Abstract
Rationale: Policy recommendations on contact investigation of HIV-seropositive patients with tuberculosis have changed several times. Current epidemiologic evidence informing these recommendations is considered low quality, and few large studies investigating the infectiousness of HIV-seropositive and -seronegative index cases have been performed in sub-Saharan Africa.
Objectives: We assessed the infectiousness of HIV-seropositive and -seronegative patients with tuberculosis to their household contacts and examined potential modifiers of this relationship.
Methods: Adults suffering from their first episode of pulmonary tuberculosis were identified in Kampala, Uganda. Field workers visited index households and enrolled consenting household contacts. Latent tuberculosis infection was measured through tuberculin skin testing, and relative risks were calculated using modified Poisson regression models. Standard assessments of interaction between latent tuberculosis infection, the HIV serostatus of index cases, and other variables were performed.
Measurements and Main Results: Latent tuberculosis infection was found in 577 of 878 (65.7%) and 717 of 974 (73.6%) household contacts of HIV-seropositive and -seronegative tuberculosis cases (relative risk, 0.89; 95% confidence interval, 0.82–0.97). On further stratification, cavitary lung disease (P < 0.0001 for interaction) and smear status (P = 0.02 for interaction) of tuberculosis cases modified the infectiousness of HIV-seropositive indexes. Cough duration of index cases did not display interaction (P = 0.499 for interaction).
Conclusions: This study suggests that HIV-seropositive tuberculosis cases may be less infectious than HIV-seronegative patients only when they are smear-negative or lack cavitary lung disease. These results may explain heterogeneity between prior studies and provide evidence suggesting that tuberculosis contact investigation should include HIV-seropositive index cases in high disease burden settings.
Keywords: Mycobacterium tuberculosis, household contacts, HIV, tuberculosis infection, infectiousness
At a Glance Commentary
Scientific Knowledge on the Subject
Several studies have compared latent Mycobacterium tuberculosis infection in contacts of HIV-seropositive and -seronegative tuberculosis cases and reported heterogeneous results. Few large studies have been reported from areas with a high burden of both HIV and tuberculosis.
What This Study Adds to the Field
In this large household contact study from urban Uganda, we found that HIV-seropositive tuberculosis cases were less infectious than HIV-seronegative patients only when they were smear-negative or did not have cavitary lung disease. These results may explain variability found in prior studies and may have implications for contact investigation in high-burden settings.
Transmission of Mycobacterium tuberculosis (tuberculosis [TB]) is driving the current global TB epidemic, especially in areas with a high burden of HIV, such as sub-Saharan Africa (1–4). Patients living with HIV have an increased risk of progressive primary disease and reactivation of TB (5, 6). Because of this risk, approximately 80% of new HIV coinfected TB cases occurring globally every year are in sub-Saharan Africa (7, 8). Moreover, in 2013, a total of 41% of patients with TB in Africa tested positive for HIV (8).
Despite the clear evidence that HIV increases the risk of TB disease and sustains a high prevalence of disease, it is less certain whether patients with TB with HIV are as efficient in transmitting new infection to contacts in their social networks. Because of this uncertainty, the role of active case finding among contacts of coinfected index cases has been contentious and policy recommendations have been inconsistent (9–11). In high-burden, low-income settings, current World Health Organization policy recommends that contact investigation be conducted when the index case is a person living with HIV. This recommendation is based on “very low-quality evidence” and further study is required to inform policy guidelines (10).
Several studies have compared latent TB infection in contacts of HIV-seropositive and -seronegative TB cases and reported heterogeneous results (11–15). Informative studies in the Dominican Republic (9) and Brazil (11) found reduced latent TB infection in household contacts of HIV-seropositive cases. In response to these findings, Espinal and colleagues (9) recommended that contact investigations in less developed countries should not be implemented in close contacts of coinfected patients. These studies, however, were unable to determine whether all or only a subset of HIV-seropositive TB cases were less infectious (16, 17). Moreover, these studies took place in settings with a low HIV burden compared with sub-Saharan Africa, where HIV prevalence is higher than 5% in most countries.
Through a large household contact study in urban Uganda, we evaluated latent TB infection rates in contacts of HIV-seropositive and -seronegative index cases and estimated rates of latent TB infection stratified by characteristics of index cases, household contacts, and the household environment. We also studied coprevalent and incident disease in these close contacts. Some of the results of this study have been previously reported in the form of an abstract (18).
Methods
Study Population and Setting
This was a prospective cohort study of household contacts of TB index cases. The study design has been described previously (5, 19, 20). Briefly, we identified newly diagnosed patients with TB greater than or equal to 18 years old from the National TB and Leprosy Program at Old Mulago Hospital in Kampala, Uganda from 1995 through 2006. Index cases were defined as the first eligible case of pulmonary TB in a household with one or more contacts. All index cases were microbiologically confirmed through a positive culture test.
Households with index cases were visited by trained field workers within 2 weeks of TB diagnosis. During this baseline visit, index cases were evaluated through a physical examination and medical history. Information was collected on age, sex, room where they sleep, cigarette smoking status, HIV serostatus, chest radiograph, and duration of cough. Extent of disease through radiographic imaging results was graded independently by an experienced clinician using the National TB Association classification system with subgroupings for cavitary and noncavitary disease (21). Sputum samples were also collected for laboratory testing of mycobacterial culture and microscopic assessment.
Household contacts of index cases were defined as any individual spending at least 7 consecutive days in the same household as the index case in the 3 months preceding diagnosis. Contacts were invited to participate; gave their written informed consent; and completed a baseline sociodemographic questionnaire and physical examination collecting data on age, sex, height, weight, cigarette smoking status, alcohol usage (yes or no), relationship to the index case (spouse, parent, child, sibling, or other), education level, past active TB, HIV status, and household characteristics (crowding, housing structure, ventilation, or smoke exposure). Nutritional status was assessed for each contact through body mass index (BMI) for adults greater than or equal to 18 years of age and through weight-for-age z scores for child contacts. Individuals were classified as underweight if their z score was less than −2 or a BMI less than 18.5, normal weight if z scores were between −2 and 2 or their BMI was greater than or equal to 18.5 and less than 25, and overweight if z scores were greater than 2 or BMI was greater than or equal to 25 (22). Bacillus Calmette-Guérin (BCG) vaccination was assessed through inspecting BCG scars and confirmed with medical records when possible.
Index cases and household contacts older than the age of 5 years were offered HIV testing with an enzyme-linked immunosorbent assay (Cambridge BiosScience, Worcester, MA). Parents gave informed consent for child contacts. Children younger than the age of 5 years were offered HIV testing if the mother was living with HIV. If the mother was negative then the child was also presumed to be negative. People living with HIV and children younger than 6 years without active TB were offered a 6-month course of isoniazid preventive therapy.
There were two outcomes at the baseline visit: latent TB infection or TB disease in the contacts. Latent TB infection was defined as positive if the skin induration reaction was greater than or equal to 10 mm in diameter (19, 23). To determine infection, a tuberculin skin test was performed by placing 0.1 ml of 5 tuberculin units of purified protein derivative (Tubersol, Connaught Laboratories, Limited, Toronto, Canada) on the volar surface of the left forearm of each participant using the Mantoux method. Two field workers independently read the diameter of induration within 48 to 72 hours using digital calipers to reduce digit preference. In a sensitivity analysis, the criteria for latent infection was varied depending on HIV status of the contact (see Table E1 in the online supplement).
Household contacts were also evaluated for coprevalent and incident disease. Coprevalent disease was defined as the identification of TB disease at the baseline visit or within 3 months of the initial evaluation. After the baseline evaluation, household contacts free of active TB were followed for up to 2 years and evaluated for incident disease. Incident disease was defined as a new case of TB disease occurring in a household contact after the first 3 months of observation.
Statistical Analytical Plan
The prevalence of latent TB infection and TB disease among household contacts were estimated using standard contingency tables and stratified by index case, household contact, and household environmental risk factors. We evaluated correlations among variables using polychoric correlation coefficients, which measure correlation between ordered levels where the latent trait can be considered continuous and normally distributed.
To reduce the pool of candidate risk factors for latent TB infection, we performed univariate item analysis on 1,800 contacts (93.1%) with complete data on all variables. We then built a multivariable regression model that included main effects and interaction terms. We added variables one at a time that were related to latent TB infection (P < 0.20). Based on a stratified analysis, we included two interaction terms that tested interaction between HIV status of the index case and sputum smear findings and cavitary disease. We used modified Poisson regression with robust standard error variance to conduct all model building. This regression model takes into account the clustering of household contacts (24) and allows for direct estimation of relative risk (RR) in observational studies (25, 26). Two-sided P values and 95% confidence intervals (CIs) were used to assess statistical significance in all models. A similar analysis was performed for coprevalent and incident TB disease.
We performed sensitivity analyses including repeating analysis with children less than 15 years old, defining latent TB infection as greater than or equal to 5-mm induration for all household contacts, and defining latent TB infection as greater than or equal to 5-mm induration for HIV-seropositive household contacts only (see online supplement).
Ethical Considerations
Institutional review boards at the Uganda National Council for Science and Technology, the Uganda National AIDS Research Subcommittee, Case Western University, and Makerere University approved this study. Informed consent was obtained for all index cases and household contacts. Parents or guardians of child contacts provided written consent in addition to verbal assent from the children.
Results
Demographic Characteristics
The study enrolled 503 index cases and their 1,941 household contacts. Eight contacts were excluded; two refused a tuberculin skin test and six did not have an index case with a known HIV test result. After exclusions, 915 (47.3%) household contacts of HIV-seropositive index cases and 1,018 (52.7%) household contacts of HIV-seronegative index cases were included.
These groups of contacts were similar with regard to most baseline sociodemographic and household characteristics (Table 1). There was a higher proportion of household contacts with HIV in the households of HIV-seropositive index cases when compared with household contacts of seronegative index cases (16.8% vs. 4.6%; P < 0.0001). HIV-seropositive index cases were younger (median, 27 vs. 32; P < 0.0001) and presented with a lower frequency of cavitary disease (45.6% vs. 63.9%; P < 0.0001) when compared with HIV-seronegative cases (see Table E3). Other characteristics of index cases were similar. Contacts of HIV-seropositive and -seronegative index cases were similar in regards to age, sex, alcohol and smoking use, BCG vaccination, closeness to the index case, and history of active TB. Differences were seen between household contacts of HIV-seropositive and -seronegative index cases in their relation to the index case (P < 0.01), household type (P < 0.01), and charcoal or fire smoke exposure (P < 0.01). Housing characteristics, such as number of household members and number of windows, were similar between contacts.
Table 1.
Demographic Characteristics of 1,933 Household Contacts of Tuberculosis Cases, Stratified by the HIV Serostatus of the Index Case
| Variable | Household Contacts of HIV-Seropositive Tuberculosis Cases [n (%)]* | Household Contacts of HIV-Seronegative Tuberculosis Cases [n (%)]* | All Contacts [n (%)]* | P Value† |
|---|---|---|---|---|
| Household contact characteristics | ||||
| N | 915 (47.3) | 1,018 (52.7) | 1,933 (100) | — |
| Median age, yr (IQR) | 11 (5–20) | 12 (5–22) | 12 (5–21) | 0.55 |
| Age group, yr | 0.05 | |||
| 0–4 | 185 (20.2) | 251 (24.7) | 436 (22.6) | |
| 5–14 | 368 (40.2) | 352 (34.6) | 720 (37.3) | |
| 15–24 | 182 (19.9) | 214 (21.0) | 396 (20.5) | |
| 25–34 | 102 (11.2) | 97 (9.5) | 199 (10.3) | |
| 35–44 | 44 (4.8) | 57 (5.6) | 101 (5.2) | |
| ≥45 | 34 (3.7) | 47 (4.6) | 81 (4.2) | |
| Sex | 0.51 | |||
| Male | 398 (43.5) | 455 (44.7) | 853 (44.5) | |
| Education level | 0.05 | |||
| None | 296 (32.4) | 365 (35.9) | 661 (34.2) | |
| Primary | 436 (47.7) | 428 (42.0) | 864 (44.7) | |
| Secondary or higher | 183 (20.0) | 225 (22.1) | 408 (21.1) | |
| Nutritional status‡ | 0.39 | |||
| Underweight | 25 (2.7) | 30 (3.0) | 55 (2.9) | |
| Normal | 813 (88.9) | 885 (86.9) | 1,698 (87.8) | |
| Overweight | 73 (8.0) | 93 (9.1) | 166 (8.6) | |
| Missing | 4 (0.4) | 10 (1.0) | 14 (0.7) | |
| BCG vaccinated§ | 0.97 | |||
| Yes | 643 (70.3) | 711 (69.8) | 1,354 (70.1) | |
| No | 236 (25.8) | 270 (26.5) | 506 (26.2) | |
| Unknown | 32 (3.5) | 33 (3.2) | 65 (3.4) | |
| Missing | 4 (0.4) | 4 (0.4) | 8 (0.4) | |
| Cigarette smoker | 0.60 | |||
| Yes | 54 (5.9) | 53 (5.2) | 107 (5.5) | |
| No | 657 (71.8) | 751 (73.8) | 1,408 (72.8) | |
| Missing | 204 (22.3) | 214 (21.0) | 418 (21.6) | |
| Relation to index case | <0.01 | |||
| Spouse | 129 (14.1) | 126 (12.4) | 255 (13.2) | |
| Parent | 24 (2.6) | 47 (4.6) | 71 (3.7) | |
| Child | 411 (44.9) | 405 (39.8) | 816 (42.2) | |
| Sibling | 78 (8.6) | 124 (12.2) | 202 (10.5) | |
| Other|| | 270 (29.6) | 312 (30.7) | 583 (30.1) | |
| Missing | 3 (0.3) | 4 (0.4) | 7 (0.4) | |
| Past active tuberculosis | 0.84 | |||
| Yes | 12 (1.3) | 14 (1.4) | 26 (1.4) | |
| No | 897 (98.0) | 995 (97.7) | 1,892 (97.9) | |
| Missing | 6 (0.7) | 9 (0.9) | 15 (0.8) | |
| Alcohol usage | 0.26 | |||
| Yes | 133 (14.5) | 123 (12.1) | 256 (13.2) | |
| No | 778 (85.0) | 889 (87.3) | 1,667 (86.2) | |
| Missing | 4 (0.4) | 6 (0.6) | 10 (0.5) | |
| HIV serostatus | <0.01 | |||
| Positive | 154 (16.8) | 47 (4.6) | 201 (10.4) | |
| Negative | 639 (69.8) | 814 (80.0) | 1,455 (75.2) | |
| Missing | 122 (13.3) | 157 (15.4) | 279 (14.4) | |
| Closeness to index case | 0.37 | |||
| Share bed | 155 (16.9) | 182 (17.9) | 337 (17.4) | |
| Share room, not bed | 404 (44.2) | 414 (40.7) | 818 (42.3) | |
| Different room | 336 (36.7) | 404 (39.7) | 740 (38.3) | |
| Missing | 20 (2.2) | 18 (1.8) | 38 (2.0) | |
| Tuberculosis infection¶ | ||||
| Latent tuberculosis infection | 577 (65.7) | 717 (73.6) | 1,294 (69.9) | 0.01 |
| Coprevalent tuberculosis | 37 (4.0) | 44 (4.3) | 81 (4.2) | 0.76 |
| All infection | 614 (67.1) | 761 (74.8) | 1,375 (71.1) | <0.01 |
| Index case characteristics | ||||
| Age group, yr | <0.01 | |||
| 18–29 | 290 (31.7) | 606 (59.5) | 896 (46.3) | |
| 30–39 | 401 (43.8) | 232 (22.8) | 633 (32.8) | |
| 40–49 | 176 (19.2) | 115 (11.3) | 291 (15.1) | |
| ≥50 | 48 (5.3) | 65 (6.4) | 113 (5.9) | |
| Sex | 0.22 | |||
| Male | 465 (50.8) | 546 (53.6) | 1,011 (52.3) | |
| Female | 450 (49.2) | 472 (46.4) | 922 (47.7) | |
| Cigarette smoker | 0.53 | |||
| Yes | 210 (23.0) | 212 (20.8) | 422 (21.8) | |
| No | 696 (76.1) | 796 (78.2) | 1,492 (77.2) | |
| Missing | 9 (1.0) | 10 (1.0) | 19 (1.0) | |
| Sputum smear status | <0.01 | |||
| Negative | 241 (26.3) | 205 (20.1) | 446 (23.1) | |
| Positive | 674 (73.7) | 813 (80.0) | 1,487 (77.0) | |
| Chest radiograph findings** | <0.01 | |||
| Normal | 52 (5.7) | 48 (4.7) | 100 (5.2) | |
| Minimal | 106 (11.6) | 71 (7.0) | 177 (9.2) | |
| Moderately advanced | 372 (40.7) | 269 (26.4) | 641 (33.2) | |
| Far advanced | 380 (41.5) | 609 (59.8) | 989 (51.2) | |
| Missing | 5 (0.6) | 21 (2.1) | 26 (1.4) | |
| Lung cavitation** | <0.01 | |||
| Cavitary disease | 422 (46.1) | 697 (68.5) | 1,119 (57.9) | |
| Noncavitary disease | 480 (52.5) | 283 (27.8) | 763 (39.5) | |
| Missing | 13 (1.4) | 38 (3.7) | 51 (2.6) | |
| Duration of cough | 0.15 | |||
| <30 d | 49 (5.4) | 69 (6.8) | 118 (6.1) | |
| ≥30 and <60 d | 201 (22.0) | 220 (21.6) | 421 (21.8) | |
| ≥60 and <90 d | 222 (24.3) | 215 (21.1) | 437 (22.6) | |
| ≥90 d | 421 (46.0) | 499 (49.0) | 920 (47.6) | |
| Missing | 22 (2.4) | 15 (1.5) | 37 (1.9) | |
| Household characteristics | ||||
| Housing type | <0.01 | |||
| Muzigo†† | 474 (51.8) | 412 (40.5) | 887 (45.8) | |
| Single-family household | 434 (47.4) | 600 (58.9) | 1,037 (54.5) | |
| Missing | 7 (0.8) | 6 (0.6) | 13 (0.7) | |
| Charcoal or fire smoke exposure | <0.01 | |||
| Inside household | 162 (17.7) | 246 (24.2) | 408 (21.1) | |
| Outside household | 680 (74.3) | 675 (66.3) | 1,355 (70.1) | |
| None | 60 (6.6) | 71 (7.0) | 131 (6.8) | |
| Missing | 13 (1.4) | 26 (2.6) | 39 (2.0) | |
| Ventilation, No. windows per room | 0.08 | |||
| >1 | 168 (18.4) | 229 (22.5) | 397 (20.5) | |
| ≤1 | 740 (80.9) | 783 (76.9) | 1,523 (78.8) | |
| Missing | 7 (0.8) | 6 (0.6) | 13 (0.7) | |
| Family size, No. in household | 0.26 | |||
| 1–5 | 405 (44.3) | 465 (45.7) | 870 (45.0) | |
| 6–10 | 408 (44.6) | 421 (41.4) | 829 (42.9) | |
| >10 | 102 (11.2) | 132 (13.0) | 234 (12.1) |
Definition of abbreviations: BCG = bacillus Calmette-Guérin; BMI = body mass index; IQR = interquartile range.
Percentages refer to within–characteristic column totals among contacts of HIV-seropositive and -seronegative tuberculosis index patients. Percentages may not total 100% because within-column percentages were rounded to the nearest integer.
We used Pearson chi-square tests to derive P value for all categorical variables. For continuous variables, we used Wilcoxon rank sum tests for comparison of two-sample medians.
Nutritional status was assessed for each contact through BMI measurements for adults greater than or equal to 18 years of age and through weight-for-age z scores for child contacts. Individuals were classified as underweight if their z score was less than −2 or a BMI less than 18.5, normal weight if z scores were between −2 and 2 or their BMI was greater than or equal to 18.5 and less than 25, and overweight if z scores were greater than 2 or BMI was greater than or equal to 25.
Evaluated through BCG scar, verified by medical records when available.
Includes other relatives, such as grandparents, grandchildren, aunts, uncles, and cousins. Also includes nonrelatives living in the household.
The denominator for “Latent tuberculosis infection” was household contacts without coprevalent disease (n = 1,852). The denominator for the “Coprevalent tuberculosis disease” row was all household contacts (n = 1,933). The “All infection” row included contacts with either latent tuberculosis infection or coprevalent disease; the denominator for this row includes all household contacts (n = 1,933). Latent tuberculosis infection was defined as a tuberculin skin test induration reaction greater than or equal to 10 mm. Coprevalent tuberculosis disease was defined as the identification of tuberculosis disease at or within 3 months of the baseline household visit.
Radiographic imaging results were graded by an experienced clinician using the 1961 National Tuberculosis Association classification system.
Muzigo households are defined as multifamily household units in the same building.
Risk of Latent TB Infection among Contacts
Overall, 1,294 (69.9%) household contacts had latent TB infection at the time of household evaluation. The proportion with latent TB infection was lower among contacts of HIV-seropositive TB cases compared with seronegative index cases (65.7% vs. 73.6%; RR, 0.89; 95% CI, 0.82–0.97) (Table 1).
In univariate analysis, the risk of latent TB infection was associated both with characteristics of household contacts and index cases. Older age, BCG vaccination, alcohol usage, and a closer relationship to the index case (spouse vs. children or siblings; shared a bed vs. living in a different room), were associated with latent TB infection among contacts (Table 2). In index cases, cavitary lesions, extent of lung disease, and HIV-seronegative status were each associated with increased proportion of latent TB infection in household contacts. Lastly, a contact in a household with one to five members was more likely to have latent TB infection than those in households with 6–10 or more than 10 members (Table 2).
Table 2.
Risk Factors for Latent Tuberculosis Infection among Household Contacts of Tuberculosis Cases*
| Variable | No. of Household Contacts | No. with Latent TB Infection (% Prevalence) | Univariable Model† (n = 1,852) |
|
|---|---|---|---|---|
| Relative Risk (95% CI) | P Value | |||
| N | 1,852 | 1,294 (69.9) | ||
| Household contact characteristics | ||||
| Age, yr (continuous) | 1,852 | — | 1.01 (1.01–1.01) | <0.01 |
| Age group, yr | ||||
| 0–4 | 395 | 233 (59.0) | 1 (Reference) | |
| 5–14 | 706 | 475 (67.3) | 1.14 (1.03–1.26) | 0.01 |
| 15–24 | 384 | 294 (76.6) | 1.30 (1.17–1.44) | <0.01 |
| 25–34 | 192 | 146 (76.0) | 1.29 (1.14–1.46) | <0.01 |
| 35–44 | 95 | 77 (81.1) | 1.37 (1.20–1.57) | <0.01 |
| ≥45 | 80 | 69 (86.3) | 1.46 (1.29–1.66) | <0.01 |
| Sex | ||||
| Male | 812 | 564 (69.5) | 1 (Reference) | |
| Female | 1,024 | 729 (71.2) | 1.02 (0.97–1.09) | 0.40 |
| Education level | ||||
| None | 606 | 374 (61.7) | 1 (Reference) | |
| Primary | 851 | 619 (72.7) | 1.18 (1.09–1.27) | <0.01 |
| Secondary or higher | 395 | 301 (76.2) | 1.23 (1.13–1.35) | <0.01 |
| Nutritional status‡ | ||||
| Underweight | 51 | 36 (70.6) | 1.02 (0.85–1.23) | 0.82 |
| Normal | 1,624 | 1,122 (69.1) | 1 (Reference) | |
| Overweight | 163 | 133 (81.6) | 1.18 (1.08–1.29) | <0.01 |
| BCG vaccinated§ | ||||
| No | 479 | 361 (75.4) | 1 (Reference) | |
| Yes | 1,301 | 888 (68.3) | 0.91 (0.85–0.97) | <0.01 |
| Unknown | 65 | 39 (60.0) | 0.80 (0.64–0.98) | 0.04 |
| Cigarette smoking | ||||
| No | 1,352 | 981 (72.6) | 1 (Reference) | |
| Yes | 102 | 80 (78.4) | 1.08 (0.97–1.20) | 0.16 |
| Relation to index case | ||||
| Spouse | 244 | 199 (81.6) | 1 (Reference) | |
| Parent | 67 | 57 (85.1) | 1.04 (0.93–1.17) | 0.47 |
| Child | 771 | 528 (68.5) | 0.84 (0.78–0.91) | <0.01 |
| Sibling | 198 | 142 (71.7) | 0.88 (0.78–0.99) | 0.04 |
| Other|| | 566 | 363 (64.1) | 0.79 (0.71–0.87) | <0.01 |
| Past active tuberculosis | ||||
| No | 1,815 | 1,266 (69.8) | 1 (Reference) | |
| Yes | 23 | 18 (76.3) | 1.12 (0.91–1.39) | 0.29 |
| Alcohol usage | ||||
| No | 1,594 | 1,077 (67.6) | 1 (Reference) | |
| Yes | 249 | 211 (84.7) | 1.25 (1.17–1.34) | <0.01 |
| HIV serostatus | ||||
| Negative | 1,397 | 987 (70.7) | 1 (Reference) | |
| Positive | 179 | 127 (71.0) | 1.00 (0.91–1.11) | 0.93 |
| Closeness to index case | ||||
| Share bed | 312 | 234 (75.0) | 1 (Reference) | |
| Share room but not bed | 783 | 551 (70.4) | 0.94 (0.87–1.01) | 0.10 |
| Different room | 722 | 485 (67.2) | 0.90 (0.82–0.98) | 0.02 |
| Index case characteristics | ||||
| Age group, yr | ||||
| 18–29 | 852 | 614 (72.1) | 1 (Reference) | |
| 30–39 | 601 | 404 (67.2) | 0.93 (0.85–1.03) | 0.16 |
| 40–49 | 287 | 202 (70.4) | 0.98 (0.86–1.11) | 0.71 |
| ≥50 | 112 | 74 (66.1) | 0.92 (0.77–1.09) | 0.33 |
| Sex | ||||
| Male | 968 | 692 (71.5) | 1 (Reference) | |
| Female | 884 | 602 (68.1) | 0.95 (0.88–1.04) | 0.26 |
| Cigarette smoker | ||||
| No | 1,431 | 991 (69.3) | 1 (Reference) | |
| Yes | 403 | 296 (73.5) | 1.06 (0.97–1. 16) | 0.20 |
| Sputum smear status | ||||
| Negative | 429 | 281 (65.5) | 1 (Reference) | |
| Positive | 1,423 | 1,013 (71.2) | 1.09 (0.98–1.21) | 0.12 |
| Chest radiograph findings¶ | ||||
| Normal | 100 | 50 (50.0) | 1 (Reference) | |
| Minimal | 177 | 97 (54.8) | 1.10 (0.82–1.46) | 0.53 |
| Moderately advanced | 641 | 425 (66.3) | 1.33 (1.03–1.72) | 0.03 |
| Far advanced | 989 | 760 (76.9) | 1.54 (1.19–1.98) | <0.01 |
| Lung cavitation¶ | ||||
| Noncavitary disease | 743 | 445 (59.9) | 1 (Reference) | |
| Cavitary disease | 1,060 | 812 (76.6) | 1.28 (1.17–1.40) | <0.01 |
| Duration of cough | ||||
| <30 d | 114 | 70 (61.4) | 1 (Reference) | |
| ≥30 and <60 d | 407 | 256 (62.9) | 1.02 (0.82–1.28) | 0.83 |
| ≥60 and <90 d | 420 | 293 (69.8) | 1.14 (0.91–1.42) | 0.26 |
| ≥90 d | 876 | 656 (74.9) | 1.22 (0.99–1.50) | 0.06 |
| HIV serostatus | ||||
| Negative | 974 | 717 (73.6) | 1 (Reference) | |
| Positive | 878 | 577 (65.7) | 0.89 (0.82–0.97) | 0.01 |
| Household characteristics | ||||
| Housing type | ||||
| Single-family household | 1,005 | 681 (67.8) | 1 (Reference) | |
| Muzigo** | 834 | 601 (72.1) | 1.06 (0.98–1.16) | 0.15 |
| Charcoal or fire smoke exposure | ||||
| Inside household | 398 | 284 (71.4) | 1 (Reference) | |
| Outside household | 1,289 | 886 (68.7) | 0.96 (0.88–1.06) | 0.44 |
| None | 127 | 94 (74.0) | 1.04 (0.87–1.23) | 0.68 |
| Ventilation, No. windows per room | ||||
| >1 | 385 | 251 (65.2) | 1 (Reference) | |
| ≤1 | 1,454 | 1,031 (70.9) | 1.09 (0.97–1.22) | 0.17 |
| Family size, No. in household | ||||
| 1–5 | 821 | 622 (75.8) | 1 (Reference) | |
| 6–10 | 803 | 535 (66.6) | 0.88 (0.81–0.95) | <0.01 |
| >10 | 228 | 137 (60.1) | 0.79 (0.67–0.94) | 0.01 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; BCG = bacillus Calmette-Guérin; TB = tuberculosis.
Column totals vary across different characteristics because of missing values for some participants. In this analysis, we included only household contacts without coprevalent disease, excluding 81 contacts. Latent tuberculosis infection was defined as an induration reaction greater than or equal to 10 mm in diameter.
The model uses a modified Poisson regression with robust error variance allowing for estimation of relative risks and adjustment for household clustering of contacts.
Nutritional status was assessed for each contact through BMI measurements for adults greater than or equal to 18 years of age and through weight-for-age z scores for child contacts. Individuals were classified as underweight if their z score was less than −2 or a BMI less than 18.5, normal weight if z scores were between −2 and 2 or their BMI was greater than or equal to 18.5 and less than 25, and overweight if z scores were greater than 2 or BMI was greater than or equal to 25.
Evaluated through BCG scar, verified by medical records when available.
Includes other relatives, such as grandparents, grandchildren, aunts, uncles, and cousins. Also includes nonrelatives living in the household.
Radiographic imaging and chest cavitation results were graded by an experienced clinician using the 1961 National Tuberculosis Association classification.
Muzigo households are defined as multifamily household units in the same building.
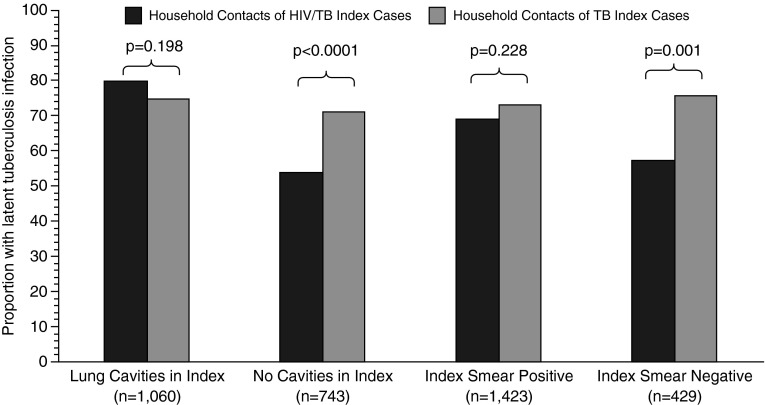
In a stratified analysis, interaction was present between HIV serostatus of the index case and the sputum smear result (P = 0.02 for interaction) and cavitary lesions (P < 0.01 for interaction); it modified the effect of HIV status in the index case on latent TB infection in contacts (Table 3, Figure 1). In an adjusted model, controlling for contact characteristics (age, education level, and alcohol use) and family size, the risk of latent TB infection was similar in contacts of HIV-seropositive cases and -seronegative cases when the index case had a positive sputum smear (RR, 0.93; 95% CI, 0.85–1.01). In contrast, the proportion of contacts with latent TB infection was lower in contacts of HIV-seropositive cases than -seronegative cases when the index case had a negative sputum smear (RR, 0.76; 95% CI, 0.64–0.90). When considering cavitary disease, the risk of latent TB infection was similar in contacts of HIV-seropositive cases and -seronegative cases when cavitary disease was present (RR, 1.03; 95% CI, 0.96–1.12), whereas the risk was reduced in contacts of HIV-seropositive cases when there was no cavitary disease (RR, 0.74; 95% CI, 0.65–0.85). No other variables, including cough duration of the index case (P = 0.4993 for interaction), interacted with latent TB infection and the HIV serostatus of the index case (see Table E2).
Table 3.
Interaction between a Latent Tuberculosis Infection, HIV Serostatus of the Index Case, and Variable Modifiers*
| Stratified by | Proportion with Latent Tuberculosis Infection (% Prevalence)† |
Stratum-Specific Crude RR‡ (95% CI) | P Value for Interaction | Stratum-Specific Adjusted RR‡§ (95% CI) | ||
|---|---|---|---|---|---|---|
| Household Contacts of HIV-Seropositive Tuberculosis Cases | Household Contacts of HIV-Seronegative Tuberculosis Cases | P Value for Interaction after Adjustment | ||||
| Sputum smear of index case | 0.02 | 0.03 | ||||
| Positive | 443/643 (68.9) | 570/780 (73.1) | 0.94 (0.86–1.04) | 0.93 (0.85–1.01) | ||
| Negative | 101/235 (57.0) | 147/194 (75.8) | 0.75 (0.63–0.90) | 0.76 (0.64–0.90) | ||
| Stratum-specific RR (95% CI) | 1.21 (1.02–1.43) | 0.96 (0.86–1.08) | ||||
| Lung cavitation of index case | <0.01 | <0.01 | ||||
| Cavitary disease | 314/394 (79.7) | 498/666 (74.8) | 1.07 (0.97–1.17) | 1.03 (0.96–1.12) | ||
| Noncavitary disease | 252/471 (53.5) | 193/272 (71.0) | 0.75 (0.65–0.87) | 0.74 (0.65–0.85) | ||
| Stratum-specific RR (95% CI) | 1.49 (1.30–1.71) | 1.05 (0.95–1.17) | ||||
Definition of abbreviations: CI = confidence interval; RR = relative risk.
All proportions are the number of individuals with a positive tuberculin skin test stratified by the HIV serostatus of the index case and the interaction factor in the left-hand column. All variables not included here were tested for interaction and are included in Table E2.
Column totals may vary across characteristics because of missing values for some participants.
The stratum-specific relative risk refers to the two proportions in that row. The reference category for each relative risk is the right-hand column: household contacts of HIV seronegative tuberculosis cases.
Adjusted for age, education level, and alcohol status of the household contact; sputum smear and cavitary status of the index case; and the number of individuals in the household. Age of household contacts and family size were added to the adjusted model as continuous variables.
Figure 1.
Prevalence of latent tuberculosis (TB) infection in household contacts after stratification of the HIV serostatus of the index case and either cavitary status (n = 1,803) or smear status (n = 1,852) of the index case. Black bars represent prevalence of latent TB infection in household contacts of TB index cases coinfected with HIV after stratification for either cavitary disease or sputum smear positivity of the index case. Gray bars represent the prevalence of latent TB infection in household contacts of HIV-seronegative TB index cases after stratification for cavitary disease or sputum smear positivity of the index case. P values represent association after adjustment for within-household clustering of contacts.
In this multivariable model, risk of latent TB infection remained associated with older age of contacts, alcohol use, and family size. Both age (RR, 1.00; 95% CI, 1.00–1.01) and alcohol use (RR, 1.12; 95% CI, 1.05–1.22) increased the risk for latent TB infection, whereas larger household size lowered the risk of latent TB infection (RR, 0.98; 95% CI, 0.97–0.99).
Risk of TB Disease among Household Contacts
HIV-seropositive household contacts were more likely to have coprevalent TB compared with those without HIV, particularly if the index case also had HIV (17/154, 11% vs. 19/639, 3%; RR, 3.71; 95% CI, 1.95–7.07) (Table 4). In addition, contacts with HIV were significantly more likely to develop incident TB than those without HIV, regardless of the HIV serostatus of the index case (Table 4). When considering all household contacts, we found no association between risk of coprevalent (4.0% vs. 4.3%; P = 0.76) or incident disease (1.8% vs. 1.9%; P = 0.97) and whether the index case had HIV (Table 4).
Table 4.
Coprevalent and Incident Tuberculosis Disease in Household Contacts of HIV-Seropositive and -Seronegative Tuberculosis Cases*
| Variable | Household Contacts of HIV-Seropositive Tuberculosis Cases | Household Contacts of HIV-Seronegative Tuberculosis Cases | Relative Risk (95% CI), P Value‡ |
|---|---|---|---|
|
Coprevalent Tuberculosis Disease† (% Prevalence) |
|||
| HIV serostatus of contact | |||
| HIV seropositive | 17/154 (11.0) | 5/47 (10.6) | 1.04 (0.41–2.63), 0.94 |
| HIV seronegative | 19/639 (3.0) | 37/814 (4.6) | 0.65 (0.37–1.14), 0.14 |
| Relative risk (95% CI), P value | 3.71 (1.95–7.07), <0.01 | 2.34 (0.93–5.90), 0.07 | |
| All HIV-tested household contacts | 36/793 (4.5) | 42/861 (4.9) | 0.93 (0.60–1.43), 0.74 |
| All household contacts§ | 37/915 (4.0) | 44/1,018 (4.3) | 0.94 (0.61–1.43), 0.76 |
| |
|||
|
Incident Tuberculosis Disease† (% Incidence) |
|||
| HIV serostatus of contact | |||
| HIV seropositive | 11/137 (8.0) | 5/42 (11.9) | 0.67 (0.25–1.79), 0.43 |
| HIV seronegative | 5/620 (0.8) | 13/777 (1.7) | 0.48 (0.17–1.33), 0.16 |
| Relative risk (95% CI), P value | 9.96 (3.55–27.89), <0.01 | 7.12 (2.73–18.55), <0.01 | |
| All HIV-tested household contacts | 16/757 (2.1) | 18/819 (2.2) | 0.96 (0.50–1.86), 0.91 |
| All household contacts§ | 16/878 (1.8) | 18/974 (1.9) | 0.99 (0.51–1.90), 0.97 |
Definition of abbreviation: CI = confidence interval.
All models use a modified Poisson regression with robust error variance allowing for estimation of relative risks and adjustment for household clustering of contacts. All proportions are individuals with a positive laboratory result stratified by the type of HIV serostatus of the index case and the contact.
Coprevalent tuberculosis disease was defined as the identification of tuberculosis disease at or within 3 months of the baseline household visit. Incident tuberculosis disease was defined as diagnosis of tuberculosis disease at subsequent household follow-up visits, conducted at 6-month intervals for 2 years. Individuals with coprevalent disease were excluded from analyses of incident disease.
The referent category for each relative risk in this column is the household contacts of HIV-seronegative tuberculosis cases.
Numbers may not add up to column totals of HIV-seropositive and -seronegative groups because some contacts did not take an HIV test and therefore are missing. All household contacts regardless of HIV serostatus or having taken an HIV test are included in this row.
Sensitivity Analysis
When we conducted several sensitivity analyses to assess the robustness of our findings we found consistent results (see Tables E4–E6). After restricting our study population to only children younger than 15 years of age, sputum smear status (P value for interaction = 0.04) and cavitary lung disease (P value for interaction = 0.002) remained statistically significant modifiers of latent TB infection and the HIV serostatus of the index case. When we varied our definition of latent TB infection our results again remained unchanged.
Discussion
In this high-burden, low-income setting with substantial ongoing TB transmission and a high burden of HIV, we found that the risk of latent TB infection among household contacts was modified by the HIV serostatus of TB index cases but the overall risk of TB disease among contacts was not. If we assume that latent TB infection among household contacts is caused by direct transmission from the index case, then we may infer that HIV infection of the index case does not affect infectiousness when the case presents with a positive sputum smear or cavitary disease, but HIV infection does seem to reduce infectiousness when the case presents with smear-negative disease or no lung cavitation. Furthermore, the risk of disease was the result of a complex interplay between the infectiousness of the index case, susceptibility of the contact, and environmental conditions.
Our findings have important implications for TB contact investigation, especially in areas where TB and HIV are endemic. The main purpose of contact investigation is to detect new cases of TB among contacts, as a form of active case finding (10, 27). Another reason for contact investigation is to identify contacts that have latent TB infection and may be at high risk for progressive primary disease (10, 27). As part of contact investigations, treatment of disease and latent infection are warranted.
When considering latent TB infection, HIV serostatus of the index case may be relevant in the evaluation of household contacts because of its effect on latent infection in contacts. Previous studies of the infectiousness of HIV-associated TB have shown diverse results (9, 12–15). In four studies (14, 15, 28, 29), the authors concluded that HIV infection of index cases did not affect infectiousness as determined by the prevalence of latent TB infection in contacts, whereas in three studies (9, 12, 30), contacts of HIV-seropositive TB cases were less likely to have latent TB infection than contacts of HIV-seronegative cases. The results of our study suggest that selection bias may account for some of the heterogeneity among these studies. Four studies that found no difference in infectiousness by HIV serostatus enrolled, by design, only index cases with sputum smear positive disease. Based on our findings, this result is expected because index cases with smear-negative disease were not included. In contrast, the studies that found a difference in infectiousness according to HIV serostatus enrolled a spectrum of cases including those with paucibacillary disease that may be less infectious.
We also found that contact age, alcohol use, and family size were associated with latent TB infection. Because the incubation period of TB can be months or years (31, 32), exposure events can accrue over time and individuals can be repeatedly infected. Our results, showing increasing prevalence of infection with age, are consistent with other studies from Uganda and elsewhere (33–35). Alcohol use increased risk of infection in our sample and has shown to be a risk factor for recent transmission in several studies (36). Reasons for this association are unclear but alcohol use may signify community-based social network mixing, thereby exposing contacts to high-risk of transmission in the community and household. Small family size likely indicates increased intimacy (and thus greater intensity of exposure) among household members and such a relationship with latent infection has been reported previously (28).
We found that the HIV serostatus of the index case is not relevant to identifying new TB disease cases among contacts. This may seem paradoxical at first, because HIV status does seem to reduce infectiousness in cases who present with paucibacillary disease. But the reduced infectiousness of paucibacillary, HIV-seropositive index cases in our study was counterbalanced by the fact that one in six of their contacts were also living with HIV, putting them at high risk for progressive primary disease. This correlation between HIV serostatus of TB index cases and their contacts may partially explain why coprevalent and incident TB disease was similar to HIV seronegative TB index cases. Indeed, in our study, HIV-seropositive contacts were two to four times more likely to have coprevalent TB disease and 7–10 times as likely to develop incident disease compared with HIV-seronegative contacts.
The interpretation of this study is subject to limitations. First, one cannot infer direct transmission of M. tuberculosis within a household from prevalence of latent infection. With appropriate information about strain types and community prevalence of latent TB infection, however, it is possible to estimate the secondary attack rate for disease and infection (5). Second, because we did not base our analysis on tuberculin skin test conversions, nondifferential misclassification of latent TB infection in household contacts is possible. To address this limitation, we performed sensitivity analysis among children younger than 15 years old and found similar results, indicating that this bias is likely minimal. Third, there may be unmeasured confounders that may affect our results. Although true of any study, we based our selection of risk factors for infection on our knowledge of working in this population for more than 20 years (5, 19, 20, 33).
During the study we did not assess level of immunosuppression among HIV-seropositive contacts or index cases. Because immunosuppression caused by HIV may lead to false-negative reactions to tuberculin skin tests, we may have underestimated the prevalence of latent infection among contacts of HIV-seropositive cases. Moreover, without CD4+ T-cell counts among index cases, we were not able to assess potential confounding of immunosuppression on transmission because atypical presentation of disease and lower smear grade are more likely when the CD4+ T-cell count is low (37, 38). Two studies of close contacts have reported that index cases with low CD4+ T-cell counts are less infectious compared with HIV-seronegative TB cases (39, 40), but this finding is not universal because one study demonstrated conflicting results (11). We postulate that many of the paucibacillary cases found in our study may represent HIV-seropositive patients with a low CD4+ T-cell count, so our findings may be compatible with these two studies (39, 40).
Our findings show an intricate and interdependent relationship between TB and HIV in an urban sub-Saharan African setting. Although previous studies have minimized the importance of contact investigation among HIV-seropositive TB cases (9), our findings support recommendations that household contact investigation and active case finding should occur regardless of the HIV serostatus of the index case (10). Moreover, household contact investigations should include active case finding for both TB and HIV infection in sub-Saharan Africa.
Acknowledgments
Acknowledgment
The authors thank the many participants and families of participants who gave time and dedication to health research. They acknowledge contributions made by study medical officers, health visitors, and data clerks. They acknowledge the invaluable contributions made by Dr. Lorna Nshuti, Dr. Roy Mugerwa, Dr. Deo Mulindwa, Allan Chiunda, Bonnie Thiel, Mark Breda, Dennis Dobbs, Hussein Kisingo, Mary Rutaro, Albert Muganda, Richard Bamuhimbisa, Yusuf Mulumba, Deborah Nsamba, Mark Breda, Barbara Kyeyune, Faith Kintu, Gladys Mpalanyi, Janet Mukose, Grace Tumusiime, Love Nakende, Pierre Peters, Keith Chervenak, Brenda Okwera, Philo Nassozi, Denise Johnson, Karen Morgan, David Guwatudde, Margaret Nakakeeto, Augustine Banyanga, Lashaunda Malone, Alfred Etwom, Mary Nsereko, Micheal Angel Mugerwa, Margaret Nansumba, Harriet Mayanja-Kizza, Moses Joloba, Roy Mugerwa, Lisa Kucharski, and Dr. Feiyou Qiu. They also acknowledge and thank Dr. Francis Adatu Engwau, former head of the Uganda National Tuberculosis and Leprosy Program, and Dr. Alphonse Okwera and thank the staff at the National Tuberculosis Treatment Centre, Mulago Hospital, the Ugandan National Tuberculosis and Leprosy Program and the Uganda Tuberculosis Investigation Bacteriological Unit, Wandegeya, for their contributions to this study. Leonardo Martinez thanks Drs. Limei Zhu and Cheng Chen for several conversations on this research topic, and Fernando Martinez and Maria Ines Pantoja for their constant support. They thank the research group Epidemiology in Action at the University of Georgia College of Public Health for their useful and instructive comments on this project and their constant encouragement. Members of this group include Allan Kizza, Florence Kizza, Anita Nsubuga, Robert Kakaire, Christian Marchello, Amara Ezeamama, Jane Mutanga, Simon Mutembo, and Andreas Handel.
Footnotes
Supported in part by the Tuberculosis Research Unit, established with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NO1-AI-95383 and HHSN266200700022C/NO1-AI-70022); the AIDS International Training and Research Program of the Fogarty International Center (TW-00011); the Center for AIDS Research (AI 36219); and an investigator-initiated grant (AI 093856) from the National Institute of Allergy and Infectious Diseases.
Author Contributions: Conception and/or design, L.M. and C.C.W. Study management, C.C.W. and S.Z. Analysis of the data, L.M. and C.C.W. Interpretation of study results, L.M., J.N.S., M.E.C., and C.C.W. Drafting the manuscript for important intellectual content, L.M., J.N.S., M.E.C., and C.C.W. Review and editing of manuscript, L.M., J.N.S., M.E.C., S.Z., and C.C.W. Approval of final version of the manuscript, L.M., J.N.S., M.E.C., S.Z., and C.C.W.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201511-2146OC on May 15, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lawn SD, Bekker LG, Middelkoop K, Myer L, Wood R. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: the need for age-specific interventions. Clin Infect Dis. 2006;42:1040–1047. doi: 10.1086/501018. [DOI] [PubMed] [Google Scholar]
- 2.Middelkoop K, Mathema B, Myer L, Shashkina E, Whitelaw A, Kaplan G, Kreiswirth B, Wood R, Bekker LG. Transmission of tuberculosis in a South African community with a high prevalence of HIV infection. J Infect Dis. 2015;211:53–61. doi: 10.1093/infdis/jiu403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaisson RE, Martinson NA. Tuberculosis in Africa: combating an HIV-driven crisis. N Engl J Med. 2008;358:1089–1092. doi: 10.1056/NEJMp0800809. [DOI] [PubMed] [Google Scholar]
- 4.Dowdy DW, Azman AS, Kendall EA, Mathema B. Transforming the fight against tuberculosis: targeting catalysts of transmission. Clin Infect Dis. 2014;59:1123–1129. doi: 10.1093/cid/ciu506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whalen CC, Zalwango S, Chiunda A, Malone L, Eisenach K, Joloba M, Boom WH, Mugerwa R. Secondary attack rate of tuberculosis in urban households in Kampala, Uganda. PLoS One. 2011;6:e16137. doi: 10.1371/journal.pone.0016137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guelar A, Gatell JM, Verdejo J, Podzamczer D, Lozano L, Aznar E, Miró JM, Mallolas J, Zamora L, González J, et al. A prospective study of the risk of tuberculosis among HIV-infected patients. AIDS. 1993;7:1345–1349. doi: 10.1097/00002030-199310000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Harries AD, Zachariah R, Corbett EL, Lawn SD, Santos-Filho ET, Chimzizi R, Harrington M, Maher D, Williams BG, De Cock KM. The HIV-associated tuberculosis epidemic: when will we act? Lancet. 2010;375:1906–1919. doi: 10.1016/S0140-6736(10)60409-6. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Global tuberculosis report. Geneva: World Health Organization; 2014. [Google Scholar]
- 9.Espinal MA, Peréz EN, Baéz J, Hénriquez L, Fernández K, Lopez M, Olivo P, Reingold AL. Infectiousness of Mycobacterium tuberculosis in HIV-1-infected patients with tuberculosis: a prospective study. Lancet. 2000;355:275–280. doi: 10.1016/S0140-6736(99)04402-5. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Recommendations for investigating contacts of persons with infectious tuberculosis in low-and middle-income countries. Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- 11.Carvalho AC, DeRiemer K, Nunes ZB, Martins M, Comelli M, Marinoni A, Kritski AL. Transmission of Mycobacterium tuberculosis to contacts of HIV-infected tuberculosis patients. Am J Respir Crit Care Med. 2001;164:2166–2171. doi: 10.1164/ajrccm.164.12.2103078. [DOI] [PubMed] [Google Scholar]
- 12.Cauthen GM, Dooley SW, Onorato IM, Ihle WW, Burr JM, Bigler WJ, Witte J, Castro KG. Transmission of Mycobacterium tuberculosis from tuberculosis patients with HIV infection or AIDS. Am J Epidemiol. 1996;144:69–77. doi: 10.1093/oxfordjournals.aje.a008856. [DOI] [PubMed] [Google Scholar]
- 13.Cruciani M, Malena M, Bosco O, Gatti G, Serpelloni G. The impact of human immunodeficiency virus type 1 on infectiousness of tuberculosis: a meta-analysis. Clin Infect Dis. 2001;33:1922–1930. doi: 10.1086/324352. [DOI] [PubMed] [Google Scholar]
- 14.Kifai EJ, Bakari M. Mantoux skin test reactivity among household contacts of HIV-infected and HIV un-infected patients with sputum smear positive TB in Dar es Salaam, Tanzania. East Afr J Public Health. 2009;6:211–218. doi: 10.4314/eajph.v6i2.51786. [DOI] [PubMed] [Google Scholar]
- 15.Klausner JD, Ryder RW, Baende E, Lelo U, Williame JC, Ngamboli K, Perriens JH, Kaboto M, Prignot J. Mycobacterium tuberculosis in household contacts of human immunodeficiency virus type 1-seropositive patients with active pulmonary tuberculosis in Kinshasa, Zaire. J Infect Dis. 1993;168:106–111. doi: 10.1093/infdis/168.1.106. [DOI] [PubMed] [Google Scholar]
- 16.Bonora S, Concia E, Allegranzi B, Biglino A, Di Perri G. Mycobacterium tuberculosis transmission and HIV status. Lancet. 2000;355:2077–, author reply 2078. doi: 10.1016/s0140-6736(05)73534-0. [DOI] [PubMed] [Google Scholar]
- 17.Espinal MA, Arthur L. Reingold. “Mycobacterium tuberculosis transmission and HIV status. - Author’s Reply. Lancet. 2000;355:2078. doi: 10.1016/s0140-6736(05)73534-0. [DOI] [PubMed] [Google Scholar]
- 18.Martinez L, Sekandi JN, Castellanos ME, Zalwango S, Whalen CC. Modifiers to the infectiousness of HIV seropositive tuberculosis patients: a household contact study from sub-Saharan Africa [abstract] Am J Respir Crit Care Med. 2016;193:A4566. doi: 10.1164/rccm.201511-2146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mudido PM, Guwatudde D, Nakakeeto MK, Bukenya GB, Nsamba D, Johnson JL, Mugerwa RD, Ellner JJ, Whalen CC. The effect of bacille Calmette-Guerin vaccination at birth on tuberculin skin test reactivity in Ugandan children. Int J Tuberc Lung Dis. 1999;3:891–895. [PubMed] [Google Scholar]
- 20.Guwatudde D, Nakakeeto M, Jones-Lopez EC, Maganda A, Chiunda A, Mugerwa RD, Ellner JJ, Bukenya G, Whalen CC. Tuberculosis in household contacts of infectious cases in Kampala, Uganda. Am J Epidemiol. 2003;158:887–898. doi: 10.1093/aje/kwg227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Thoracic Society. National Tuberculosis Association of the USA diagnostic standards and classification of tuberculosis. New York: National Tuberculosis Association; 1961. [Google Scholar]
- 22.World Health OrganizationChild growth standards [accessed 2015 May 15]. Available from: http://www.who.int/childgrowth/standards/bmi_for_age/en/index.html
- 23.Bugiani M, Borraccino A, Migliore E, Carosso A, Piccioni P, Cavallero M, Caria E, Salamina G, Arossa W. Tuberculin reactivity in adult BCG-vaccinated subjects: a cross-sectional study. Int J Tuberc Lung Dis. 2003;7:320–326. [PubMed] [Google Scholar]
- 24.Narain R, Nair SS, Rao GR, Chandrasekhar P. Distribution of tuberculous infection and disease among households in a rural community. Bull World Health Organ. 1966;34:639–654. [PMC free article] [PubMed] [Google Scholar]
- 25.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 26.Zocchetti C, Consonni D, Bertazzi PA. Relationship between prevalence rate ratios and odds ratios in cross-sectional studies. Int J Epidemiol. 1997;26:220–223. doi: 10.1093/ije/26.1.220. [DOI] [PubMed] [Google Scholar]
- 27.Hill PC, Ota MO. Tuberculosis case-contact research in endemic tropical settings: design, conduct, and relevance to other infectious diseases. Lancet Infect Dis. 2010;10:723–732. doi: 10.1016/S1473-3099(10)70164-X. [DOI] [PubMed] [Google Scholar]
- 28.Lienhardt C, Fielding K, Sillah J, Tunkara A, Donkor S, Manneh K, Warndorff D, McAdam KP, Bennett S. Risk factors for tuberculosis infection in sub-Saharan Africa: a contact study in the Gambia. Am J Respir Crit Care Med. 2003;168:448–455. doi: 10.1164/rccm.200212-1483OC. [DOI] [PubMed] [Google Scholar]
- 29.Gustafson P, Lisse I, Gomes V, Vieira CS, Lienhardt C, Nauclér A, Jensen H, Aaby P. Risk factors for positive tuberculin skin test in Guinea-Bissau. Epidemiology. 2007;18:340–347. doi: 10.1097/01.ede.0000259987.46912.2b. [DOI] [PubMed] [Google Scholar]
- 30.Naing NN, Mohammad WZW, Salleh R, Ahmad N, Iyawoo K, Shaban H, Simon GK, Joseph MS, Abu Bakar MH. A multi-centered study of the influence of HIV on transmission of tuberculosis to household contacts in three states of Malaysia. Int Med J. 2007;14:273–279. [Google Scholar]
- 31.Borgdorff MW, Sebek M, Geskus RB, Kremer K, Kalisvaart N, van Soolingen D. The incubation period distribution of tuberculosis estimated with a molecular epidemiological approach. Int J Epidemiol. 2011;40:964–970. doi: 10.1093/ije/dyr058. [DOI] [PubMed] [Google Scholar]
- 32.Vynnycky E, Fine PE. Lifetime risks, incubation period, and serial interval of tuberculosis. Am J Epidemiol. 2000;152:247–263. doi: 10.1093/aje/152.3.247. [DOI] [PubMed] [Google Scholar]
- 33.Kizza FN, List J, Nkwata AK, Okwera A, Ezeamama AE, Whalen CC, Sekandi JN. Prevalence of latent tuberculosis infection and associated risk factors in an urban African setting. BMC Infect Dis. 2015;15:165. doi: 10.1186/s12879-015-0904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez L, Arman A, Haveman N, Lundgren A, Cabrera L, Evans CA, Pelly TF, Saito M, Callacondo D, Oberhelman R, et al. Changes in tuberculin skin test positivity over 20 years in periurban shantytowns in Lima, Peru. Am J Trop Med Hyg. 2013;89:507–515. doi: 10.4269/ajtmh.13-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wood R, Liang H, Wu H, Middelkoop K, Oni T, Rangaka MX, Wilkinson RJ, Bekker LG, Lawn SD. Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int J Tuberc Lung Dis. 2010;14:406–412. [PMC free article] [PubMed] [Google Scholar]
- 36.Lönnroth K, Williams BG, Stadlin S, Jaramillo E, Dye C. Alcohol use as a risk factor for tuberculosis: a systematic review. BMC Public Health. 2008;8:289. doi: 10.1186/1471-2458-8-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chamie G, Luetkemeyer A, Walusimbi-Nanteza M, Okwera A, Whalen CC, Mugerwa RD, Havlir DV, Charlebois ED. Significant variation in presentation of pulmonary tuberculosis across a high resolution of CD4 strata. Int J Tuberc Lung Dis. 2010;14:1295–1302. [PMC free article] [PubMed] [Google Scholar]
- 38.Perlman DC, el-Sadr WM, Nelson ET, Matts JP, Telzak EE, Salomon N, Chirgwin K, Hafner R The Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA). The AIDS Clinical Trials Group (ACTG) Variation of chest radiographic patterns in pulmonary tuberculosis by degree of human immunodeficiency virus-related immunosuppression. Clin Infect Dis. 1997;25:242–246. doi: 10.1086/514546. [DOI] [PubMed] [Google Scholar]
- 39.Huang CC, Tchetgen ET, Becerra MC, Cohen T, Hughes KC, Zhang Z, Calderon R, Yataco R, Contreras C, Galea J, et al. The effect of HIV-related immunosuppression on the risk of tuberculosis transmission to household contacts. Clin Infect Dis. 2014;58:765–774. doi: 10.1093/cid/cit948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kenyon TA, Creek T, Laserson K, Makhoa M, Chimidza N, Mwasekaga M, Tappero J, Lockman S, Moeti T, Binkin N. Risk factors for transmission of Mycobacterium tuberculosis from HIV-infected tuberculosis patients, Botswana. Int J Tuberc Lung Dis. 2002;6:843–850. [PubMed] [Google Scholar]