar]
- 10.Eggers KM, Kempf T, Lagerqvist B, Lindahl B, Olofsson S, Jantzen F, Peter T, Allhoff T, Siegbahn A, Venge P, et al. Growth-differentiation factor-15 for long-term risk prediction in patients stabilized after an episode of non-ST-segment-elevation acute coronary syndrome. Circ Cardiovasc Genet. 2010;3:88–96. doi: 10.1161/CIRCGENETICS.109.877456. [DOI] [PubMed] [Google Scholar]
- 11.Tuder RM, Davis LA, Graham BB. Targeting energetic metabolism: a new frontier in the pathogenesis and treatment of pulmonary hypertension. Am J Respir Crit Care Med. 2012;185:260–266. doi: 10.1164/rccm.201108-1536PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Botney MD, Bahadori L, Gold LI. Vascular remodeling in primary pulmonary hypertension. Potential role for transforming growth factor-beta. Am J Pathol. 1994;144:286–295. [PMC free article] [PubMed] [Google Scholar]
- 13.Zaiman AL, Podowski M, Medicherla S, Gordy K, Xu F, Zhen L, Shimoda LA, Neptune E, Higgins L, Murphy A, et al. Role of the TGF-beta/Alk5 signaling pathway in monocrotaline-induced pulmonary hypertension. Am J Respir Crit Care Med. 2008;177:896–905. doi: 10.1164/rccm.200707-1083OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YF, Feng JA, Li P, Xing D, Zhang Y, Serra R, Ambalavanan N, Majid-Hassan E, Oparil S. Dominant negative mutation of the TGF-beta receptor blocks hypoxia-induced pulmonary vascular remodeling. J Appl Physiol (1985) 2006;100:564–571. doi: 10.1152/japplphysiol.00595.2005. [DOI] [PubMed] [Google Scholar]
- 15.Sheares KK, Jeffery TK, Long L, Yang X, Morrell NW. Differential effects of TGF-beta1 and BMP-4 on the hypoxic induction of cyclooxygenase-2 in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L919–L927. doi: 10.1152/ajplung.00012.2004. [DOI] [PubMed] [Google Scholar]
- 16.Graham BB, Chabon J, Gebreab L, Poole J, Debella E, Davis L, Tanaka T, Sanders L, Dropcho N, Bandeira A, et al. Transforming growth factor-β signaling promotes pulmonary hypertension caused by Schistosoma mansoni. Circulation. 2013;128:1354–1364. doi: 10.1161/CIRCULATIONAHA.113.003072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas M, Docx C, Holmes AM, Beach S, Duggan N, England K, Leblanc C, Lebret C, Schindler F, Raza F, et al. Activin-like kinase 5 (ALK5) mediates abnormal proliferation of vascular smooth muscle cells from patients with familial pulmonary arterial hypertension and is involved in the progression of experimental pulmonary arterial hypertension induced by monocrotaline. Am J Pathol. 2009;174:380–389. doi: 10.2353/ajpath.2009.080565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderton MJ, Mellor HR, Bell A, Sadler C, Pass M, Powell S, Steele SJ, Roberts RR, Heier A. Induction of heart valve lesions by small-molecule ALK5 inhibitors. Toxicol Pathol. 2011;39:916–924. doi: 10.1177/0192623311416259. [DOI] [PubMed] [Google Scholar]
- 19.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 20.Shin JA, Hong OK, Lee HJ, Jeon SY, Kim JW, Lee SH, Cho JH, Lee JM, Choi YH, Chang SA, et al. Transforming growth factor-β induces epithelial to mesenchymal transition and suppresses the proliferation and transdifferentiation of cultured human pancreatic duct cells. J Cell Biochem. 2011;112:179–188. doi: 10.1002/jcb.22929. [DOI] [PubMed] [Google Scholar]
- 21.Azhar M, Runyan RB, Gard C, Sanford LP, Miller ML, Andringa A, Pawlowski S, Rajan S, Doetschman T. Ligand-specific function of transforming growth factor beta in epithelial-mesenchymal transition in heart development. Dev Dyn. 2009;238:431–442. doi: 10.1002/dvdy.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camenisch TD, Molin DG, Person A, Runyan RB, Gittenberger-de Groot AC, McDonald JA, Klewer SE. Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev Biol. 2002;248:170–181. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- 23.Saito RA, Watabe T, Horiguchi K, Kohyama T, Saitoh M, Nagase T, Miyazono K. Thyroid transcription factor-1 inhibits transforming growth factor-beta-mediated epithelial-to-mesenchymal transition in lung adenocarcinoma cells. Cancer Res. 2009;69:2783–2791. doi: 10.1158/0008-5472.CAN-08-3490. [DOI] [PubMed] [Google Scholar]
- 24.Kokudo T, Suzuki Y, Yoshimatsu Y, Yamazaki T, Watabe T, Miyazono K. Snail is required for TGFbeta-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells. J Cell Sci. 2008;121:3317–3324. doi: 10.1242/jcs.028282. [DOI] [PubMed] [Google Scholar]
- 25.Townsend TA, Robinson JY, Deig CR, Hill CR, Misfeldt A, Blobe GC, Barnett JV. BMP-2 and TGFβ2 shared pathways regulate endocardial cell transformation. Cells Tissues Organs. 2011;194:1–12. doi: 10.1159/000322035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azhar M, Brown K, Gard C, Chen H, Rajan S, Elliott DA, Stevens MV, Camenisch TD, Conway SJ, Doetschman T. Transforming growth factor Beta2 is required for valve remodeling during heart development. Dev Dyn. 2011;240:2127–2141. doi: 10.1002/dvdy.22702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Townsend TA, Robinson JY, How T, DeLaughter DM, Blobe GC, Barnett JV. Endocardial cell epithelial-mesenchymal transformation requires Type III TGFβ receptor interaction with GIPC. Cell Signal. 2012;24:247–256. doi: 10.1016/j.cellsig.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yung LM, Paskin-Flerlage SD, Nikolic I, Pearsall RS, Kumar R, Yu PB.A selective transforming growth factor-β and growth differentiation factor-15 ligand trap attenuates pulmonary hypertension. Presented at the American Heart Association Scientific Sessions. November 7–11, 2014, Chicago, IL. p. A17285 [Google Scholar]
- 30.Mitchell D, Pobre EG, Mulivor AW, Grinberg AV, Castonguay R, Monnell TE, Solban N, Ucran JA, Pearsall RS, Underwood KW, et al. ALK1-Fc inhibits multiple mediators of angiogenesis and suppresses tumor growth. Mol Cancer Ther. 2010;9:379–388. doi: 10.1158/1535-7163.MCT-09-0650. [DOI] [PubMed] [Google Scholar]
- 31.Megalou AJ, Glava C, Vilaeti AD, Oikonomidis DL, Baltogiannis GG, Papalois A, Vlahos AP, Kolettis TM. Transforming growth factor-β inhibition and endothelin receptor blockade in rats with monocrotaline-induced pulmonary hypertension. Pulm Circ. 2012;2:461–469. doi: 10.4103/2045-8932.105034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long L, Crosby A, Yang X, Southwood M, Upton PD, Kim DK, Morrell NW. Altered bone morphogenetic protein and transforming growth factor-beta signaling in rat models of pulmonary hypertension: potential for activin receptor-like kinase-5 inhibition in prevention and progression of disease. Circulation. 2009;119:566–576. doi: 10.1161/CIRCULATIONAHA.108.821504. [DOI] [PubMed] [Google Scholar]
- 33.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation. 2010;121:2747–2754. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 34.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King KE, Iyemere VP, Weissberg PL, Shanahan CM. Krüppel-like factor 4 (KLF4/GKLF) is a target of bone morphogenetic proteins and transforming growth factor beta 1 in the regulation of vascular smooth muscle cell phenotype. J Biol Chem. 2003;278:11661–11669. doi: 10.1074/jbc.M211337200. [DOI] [PubMed] [Google Scholar]
- 36.Grafe I, Yang T, Alexander S, Homan EP, Lietman C, Jiang MM, Bertin T, Munivez E, Chen Y, Dawson B, et al. Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat Med. 2014;20:670–675. doi: 10.1038/nm.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, ap Rhys CM, Holm TM, Loeys BL, et al. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13:204–210. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JM, Mecham RP, Judge DP, et al. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest. 2004;114:1586–1592. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallo EM, Loch DC, Habashi JP, Calderon JF, Chen Y, Bedja D, van Erp C, Gerber EE, Parker SJ, Sauls K, et al. Angiotensin II-dependent TGF-β signaling contributes to Loeys-Dietz syndrome vascular pathogenesis. J Clin Invest. 2014;124:448–460. doi: 10.1172/JCI69666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu CF, Chiang WC, Lai CF, Chang FC, Chen YT, Chou YH, Wu TH, Linn GR, Ling H, Wu KD, et al. Transforming growth factor β-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am J Pathol. 2013;182:118–131. doi: 10.1016/j.ajpath.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ling H, Roux E, Hempel D, Tao J, Smith M, Lonning S, Zuk A, Arbeeny C, Ledbetter S. Transforming growth factor β neutralization ameliorates pre-existing hepatic fibrosis and reduces cholangiocarcinoma in thioacetamide-treated rats. PLoS One. 2013;8:e54499. doi: 10.1371/journal.pone.0054499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grygielko ET, Martin WM, Tweed C, Thornton P, Harling J, Brooks DP, Laping NJ. Inhibition of gene markers of fibrosis with a novel inhibitor of transforming growth factor-beta type I receptor kinase in puromycin-induced nephritis. J Pharmacol Exp Ther. 2005;313:943–951. doi: 10.1124/jpet.104.082099. [DOI] [PubMed] [Google Scholar]
- 43.Kim DK, Sheen YY, Jin CH, Park CY, Sreenu D, Rao KS, Krishnaiah M, Subrahmanyam VB.2-pyridyl substituted imidazoles as therapeutic alk5 and/or alk4 inhibitors. World Intellectual Property Organization. Patents C07D471/04, A61P25/28, A61P13/12, A61K31/437, A61P19/10, A61P11/00, C07D401/14, C07D413/14 ed. Ewha University-Industry Collaboration Foundation; 2011 [Google Scholar]
- 44.Loots GG, Keller H, Leupin O, Murugesh D, Collette NM, Genetos DC. TGF-β regulates sclerostin expression via the ECR5 enhancer. Bone. 2012;50:663–669. doi: 10.1016/j.bone.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esparza-Lopez J, Montiel JL, Vilchis-Landeros MM, Okadome T, Miyazono K, López-Casillas F. Ligand binding and functional properties of betaglycan, a co-receptor of the transforming growth factor-beta superfamily. Specialized binding regions for transforming growth factor-beta and inhibin A. J Biol Chem. 2001;276:14588–14596. doi: 10.1074/jbc.M008866200. [DOI] [PubMed] [Google Scholar]
- 46.Brown CB, Boyer AS, Runyan RB, Barnett JV. Requirement of type III TGF-beta receptor for endocardial cell transformation in the heart. Science. 1999;283:2080–2082. doi: 10.1126/science.283.5410.2080. [DOI] [PubMed] [Google Scholar]
- 47.Blobe GC, Schiemann WP, Pepin MC, Beauchemin M, Moustakas A, Lodish HF, O’Connor-McCourt MD. Functional roles for the cytoplasmic domain of the type III transforming growth factor beta receptor in regulating transforming growth factor beta signaling. J Biol Chem. 2001;276:24627–24637. doi: 10.1074/jbc.M100188200. [DOI] [PubMed] [Google Scholar]
- 48.Gomez-Arroyo JG, Farkas L, Alhussaini AA, Farkas D, Kraskauskas D, Voelkel NF, Bogaard HJ. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol. 2012;302:L363–L369. doi: 10.1152/ajplung.00212.2011. [DOI] [PubMed] [Google Scholar]
- 49.Wang XB, Wang W, Zhu XC, Ye WJ, Cai H, Wu PL, Huang XY, Wang LX. The potential of asiaticoside for TGF-β1/Smad signaling inhibition in prevention and progression of hypoxia-induced pulmonary hypertension. Life Sci. 2015;137:56–64. doi: 10.1016/j.lfs.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 50.Selimovic N, Bergh CH, Andersson B, Sakiniene E, Carlsten H, Rundqvist B. Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Eur Respir J. 2009;34:662–668. doi: 10.1183/09031936.00174908. [DOI] [PubMed] [Google Scholar]
- 51.Gore B, Izikki M, Mercier O, Dewachter L, Fadel E, Humbert M, Dartevelle P, Simonneau G, Naeije R, Lebrin F, et al. Key role of the endothelial TGF-β/ALK1/endoglin signaling pathway in humans and rodents pulmonary hypertension. PLoS One. 2014;9:e100310. doi: 10.1371/journal.pone.0100310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phillips JA, 3rd, Poling JS, Phillips CA, Stanton KC, Austin ED, Cogan JD, Wheeler L, Yu C, Newman JH, Dietz HC, Loyd JE. Synergistic heterozygosity for TGFbeta1 SNPs and BMPR2 mutations modulates the age at diagnosis and penetrance of familial pulmonary arterial hypertension. Genet Med. 2008;10:359–365. doi: 10.1097/GIM.0b013e318172dcdf. [DOI] [PubMed] [Google Scholar]
- 53.Ciuclan L, Bonneau O, Hussey M, Duggan N, Holmes AM, Good R, Stringer R, Jones P, Morrell NW, Jarai G, et al. A novel murine model of severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2011;184:1171–1182. doi: 10.1164/rccm.201103-0412OC. [DOI] [PubMed] [Google Scholar]
- 54.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. 2009;104:236–244. doi: 10.1161/CIRCRESAHA.108.182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, Trembath RC, Jennings S, Barker L, Nicklin P, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. 2010;122:920–927. doi: 10.1161/CIRCULATIONAHA.109.933762. [DOI] [PubMed] [Google Scholar]
- 56.Long L, Ormiston ML, Yang X, Southwood M, Gräf S, Machado RD, Mueller M, Kinzel B, Yung LM, Wilkinson JM, et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med. 2015;21:777–785. doi: 10.1038/nm.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]