Abstract
Respiratory diseases are highly complex, being driven by host–environment interactions and manifested by inflammatory, structural, and functional abnormalities that vary over time. Traditional reductionist approaches have contributed vastly to our knowledge of biological systems in health and disease to date; however, they are insufficient to provide an understanding of the behavior of the system as a whole. In this Pulmonary Perspective, we discuss systems biology approaches, especially but not limited to the study of the lung as a complex system. Such integrative approaches take into account the large number of dynamic subunits and their interactions found in biological systems. Borrowing methods from physics and mathematics, it is possible to study the collective behavior of these systems over time and in a multidimensional manner. We first examine the physiological basis for complexity in the respiratory system and its implications for disease. We then expand on the potential applications of systems biology methods to study complex systems, within the context of diagnosis and monitoring of respiratory diseases including asthma, chronic obstructive pulmonary disease (COPD), and critical illness. We summarize the significant advances made in recent years using systems approaches for disease phenotyping, applied to data ranging from the molecular to clinical level, obtained from large-scale asthma and COPD networks. We describe new studies using temporal complexity patterns to characterize asthma and COPD and predict exacerbations as well as predict adverse outcomes in critical care. We highlight new methods that are emerging with this approach and discuss remaining questions that merit greater attention in the field.
Keywords: diagnosis, monitoring, complex systems, lung function, biomarkers
The respiratory system is highly complex, both in health and disease. This has long been recognized in the clinic, because chronic diseases such as asthma and chronic obstructive pulmonary disease (COPD) are typically complex, multifactorial, and influenced by external, environmental, and internal inflammatory stimuli. Furthermore, they tend to manifest themselves not in an instant but over time (e.g., the temporal behavior of asthma fluctuates over time in response to constantly changing stimuli) (1). The experienced clinician intuitively knows to characterize and monitor disease stability on the basis of multidimensional, constantly changing factors (2). Likewise, in intensive care, a multitude of physiological signals are routinely assessed over time to determine prognosis and direct intervention.
For these reasons, the respiratory system is increasingly conceptualized as a complex system. In biology, complex systems are dynamic systems comprising multiple nonlinearly interacting subunits that operate under nonequilibrium conditions (3). Interactions between the subunits exhibit emergence (i.e., the interactions between individual subunits result in more complex, organized behavior that cannot be readily deduced from the components alone). These interactions are often characterized by self-similar (fractal) spatial structures and/or temporal behavior with repeating motifs extending over substantial parts of the structure or a time period (long-range correlations; i.e., “memory”) and distributions with long tails that follow a power law, allowing for “rare” sizes or events to occur (3). Fractal structures are ubiquitous in nature (e.g., lightning, deltas, trees, mountains, clouds, coastlines). Within the respiratory system, obvious self-similar structures are the airway and vascular trees (4), and complex temporal behavior is present in the dynamics of breathing (5).
Systems biology may be defined as a discipline that attempts to understand biological systems, complex or otherwise, from the interactions of their components, using computational and mathematical tools, which often overlap with those of complexity science. In this article, we discuss the use of systems biology approaches, especially but not limited to the study of the lung as a complex system. Such approaches have captured the imagination of a rapidly growing number of medical researchers (1, 2, 6–8). Although traditional reductionist approaches have contributed vastly to our knowledge of biological systems to date, they limit our understanding of the behavior of the system as a whole. Systems biology takes into account the known components of a biological entity, be it at the molecular, cellular, organ, individual, or even population level, and studies the interactions between the different components and their collective behavior over time. This has been facilitated by advances in computing and information technology that allow us to model, collect, process, and interpret ever-larger amounts of information.
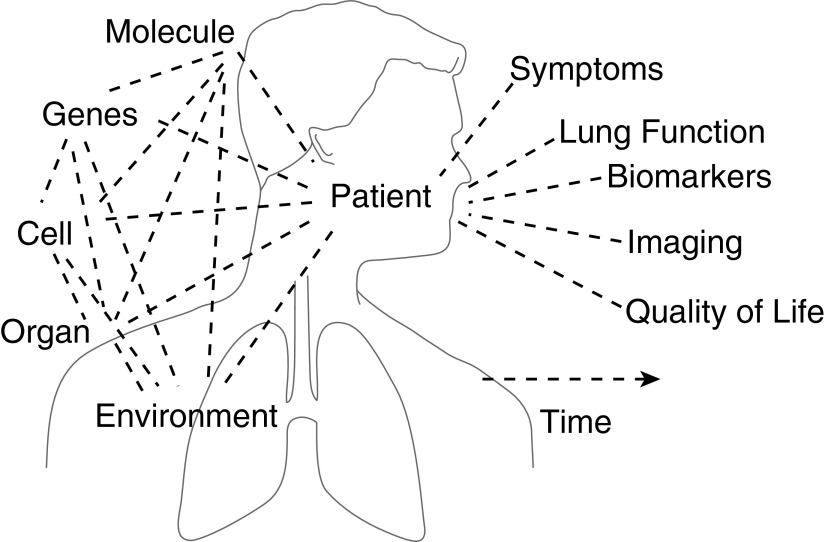
This Pulmonary Perspective summarizes a Scientific Symposium entitled “Systems Biology and Clinical Practice: The Twain Shall Meet?” that was held in May 2015 during the American Thoracic Society International Conference in Denver, Colorado. Here, we first examine the physiological basis for complexity in the respiratory system and its implications for disease. We then discuss current applications of the systems biology approach to asthma and COPD and in critical care. Finally, we provide an overview of future potential contributions in the respiratory and critical care fields (Figure 1).
Figure 1.
A vision for how systems biology can meet clinical practice: physicians will collect information on their patients with asthma and chronic obstructive pulmonary disease or in the intensive care unit related to variation in symptoms, lung function, biomarkers, structure, quality of life, and other aspects of disease, which can then be integrated with information from environmental and psychosocial factors, using advanced analyses to comprehensively describe behavior over time. This approach enables them to arrive at more objective and precise clinical decisions and treatment strategies and even provide accurate prognostic indicators.
Some of the results of these studies have been previously reported in the form of an abstract (9).
How Does Complexity Arise in the Respiratory System?
The complexity of the structures and biological processes in the healthy lung arises from interactions observed at both the microscopic and macroscopic scales, such as extracellular matrix maintenance, surfactant secretion, and control of breathing. To gain insight into how the complexity of the normal lung transitions into pathological states, it is useful to understand the processes that contribute to the complexity of the normal lung.
From Micro- to Macroscopic Scales, and Vice Versa
Generally, complex behavior emerges from nonlinear interactions among the subunits of a system when pushed away from equilibrium. There are many known examples of mechanisms that contribute to complexity within the lung. First, in adaptive systems composed of many autonomous “agents,” nonlinear interactions can lead to complex behavior (e.g., the collective migration of epithelial cells during wound closure) (10, 11). Second, such agents can also build complex structures; indeed, the adaptive behavior of lung cells during embryonic development produces growth patterns from which the complex fractal airway and vascular trees arise (12). Third, signals propagating through an existing complex structure result in complex temporal behavior (13). For example, airways open in an avalanche-like progression with a size distribution that follows a power law, which means that the probability that a large alveolar region simultaneously pops open during inflation of a partially collapsed lung is not negligible (14). The avalanche behavior is a consequence of pressure waves sequentially opening airways while propagating through the tree. Fourth, complexity may arise from network behavior; the origin of long-range correlations in tidal volume can be traced back to the highly nonlinear interactions among the five respiratory neuron groups forming a network in the respiratory rhythm generator (15). Therefore, complexity is deeply embedded in multiple instances in the respiratory system, even in health.
The link between the origins and consequences of complexity may also be bidirectional. Not only has complexity at the macroscale been attributed to nonlinear microscopic processes interacting with the environment (16) but also it could influence microscale processes. For example, type II alveolar epithelial cells in the lung secrete surfactant, which reduces surface tension at the air–liquid interface, allowing normal breathing by minimizing alveolar collapse. A single large stretch during a deep inspiration has been shown to be a potent stimulator of surfactant release (17). More importantly, the variability of the breathing pattern itself has also been found to up-regulate surfactant secretion (18), which may have a significant impact on how patients will be mechanically ventilated in the future (19). Macroscopic complexity in physiological variables, such as mechanical forces of tidal volume or blood pressure acting on cells (20), can regulate normal cell function. Therefore, changes in macroscale complexity during the course of disease may adversely affect signaling and tissue remodeling at the microscale.
Influence of Intrinsic versus Extrinsic Factors
We have gained much information from mechanistic association studies regarding the factors influencing observable macroscopic behavior (e.g., variability of airway caliber and airway function over time). Short-term variations in airway function are dependent mainly on intrinsic factors, such as mean airway caliber, posture, bronchial reactivity, airway smooth muscle loading, end-expiratory lung volume, and viscoelastic properties of lung, as well as extrinsic factors, such as methacholine and bronchodilators (1, 15, 21–24). The sources of these variations are difficult to disentangle, are different between asthma and COPD, and have been associated with disease severity and inflammatory phenotypes (25). Whether short-term variations of airway caliber are related to airway remodeling is still unknown. Meanwhile, from larger epidemiological studies, we are starting to see that long-term day-to-day variations of lung function are strongly influenced by the interaction of the respiratory system with extrinsic environmental factors, via correlations with, for example, air pollution, viral infections, pollen, medication, and diurnal changes of stimuli. We know that intrinsic factors, such as sex and airway structure, as indicated by mean airway caliber, as well as asthma control and disease severity (summarized in References 1, 23, 26), also play a part.
Relevance to Health and Disease
In health, a complex system may be tolerant to gradual and small changes in one of its subsystems or the interactions among subsystems. However, due to the nonlinear and networked nature of the interactions, such small changes can often trigger grossly amplified global effects once a threshold is reached. This is when clinical symptoms first appear and disease manifests itself (27). An understanding of the origins and details of complexity of the healthy respiratory system can provide insight into how pathological states emerge and hence help inform the practical application of systems biology approaches toward more effective strategies for diagnosis, monitoring, and treatment of pathological conditions.
What Can Systems Biology Do for Me? A Respiratory Clinician’s Wish List
What are the key clinical questions that could potentially be answered by a systems approach? Airway disease provides some striking examples of clinical problems for which such an approach could promote understanding of underlying mechanisms and improve outcomes.
Diagnosis
The clinician’s wish list begins with diagnosis, as respiratory symptoms are nonspecific, and objective measures are often unavailable. Asthma is characterized by typical patterns of symptoms (e.g., triggered by exercise/laughter/cold air) and increased lung function variability. However, respiratory symptoms may be due to factors other than bronchoconstriction, contributing to the poor correlation between symptoms and lung function (28, 29), and to difficulty in diagnosis. Furthermore, although asthma is most characteristically associated with variability in symptoms and lung function, and COPD most characteristically by progressive symptoms and airflow limitation, both conditions are heterogeneous (30, 31), and their clinical features overlap, as evidenced by the 15 to 20% of patients who share both diagnoses (32).
Systems biology approaches should enable us to uncover hidden patterns or information, and the application of systems biology to a broad range of available measures, from symptoms through to exhaled breath, provides hope for point-of-care diagnosis of patients presenting with respiratory symptoms (33). Clinical recognition of temporal symptom patterns has already led to computerized peak flow algorithms for diagnosis of occupational asthma using machine learning (34). For wheezing in preschool children, which may represent anything from self-limiting viral infections to the initial manifestation of lifelong asthma, recent guidelines recommend a probability-based approach to diagnose asthma on the basis of wheezing episodes, interval symptoms, atopy, and family history (35). However, at present, this process involves the clinician mentally combining a limited set of subjective and objective data from each individual child. Applying systems biology approaches to the extensive clinical and biomarker data collected over time from cohort studies may generate new tools for diagnosis of such children.
Monitoring
Once a diagnosis of airway disease is made, we need sophisticated analyses for monitoring progress and evaluating treatment response. In clinical trials, analysis of symptoms is often limited to frequency (e.g., number of days/week) (36). In clinical trials, mixed-model analysis of lung function is increasingly used to take advantage of multiple data points (36). However, for clinical practice, lung function analysis is often simplistic, dating back to the era of paper diaries (with their high rates of data fabrication) and manual calculations, resulting in loss of information. Common metrics, such as diurnal peak expiratory flow (PEF) variability, which simplifies twice-daily (or more frequent) measurements down to a single averaged number per week, can conceal clinically important differences in underlying mechanisms, as seen by the lack of increase in diurnal PEF variability during severe viral asthma exacerbations (37). Conventional criteria for identifying worsening asthma, if simply based on percent predicted PEF, fail to take into account the patient’s background level of variation in symptoms or lung function (38). The availability of repeated measures allows for systems approaches, particularly with expanding use of electronic medical records and ambulatory monitoring. Such approaches may in addition include fluctuations in biological disease markers, as a strength of these methods is the ability to identify complex relationships. For example, in newly diagnosed asthma or COPD, can patients at risk of progression to severe disease or accelerated decline in lung function be identified early, before the underlying mechanisms are obscured by time and treatment? After a change in treatment, which short-term measures will identify a positive response or a loss of control? With increasing interest in both patient-centered measures and personalized treatment, can sophisticated analyses allow identification of specific treatable mechanisms from specific symptom features? Some possibilities offered by systems approaches are discussed in the next sections.
Treatment
A final example in the clinician’s wish list, relevant to a broad range of diseases, is a better understanding about patterns of medication adherence and their relationship with other variables and with treatment response. Adherence is often estimated from dispensing records or patient self-report, both of which overestimate actual use. Much greater accuracy and granularity is provided by electronic inhaler monitoring, but as yet we lack suitable methods of analysis. Current adherence metrics typically average adherence over many months, but this conceals gaps or trends in usage (39); “50% adherence” may indicate erratic usage with long gaps or regular low-dose treatment, and these patterns have different risk implications. Visual inspection of medication data can identify basic adherence patterns (40), and advanced metrics could describe these patterns of medication use more comprehensively, but a systems approach could allow examination of additional relevant data, also taking into account the complex relationships between medication usage and patient characteristics (including personality and attitudes); day-to-day exposures such as pollution, temperature, and pollens; and medication type. It could then comprehensively assess their combined effect on clinically important outcomes, such as symptom control and risk of adverse outcomes.
The examples provided above are only a start. Human beings are complex organisms with complex exposures, and our approach to understanding disease mechanisms and treatment strategies should not be limited by simplistic analysis methods. A dialogue between clinicians and systems biology scientists will stimulate progress in both fields. In the next few sections, we discuss how far systems biology has come in addressing the wish list.
Phenotypic Fingerprinting: Diagnosis in Asthma and COPD
The diagnostic approach to asthma and COPD is highly illustrative of the ambiguity that still surrounds patient classification even today. After difficulties in clearly distinguishing these two entities during the mid-20th century and introducing a complex phenotype-driven approach (41), decades of subsequent efforts produced globally agreed criteria to define asthma (30) and COPD (31). By itself, this represented a first and solid step toward subclassification of obstructive airway diseases. Even though this is fulfilling its purpose of globally improving the standards of care tremendously well, the inevitable overlap of disease features between asthma and COPD in individual patients has regained wide recognition (30, 32), thereby promoting a new phenotype-driven approach on the basis of clinical and biological features that are predictive of therapy responsiveness regardless of the diagnostic label of asthma or COPD (42).
Phenotype or Endotype?
Patients vary with respect to their clinically observable characteristics, which can be described as relatively stable (but not invariable) phenotypes. There is little doubt that adding biological information to clinical features improves the phenotyping of asthma and COPD (43), for which high-throughput platforms for capturing molecular mixtures are currently available (44). This not only allows probabilistic phenotyping of individual patients but also, importantly, enables discovery of unidentified molecular networks in disease (45, 46). Such hypothesis generation greatly facilitates traditional mechanistic research, which adds information regarding causative pathways to the various disease phenotypes to arrive at disease endotypes (47). In fact, as yet there are very few known endotypes for asthma and COPD, other than those based on blocking single cytokines (e.g., IL-5, IL-13), which still does not unravel the causative involvement of comprehensive biological mechanisms (e.g., distinct type 2 cytokine pathways). Molecular disease fingerprinting will provide the new phenotypes for unraveling the disease endotypes.
The Data
Building the data has understandably taken time, but now proof-of-principle papers are being published, mainly in severe asthma to date. The systems biology approach used by Ghebre and colleagues (48) showed that, using a limited array for sputum cytokines in patients with a clinical diagnosis of severe asthma or moderate to severe COPD, it was possible to distinguish novel groups of patients regardless of their original diagnostic label. Importantly, the Ghebre study included validation in an independent cohort, which is essential and often still lacking in this research area (49). Among patients with COPD, such an approach also appears to be effective. Gene expression profiling captures phenotypes of patients in relation to frequent exacerbations (50) and sensitivity to inhaled steroids (51), thereby discovering novel biological pathways in COPD. Similar findings have recently been published among children with severe asthma when using gene expression profiles of peripheral blood leukocytes (52) and in adults with asthma on the basis of transcriptomics of epithelial cells or laser-dissected airway smooth muscle (53, 54). The next step will be to apply similar methodology to broader population samples.
Added Value?
The bottom line of these studies seems to be that comprehensive assessment of molecular fingerprints at the RNA or protein level may provide added value to currently existing, mostly singular molecular markers of disease. When integrated with clinical information, such molecular fingerprints will contribute substantially to a gradual shift from conventional clinical diagnoses to stratified and individualized phenotyping of patients (6). The U-BIOPRED (Unbiased BIOmarkers in PREDiction of respiratory disease outcomes) project is currently delivering its molecular fingerprints and its bioclinical handprints for severe asthma (www.ubiopred.eu). As more prospective studies arise, the systems approach enables us to be better at interpreting cellular, molecular to whole physiology studies from a holistic point of view, aided by multiscale modeling (55), and integrating these results with traditional clinical understanding. Furthermore, this concept is applicable in other areas of respiratory medicine (43)—in fact, medicine in general.
Variability Analysis: Monitoring in Asthma and COPD
As a complex system, the lung involves dynamic changes in structure and function that result in the emergence of the clinical manifestations, or phenotype, of diseases like asthma and COPD (2). In the next few sections we demonstrate that monitoring of the variability of physiologic variables over time enables us to more comprehensively describe the complex behavior of the lung. It should be noted that temporal variations over time are not a necessary condition of complexity; however, they occur frequently in biological systems and are often inadequately addressed using simplistic measures and basic statistical approaches. Many of the methods that can be used to study temporal behavior are borrowed from complexity science and have been associated with key clinical outcomes. Thus, coupled with the consideration of multiple variables, variability over time is a key component within the systems biology approach to studying respiratory diseases. This potentially leads to a deeper understanding of the pathophysiology of disease and, in the case of asthma and possibly COPD, an opportunity to predict future control.
Assessment of Disease Status and Future Risk
Fluctuations in lung function are a critical component of asthma. For many years, the diurnal variation in PEF has been recognized to be more severe in patients with greater symptoms of asthma and in those with more difficult to control, or brittle, asthma (56, 57). Indeed, monitoring the variability in PEF within the first 2 weeks after withdrawal of inhaled corticosteroids may help predict loss of asthma control (58). Similarly, a drop in PEF combined with increased symptoms during a 1-week period have been shown to define a so-called “action point” beyond which a worsening of asthma will occur within the next 5 days (59). However, as indicated above, these studies were based on simple PEF metrics. Detrended fluctuation analysis (DFA), has been used to show that intrinsic fluctuations in PEF over time demonstrate complex fractal-type (self-similar) behavior (60), also termed long-range correlations (i.e., variability of PEF measured over a long time window relates to the variability of PEF over shorter time scales). Analysis of these fluctuations can in turn be used to predict future lung function: In both mild-moderate and severe asthma, asthma control assessed using a Global Initiative for Asthma–based score was associated with higher fluctuation content (i.e., greater self-similarity) (61). Furthermore, the fluctuation properties of PEF can be used to generate conditional probabilities of a future asthma exacerbation for an individual within the next month (62).
In contrast, PEF variability has not to date been useful for monitoring of patients with COPD, with mixed results relating to symptoms in comparison to asthma (63). As in asthma, a drop in PEF precedes an exacerbation of COPD, but its predictive value for exacerbation is low (64). Nevertheless, using DFA, PEF fluctuations in patients with COPD have been shown to be related to exacerbation frequency (65). Advanced analyses of PEF may provide more information where simple measures of PEF variability have failed, particularly when integrated with other clinical measures and biomarkers.
More Sensitive Lung Function Measures?
Lung function can also be characterized by respiratory impedance (pressure over flow at a given frequency), measured by the forced oscillation technique (FOT). In 2001, Que and colleagues demonstrated that respiratory system resistance measured by FOT was more variable in patients with asthma than in healthy control subjects, even across time spans as short as 15 minutes (66). This variability has subsequently been demonstrated in a number of other studies involving both adults and children (22, 67–69). One study has even suggested that home monitoring of FOT for just 2 minutes a day over only 8 days could yield sufficient information to calculate a conditional probability of lung function deterioration over the subsequent week (70). More recently, it was even possible to obtain individualized measures of risk (71). Increasing FOT impedance has also been associated with loss of airflow pattern complexity in patients with increasing severity of airflow limitation (72).
Implications for COPD?
When measured using FOT, patients with COPD have increased day-to-day variability in impedance compared with healthy control subjects, and even higher than that of patients with asthma (73), despite the fact that COPD is traditionally viewed as a disease of progressive obstruction. Although DFA of respiratory system impedance was not able to differentiate COPD from asthma or healthy control subjects, a distance-based time series analysis was able to distinguish asthma and COPD in the majority of cases (22). In moderate COPD, there are larger differences between inspiratory and expiratory FOT reactance compared with patients with asthma and healthy control subjects (74), and this difference is more variable over time and correlates well with dyspnea (75). Home monitoring of lung function by FOT in patients with COPD has recently been shown to be feasible and reliable (76), suggesting a great potential role for monitoring of FOT variability in COPD.
In terms of disease monitoring, we are seeing a rapid increase in the number of technologies that enable home monitoring of PEF or FOT impedance and communication via smartphone applications (e.g., Asthma Health App, http://apps.icahn.mssm.edu/asthma/). If incorporated into the systems approach of making sense of data from multiple sources and comprehensively describing behavior over time, this may revolutionize the way we routinely care for patients with asthma, COPD, and other chronic lung diseases.
Temporal Phenotyping: Extending Variability Analyses for Both Diagnosis and Monitoring
We have already seen that fractal-type fluctuations of lung function over time may help to assess disease status and risk (58, 60, 62, 65, 72, 77, 78) and to monitor therapy success (58, 60, 79). Furthermore, in asthma, fluctuations in inflammatory markers such as fractional exhaled nitric oxide are related to symptoms (62, 80), and the degree of concordance between the temporal changes in the biomarker and the temporal changes in daily symptoms may even serve as a measure of disease stability (62). There is also some evidence that such analyses could be used to phenotype asthma and COPD (61, 65). Can these analyses add to the increased importance of phenotyping and endotyping in the context of personalized health and phenotype-specific treatment?
The Current Limitations of Cluster Analyses
Cluster analyses represent one example in which systems biology approaches have gained increasing acceptance. Powerful observer-independent statistical clustering methods have been used to phenotype patients with asthma on the basis of symptoms and biomarkers (81–83). All of these clustering methods, however, are population based, cross-sectional, and measured at one point in time. They do not consider temporal fluctuations or trends in symptoms and biomarkers. They have also largely been conducted in patients with a single diagnostic label. Furthermore, very often it is not considered that the clinical phenotype—and thus the related omics endotypes—may change in the context of changes in the environment. In a dynamic chronic disease such as asthma, in which symptoms are strongly determined by the environmental interaction, it is therefore crucially important to develop observer-independent clustering methods, which quantify and characterize this interaction (“interactome”) with the environment.
Combining Variability Analyses with Clustering
Time series of fluctuations in day-to-day lung function may be an ideal basis to develop such a method of temporal phenotyping of asthma and its relationship with changes in the environment. Delgado-Eckert and colleagues (9) have developed a fluctuation-based clustering (FBC) method and prospectively validated it in the EFRAIM (Mechanisms of Early Protective Exposures on Allergy Development study) cohort of 696 children with asthma. Using the earth-movers distance method (84), which has been used in other fields such as astronomy, the authors clustered the patients on the basis of their twice-daily FEV1 and PEF fluctuation and distribution properties within a period of 4 weeks. In addition, the method uses a data-driven cluster stability algorithm that allows the inclusion of patients with missing measurements. To clinically validate the FBC method in a blinded manner, they compared the predominant clinical characteristics of the clusters found with previously published clinical asthma phenotypes in this cohort (85). Out of the four clusters derived, two clusters contained a significant overrepresentation of children with well-known clinical characteristics of asthma, whereas children from a farming environment and healthy control subjects predominated the other clusters. Because patient-related features and environmental features influence the FBC clusters, the method may represent an interesting novel clustering dimension, which takes into account how a chronic disease interacts with the environment and how stable it is over time.
This method is in its infancy and holds promising future implications for how we examine disease over time in a comprehensive, truly multidimensional manner. It may help us better classify disease status on the basis of other time-varying variables (e.g., pattern of adherence to treatment or symptom or treatment changes over time).
Variability Analysis: Monitoring in the Intensive Care Unit
The systems approach lends itself particularly well to the wealth of physiological data collected in the intensive care unit (ICU) setting. By measuring the degree and character of fluctuations of the inter-beat and inter-breath time intervals time series over windows in time, discarding poor-quality waveforms, beats or breaths, it is possible to monitor heart and respiratory rate variability (HRV and RRV) over time as a means to evaluate the integrity of the whole system. There has been extensive research highlighting the association between loss of variability and illness incidence and severity, showing that HRV and RRV assessment may provide added value when compared with traditional forms of clinical assessment by improving our ability to diagnose onset or severity of critical illness and predict extubation outcomes. In this way, HRV and RRV monitoring may help reduce the risk of patient deterioration, morbidity, mortality, and healthcare burden (86–89).
A variety of time series analysis techniques exist to quantify and characterize patterns of variation over intervals in time (90). Predictive modeling using machine learning techniques can then be used to transform multivariate variability metrics into clinically relevant measures, such as probability or likelihood of a clinical event, useful for decision support.
Clinical Impact
The potential clinical value of monitoring variability has been investigated with respect to early warning of sepsis (24, 91, 92) as well as prognostication of organ failure and the impact of sedation (93, 94). In addition, increased age leads to degradation of the multiscale self-similar behavior seen in RRV, with loss of long-range fractal scaling, signifying loss of complexity (5). Altered RRV is associated with the presence and severity of lung illness, including restrictive lung disease (95) and the prediction of severity of asthma exacerbations (60). Reduced variability may indicate loss of adaptability or ability to tolerate an increased workload of breathing; several small studies (96, 97), in addition to a recent large multicenter study (98), have demonstrated that reduced RRV (degree and complexity) during a spontaneous breathing trial before extubation is associated with an increased risk of postextubation failure, defined as reintubation within 48 hours. These data suggest that RRV offers added value over traditional methods, with evidence of both superior and complementary predictive capacity (98). To what degree these findings will be reproducible in other populations and what impact this information might have on extubation decisions remain to be determined.
The role of variability monitoring in ICU monitoring and the physiologic meaning of variability remain exciting avenues of research. Although fairly compelling observational studies exist, interventional trials are now required to determine the impact of this approach on patient care outcomes and cost.
Conclusions and Implications
In summary:
-
1.
Systems biology approaches and complexity science are relevant to studying the respiratory system, as respiratory diseases are typically complex, influenced by a multitude of intrinsic factors and extrinsic stimuli, and vary over time. Purely reductionist approaches are useful, yet need to be complemented by systems approaches that involve quantitative and integrative methods that study the system as a whole.
-
2.
A multitude of potential sources of complexity are known to exist in the respiratory system. Understanding the general nonlinear mechanisms by which complexity arises and how they interact can provide insight into how pathological states emerge and hence may help us better develop targeted intervention and treatment strategies.
-
3.
Systems biology approaches enable us to integrate new molecular results with traditional clinical measures, bridging the gap between the two and facilitating the concept of molecular phenotyping in diagnosis of asthma and COPD. Such approaches have included the use of techniques to monitor and identify subtle changes in patterns in lung function and other biomarkers over time in asthma, COPD, and critical illness. Thus, a systems approach provides added value to conventional methods of both diagnosis and monitoring.
-
4.
Novel methods, such as temporal phenotyping of the underlying complex system, will further add to the understanding of disease phenotypes and their stability over time and potentially offer predictors of future behavior.
The Future for Systems Biology: Where to from Here?
We have demonstrated the clinical utility of systems biology approaches, accounting for the complexity of the lung, in diagnosis, monitoring, and treatment. Such tools are already available at our disposal. However, one limitation is that so far systems biology approaches have mostly been applied in observational studies, which are often only hypothesis generating in nature. To take further advantage of the systems approach, future studies need to include prospective validation of risk groups identified by systems approaches, by studying their long-term outcomes. We now have sufficient evidence to run investigator-initiated clinical trials to show that treatment strategies based on such risk groups are more effective than conventional “all in one” treatment approaches. The design of such future clinical studies will be markedly different from classical approaches, involving larger data sets of well-characterized patients observed over longer time periods using serial measurements of biomarkers and advanced analyses to comprehensively describe behavior over time.
The development of novel integrative and quantitative techniques inspired by the systems approach, coupled with increasing availability of data from present and future clinical trials, will continue to spur on the field of respiratory medicine. It is our vision that in the near future, physicians will collect information on their patients with asthma and COPD or in the ICU related to variation in symptoms, lung function, biomarkers, structure, quality of life, and other aspects of disease, which can then be integrated with information from environment and psychosocial factors (Figure 1). This innovative phenotypic information goes beyond traditional diagnoses, to the benefit of patients and caregivers. They can then make full use of this information to arrive at clinical decisions and treatment strategies and even provide prognostic indicators with clinically useful accuracy.
Footnotes
Supported by National Institutes of Health grant NIH HL 126040 (B.S.).
Summary of a Scientific Symposium held at the American Thoracic Society International Conference in Denver, Colorado, on May 20, 2015.
Author Contributions: All authors contributed equally to the writing of this manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201511-2288PP on August 24, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Frey U, Maksym G, Suki B. Temporal complexity in clinical manifestations of lung disease. J Appl Physiol (1985) 2011;110:1723–1731. doi: 10.1152/japplphysiol.01297.2010. [DOI] [PubMed] [Google Scholar]
- 2.Frey U, Suki B. Complexity of chronic asthma and chronic obstructive pulmonary disease: implications for risk assessment, and disease progression and control. Lancet. 2008;372:1088–1099. doi: 10.1016/S0140-6736(08)61450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suki B Bates JH Frey U Complexity and emergent phenomena Compr Physiol 20111995–1029 [DOI] [PubMed] [Google Scholar]
- 4.Glenny RW, Robertson HT, Yamashiro S, Bassingthwaighte JB. Applications of fractal analysis to physiology. J Appl Physiol (1985) 1991;70:2351–2367. doi: 10.1152/jappl.1991.70.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng CK, Mietus JE, Liu Y, Lee C, Hausdorff JM, Stanley HE, Goldberger AL, Lipsitz LA. Quantifying fractal dynamics of human respiration: age and gender effects. Ann Biomed Eng. 2002;30:683–692. doi: 10.1114/1.1481053. [DOI] [PubMed] [Google Scholar]
- 6.Agustí A, Antó JM, Auffray C, Barbé F, Barreiro E, Dorca J, Escarrabill J, Faner R, Furlong LI, Garcia-Aymerich J, et al. Personalized respiratory medicine: exploring the horizon, addressing the issues. Summary of a BRN-AJRCCM workshop held in Barcelona on June 12, 2014. Am J Respir Crit Care Med. 2015;191:391–401. doi: 10.1164/rccm.201410-1935PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaminsky DA, Irvin CG, Sterk PJ. Complex systems in pulmonary medicine: a systems biology approach to lung disease. J Appl Physiol (1985) 2011;110:1716–1722. doi: 10.1152/japplphysiol.01310.2010. [DOI] [PubMed] [Google Scholar]
- 8.Seely AJ, Macklem P. Fractal variability: an emergent property of complex dissipative systems. Chaos. 2012;22:013108. doi: 10.1063/1.3675622. [DOI] [PubMed] [Google Scholar]
- 9.Delgado-Eckert E, Kumar E, Fuchs O, Pekkanen J, Dalphin JC, Riedler J, Lauener R, Karvonen AM, Genuneit J, Von Mutius E, et al. PASTURE Study Group. Asthma phenotypes determined by a novel fluctuation based clustering method using a time window of lung function observations [abstract] Eur Respir J. 2015;46:OA1473. [Google Scholar]
- 10.Tambe DT, Hardin CC, Angelini TE, Rajendran K, Park CY, Serra-Picamal X, Zhou EH, Zaman MH, Butler JP, Weitz DA, et al. Collective cell guidance by cooperative intercellular forces. Nat Mater. 2011;10:469–475. doi: 10.1038/nmat3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perotin JM, Adam D, Vella-Boucaud J, Delepine G, Sandu S, Jonvel AC, Prevost A, Berthiot G, Pison C, Lebargy F, et al. Delay of airway epithelial wound repair in COPD is associated with airflow obstruction severity. Respir Res. 2014;15:151. doi: 10.1186/s12931-014-0151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glenny RW. Emergence of matched airway and vascular trees from fractal rules. J Appl Physiol (1985) 2011;110:1119–1129. doi: 10.1152/japplphysiol.01293.2010. [DOI] [PubMed] [Google Scholar]
- 13.Suki B. Fluctuations and power laws in pulmonary physiology. Am J Respir Crit Care Med. 2002;166:133–137. doi: 10.1164/rccm.200202-152pp. [DOI] [PubMed] [Google Scholar]
- 14.Suki B, Barabási AL, Hantos Z, Peták F, Stanley HE. Avalanches and power-law behaviour in lung inflation. Nature. 1994;368:615–618. doi: 10.1038/368615a0. [DOI] [PubMed] [Google Scholar]
- 15.Dellaca RL, Aliverti A, Lo Mauro A, Lutchen KR, Pedotti A, Suki B. Correlated variability in the breathing pattern and end-expiratory lung volumes in conscious humans. Plos One. 2015;10:e0116317. doi: 10.1371/journal.pone.0116317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glass L. Synchronization and rhythmic processes in physiology. Nature. 2001;410:277–284. doi: 10.1038/35065745. [DOI] [PubMed] [Google Scholar]
- 17.Wirtz HR, Dobbs LG. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science. 1990;250:1266–1269. doi: 10.1126/science.2173861. [DOI] [PubMed] [Google Scholar]
- 18.Arold SP, Bartolák-Suki E, Suki B. Variable stretch pattern enhances surfactant secretion in alveolar type II cells in culture. Am J Physiol Lung Cell Mol Physiol. 2009;296:L574–L581. doi: 10.1152/ajplung.90454.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spieth PM, Güldner A, Huhle R, Beda A, Bluth T, Schreiter D, Ragaller M, Gottschlich B, Kiss T, Jaber S, et al. Short-term effects of noisy pressure support ventilation in patients with acute hypoxemic respiratory failure. Crit Care. 2013;17:R261. doi: 10.1186/cc13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartolák-Suki E, Imsirovic J, Parameswaran H, Wellman TJ, Martinez N, Allen PG, Frey U, Suki B. Fluctuation-driven mechanotransduction regulates mitochondrial-network structure and function. Nat Mater. 2015;14:1049–1057. doi: 10.1038/nmat4358. [DOI] [PubMed] [Google Scholar]
- 21.Gobbi A, Pellegrino R, Gulotta C, Antonelli A, Pompilio P, Crimi C, Torchio R, Dutto L, Parola P, Dellacà RL, et al. Short-term variability in respiratory impedance and effect of deep breath in asthmatic and healthy subjects with airway smooth muscle activation and unloading. J Appl Physiol (1985) 2013;115:708–715. doi: 10.1152/japplphysiol.00013.2013. [DOI] [PubMed] [Google Scholar]
- 22.Muskulus M, Slats AM, Sterk PJ, Verduyn-Lunel S. Fluctuations and determinism of respiratory impedance in asthma and chronic obstructive pulmonary disease. J Appl Physiol (1985) 2010;109:1582–1591. doi: 10.1152/japplphysiol.01414.2009. [DOI] [PubMed] [Google Scholar]
- 23.Diba C, Salome CM, Reddel HK, Thorpe CW, Toelle B, King GG. Short-term variability of airway caliber: a marker of asthma? J Appl Physiol (1985) 2007;103:296–304. doi: 10.1152/japplphysiol.00420.2006. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad S, Tejuja A, Newman KD, Zarychanski R, Seely AJ. Clinical review: a review and analysis of heart rate variability and the diagnosis and prognosis of infection. Crit Care. 2009;13:232. doi: 10.1186/cc8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slats AM, Janssen K, van Schadewijk A, van der Plas DT, Schot R, van den Aardweg JG, de Jongste JC, Hiemstra PS, Mauad T, Rabe KF, et al. Bronchial inflammation and airway responses to deep inspiration in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:121–128. doi: 10.1164/rccm.200612-1814OC. [DOI] [PubMed] [Google Scholar]
- 26.Künzli N, Stutz EZ, Perruchoud AP, Brändli O, Tschopp JM, Bolognini G, Karrer W, Schindler C, Ackermann-Liebrich U, Leuenberger P The SPALDIA Team. Peak flow variability in the SAPALDIA study and its validity in screening for asthma-related conditions. Am J Respir Crit Care Med. 1999;160:427–434. doi: 10.1164/ajrccm.160.2.9807008. [DOI] [PubMed] [Google Scholar]
- 27.Bates JH, Davis GS, Majumdar A, Butnor KJ, Suki B. Linking parenchymal disease progression to changes in lung mechanical function by percolation. Am J Respir Crit Care Med. 2007;176:617–623. doi: 10.1164/rccm.200611-1739OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brand PL, Duiverman EJ, Waalkens HJ, van Essen-Zandvliet EE, Kerrebijn KF Dutch CNSLD Study Group. Peak flow variation in childhood asthma: correlation with symptoms, airways obstruction, and hyperresponsiveness during long-term treatment with inhaled corticosteroids. Thorax. 1999;54:103–107. doi: 10.1136/thx.54.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerstjens HA, Brand PL, de Jong PM, Koëter GH, Postma DS The Dutch CNSLD Study Group. Influence of treatment on peak expiratory flow and its relation to airway hyperresponsiveness and symptoms. Thorax. 1994;49:1109–1115. doi: 10.1136/thx.49.11.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddel HK, Bateman ED, Becker A, Boulet LP, Cruz AA, Drazen JM, Haahtela T, Hurd SS, Inoue H, de Jongste JC, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J. 2015;46:622–639. doi: 10.1183/13993003.00853-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 32.Bateman ED, Reddel HK, van Zyl-Smit RN, Agusti A. The asthma–COPD overlap syndrome: towards a revised taxonomy of chronic airways diseases? Lancet Respir Med. 2015;3:719–728. doi: 10.1016/S2213-2600(15)00254-4. [DOI] [PubMed] [Google Scholar]
- 33.Boots AW, Bos LD, van der Schee MP, van Schooten FJ, Sterk PJ. Exhaled molecular fingerprinting in diagnosis and monitoring: validating volatile promises. Trends Mol Med. 2015;21:633–644. doi: 10.1016/j.molmed.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Baldwin DR, Gannon P, Bright P, Newton DT, Robertson A, Venables K, Graneek B, Barker RD, Cartier A, Malo JL, et al. Interpretation of occupational peak flow records: level of agreement between expert clinicians and Oasys-2. Thorax. 2002;57:860–864. doi: 10.1136/thorax.57.10.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brand PL, Caudri D, Eber E, Gaillard EA, Garcia-Marcos L, Hedlin G, Henderson J, Kuehni CE, Merkus PJ, Pedersen S, et al. Classification and pharmacological treatment of preschool wheezing: changes since 2008. Eur Respir J. 2014;43:1172–1177. doi: 10.1183/09031936.00199913. [DOI] [PubMed] [Google Scholar]
- 36.Reddel HK, Taylor DR, Bateman ED, Boulet L-P, Boushey HA, Busse WW, Casale TB, Chanez P, Enright PL, Gibson PG, et al. American Thoracic Society/European Respiratory Society Task Force on Asthma Control and Exacerbations. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 37.Reddel H, Ware S, Marks G, Salome C, Jenkins C, Woolcock A.Differences between asthma exacerbations and poor asthma control Lancet 1999353364–369.[Published erratum appears in Lancet 353:758.] [DOI] [PubMed] [Google Scholar]
- 38.Jansen J, McCaffery KJ, Hayen A, Ma D, Reddel HK. Impact of graphic format on perception of change in biological data: implications for health monitoring in conditions such as asthma. Prim Care Respir J. 2012;21:94–100. doi: 10.4104/pcrj.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foster JM, Usherwood T, Smith L, Sawyer SM, Xuan W, Rand CS, Reddel HK. Inhaler reminders improve adherence with controller treatment in primary care patients with asthma. J Allergy Clin Immunol. 2014;134:1260–1268.e3. doi: 10.1016/j.jaci.2014.05.041. [DOI] [PubMed] [Google Scholar]
- 40.Lacasse Y, Archibald H, Ernst P, Boulet LP. Patterns and determinants of compliance with inhaled steroids in adults with asthma. Can Respir J. 2005;12:211–217. doi: 10.1155/2005/375454. [DOI] [PubMed] [Google Scholar]
- 41.Sluiter HJ, Koëter GH, de Monchy JG, Postma DS, de Vries K, Orie NG. The Dutch hypothesis (chronic non-specific lung disease) revisited. Eur Respir J. 1991;4:479–489. [PubMed] [Google Scholar]
- 42.Agusti A, Bel E, Thomas M, Vogelmeier C, Brusselle G, Holgate S, Humbert M, Jones P, Gibson PG, Vestbo J, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47:410–419. doi: 10.1183/13993003.01359-2015. [DOI] [PubMed] [Google Scholar]
- 43.Auffray C, Adcock IM, Chung KF, Djukanovic R, Pison C, Sterk PJ. An integrative systems biology approach to understanding pulmonary diseases. Chest. 2010;137:1410–1416. doi: 10.1378/chest.09-1850. [DOI] [PubMed] [Google Scholar]
- 44.Wheelock CE, Goss VM, Balgoma D, Nicholas B, Brandsma J, Skipp PJ, Snowden S, Burg D, D’Amico A, Horvath I, et al. U-BIOPRED Study Group. Application of ’omics technologies to biomarker discovery in inflammatory lung diseases. Eur Respir J. 2013;42:802–825. doi: 10.1183/09031936.00078812. [DOI] [PubMed] [Google Scholar]
- 45.Periyasamy S, Gray A, Kille P. The bottom-up approach to defining life: deciphering the functional organization of biological cells via multi-objective representation of biological complexity from molecules to cells. Front Physiol. 2013;4:369. doi: 10.3389/fphys.2013.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sung J, Wang Y, Chandrasekaran S, Witten DM, Price ND. Molecular signatures from omics data: from chaos to consensus. Biotechnol J. 2012;7:946–957. doi: 10.1002/biot.201100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372:1107–1119. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- 48.Ghebre MA, Bafadhel M, Desai D, Cohen SE, Newbold P, Rapley L, Woods J, Rugman P, Pavord ID, Newby C, et al. Biological clustering supports both “Dutch” and “British” hypotheses of asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2015;135:63–72. doi: 10.1016/j.jaci.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McShane LM, Cavenagh MM, Lively TG, Eberhard DA, Bigbee WL, Williams PM, Mesirov JP, Polley MY, Kim KY, Tricoli JV, et al. Criteria for the use of omics-based predictors in clinical trials: explanation and elaboration. BMC Med. 2013;11:220. doi: 10.1186/1741-7015-11-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh D, Fox SM, Tal-Singer R, Bates S, Riley JH, Celli B. Altered gene expression in blood and sputum in COPD frequent exacerbators in the ECLIPSE cohort. Plos One. 2014;9:e107381. doi: 10.1371/journal.pone.0107381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van den Berge M, Steiling K, Timens W, Hiemstra PS, Sterk PJ, Heijink IH, Liu G, Alekseyev YO, Lenburg ME, Spira A, et al. Airway gene expression in COPD is dynamic with inhaled corticosteroid treatment and reflects biological pathways associated with disease activity. Thorax. 2014;69:14–23. doi: 10.1136/thoraxjnl-2012-202878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Persson H, Kwon AT, Ramilowski JA, Silberberg G, Söderhäll C, Orsmark-Pietras C, Nordlund B, Konradsen JR, de Hoon MJ, Melén E, et al. Transcriptome analysis of controlled and therapy-resistant childhood asthma reveals distinct gene expression profiles. J Allergy Clin Immunol. 2015;136:638–648. doi: 10.1016/j.jaci.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 53.Wagener AH, Zwinderman AH, Luiten S, Fokkens WJ, Bel EH, Sterk PJ, van Drunen CM. The impact of allergic rhinitis and asthma on human nasal and bronchial epithelial gene expression. Plos One. 2013;8:e80257. doi: 10.1371/journal.pone.0080257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yick CY, Zwinderman AH, Kunst PW, Grünberg K, Mauad T, Chowdhury S, Bel EH, Baas F, Lutter R, Sterk PJ. Gene expression profiling of laser microdissected airway smooth muscle tissue in asthma and atopy. Allergy. 2014;69:1233–1240. doi: 10.1111/all.12452. [DOI] [PubMed] [Google Scholar]
- 55.Burrowes KS, De Backer J, Smallwood R, Sterk PJ, Gut I, Wirix-Speetjens R, Siddiqui S, Owers-Bradley J, Wild J, Maier D, et al. Multi-scale computational models of the airways to unravel the pathophysiological mechanisms in asthma and chronic obstructive pulmonary disease (AirPROM) Interface Focus. 2013;3:20120057. doi: 10.1098/rsfs.2012.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ayres JG, Miles JF, Barnes PJ. Brittle asthma. Thorax. 1998;53:315–321. doi: 10.1136/thx.53.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boezen HM, Schouten JP, Postma DS, Rijcken B. Relation between respiratory symptoms, pulmonary function and peak flow variability in adults. Thorax. 1995;50:121–126. doi: 10.1136/thx.50.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thamrin C, Taylor DR, Jones SL, Suki B, Frey U. Variability of lung function predicts loss of asthma control following withdrawal of inhaled corticosteroid treatment. Thorax. 2010;65:403–408. doi: 10.1136/thx.2009.129668. [DOI] [PubMed] [Google Scholar]
- 59.Honkoop PJ, Taylor DR, Smith AD, Snoeck-Stroband JB, Sont JK. Early detection of asthma exacerbations by using action points in self-management plans. Eur Respir J. 2013;41:53–59. doi: 10.1183/09031936.00205911. [DOI] [PubMed] [Google Scholar]
- 60.Frey U, Brodbeck T, Majumdar A, Taylor DR, Town GI, Silverman M, Suki B. Risk of severe asthma episodes predicted from fluctuation analysis of airway function. Nature. 2005;438:667–670. doi: 10.1038/nature04176. [DOI] [PubMed] [Google Scholar]
- 61.Thamrin C, Nydegger R, Stern G, Chanez P, Wenzel SE, Watt RA, FitzPatrick S, Taylor DR, Frey U. Associations between fluctuations in lung function and asthma control in two populations with differing asthma severity. Thorax. 2011;66:1036–1042. doi: 10.1136/thx.2010.156489. [DOI] [PubMed] [Google Scholar]
- 62.Stern G, de Jongste J, van der Valk R, Baraldi E, Carraro S, Thamrin C, Frey U. Fluctuation phenotyping based on daily fraction of exhaled nitric oxide values in asthmatic children. J Allergy Clin Immunol. 2011;128:293–300. doi: 10.1016/j.jaci.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 63.Brand PL, Postma DS, Kerstjens HA, Koëter GH, Group TDCS The Dutch CNSLD Study Group. Relationship of airway hyperresponsiveness to respiratory symptoms and diurnal peak flow variation in patients with obstructive lung disease. Am Rev Respir Dis. 1991;143:916–921. doi: 10.1164/ajrccm/143.5_Pt_1.916. [DOI] [PubMed] [Google Scholar]
- 64.van den Berge M, Hop WC, van der Molen T, van Noord JA, Creemers JP, Schreurs AJ, Wouters EF, Postma DS COSMIC (COPD and Seretide: a Multi-Center Intervention and Characterization) study group. Prediction and course of symptoms and lung function around an exacerbation in chronic obstructive pulmonary disease. Respir Res. 2012;13:44. doi: 10.1186/1465-9921-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Donaldson GC, Seemungal TA, Hurst JR, Wedzicha JA. Detrended fluctuation analysis of peak expiratory flow and exacerbation frequency in COPD. Eur Respir J. 2012;40:1123–1129. doi: 10.1183/09031936.00180811. [DOI] [PubMed] [Google Scholar]
- 66.Que CL, Kenyon CM, Olivenstein R, Macklem PT, Maksym GN. Homeokinesis and short-term variability of human airway caliber. J Appl Physiol (1985) 2001;91:1131–1141. doi: 10.1152/jappl.2001.91.3.1131. [DOI] [PubMed] [Google Scholar]
- 67.Lall CA, Cheng N, Hernandez P, Pianosi PT, Dali Z, Abouzied A, Maksym GN. Airway resistance variability and response to bronchodilator in children with asthma. Eur Respir J. 2007;30:260–268. doi: 10.1183/09031936.00064006. [DOI] [PubMed] [Google Scholar]
- 68.Robinson PD, Brown NJ, Turner M, Van Asperen P, Selvadurai H, King GG. Increased day-to-day variability of forced oscillatory resistance in poorly controlled or persistent pediatric asthma. Chest. 2014;146:974–981. doi: 10.1378/chest.14-0288. [DOI] [PubMed] [Google Scholar]
- 69.Trübel H, Banikol WK. Variability analysis of oscillatory airway resistance in children. Eur J Appl Physiol. 2005;94:364–370. doi: 10.1007/s00421-005-1372-x. [DOI] [PubMed] [Google Scholar]
- 70.Gulotta C, Suki B, Brusasco V, Pellegrino R, Gobbi A, Pedotti A, Dellacà RL. Monitoring the temporal changes of respiratory resistance: a novel test for the management of asthma. Am J Respir Crit Care Med. 2012;185:1330–1331. doi: 10.1164/ajrccm.185.12.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gobbi A, Dellaca RL, King GG, Thamrin C. Towards predicting individual risk in asthma using daily home monitoring of resistance. Am J Respir Crit Care Med. In press doi: 10.1164/rccm.201603-0537LE. [DOI] [PubMed] [Google Scholar]
- 72.Veiga J, Lopes AJ, Jansen JM, Melo PL. Fluctuation analysis of respiratory impedance waveform in asthmatic patients: effect of airway obstruction. Med Biol Eng Comput. 2012;50:1249–1259. doi: 10.1007/s11517-012-0957-x. [DOI] [PubMed] [Google Scholar]
- 73.Timmins SC, Coatsworth N, Palnitkar G, Thamrin C, Farrow CE, Schoeffel RE, Berend N, Diba C, Salome CM, King GG. Day-to-day variability of oscillatory impedance and spirometry in asthma and COPD. Respir Physiol Neurobiol. 2013;185:416–424. doi: 10.1016/j.resp.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 74.Paredi P, Goldman M, Alamen A, Ausin P, Usmani OS, Pride NB, Barnes PJ. Comparison of inspiratory and expiratory resistance and reactance in patients with asthma and chronic obstructive pulmonary disease. Thorax. 2010;65:263–267. doi: 10.1136/thx.2009.120790. [DOI] [PubMed] [Google Scholar]
- 75.Aarli BB, Calverley PM, Jensen RL, Eagan TM, Bakke PS, Hardie JA. Variability of within-breath reactance in COPD patients and its association with dyspnoea. Eur Respir J. 2015;45:625–634. doi: 10.1183/09031936.00051214. [DOI] [PubMed] [Google Scholar]
- 76.Timmins SC, Diba C, Thamrin C, Berend N, Salome CM, King GG. The feasibility of home monitoring of impedance with the forced oscillation technique in chronic obstructive pulmonary disease subjects. Physiol Meas. 2013;34:67–81. doi: 10.1088/0967-3334/34/1/67. [DOI] [PubMed] [Google Scholar]
- 77.Gonem S, Umar I, Burke D, Desai D, Corkill S, Owers-Bradley J, Brightling CE, Siddiqui S. Airway impedance entropy and exacerbations in severe asthma. Eur Respir J. 2012;40:1156–1163. doi: 10.1183/09031936.00228611. [DOI] [PubMed] [Google Scholar]
- 78.Veiga J, Faria RC, Esteves GP, Lopes AJ, Jansen JM, Melo PL. Approximate entropy as a measure of the airflow pattern complexity in asthma. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:2463–2466. doi: 10.1109/IEMBS.2010.5626547. [DOI] [PubMed] [Google Scholar]
- 79.Thamrin C, Stern G, Strippoli MP, Kuehni CE, Suki B, Taylor DR, Frey U. Fluctuation analysis of lung function as a predictor of long-term response to beta2-agonists. Eur Respir J. 2009;33:486–493. doi: 10.1183/09031936.00106208. [DOI] [PubMed] [Google Scholar]
- 80.van der Valk RJ, Baraldi E, Stern G, Frey U, de Jongste JC. Daily exhaled nitric oxide measurements and asthma exacerbations in children. Allergy. 2012;67:265–271. doi: 10.1111/j.1398-9995.2011.02734.x. [DOI] [PubMed] [Google Scholar]
- 81.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, Wardlaw AJ, Green RH. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moore WC. The natural history of asthma phenotypes identified by cluster analysis: looking for chutes and ladders. Am J Respir Crit Care Med. 2013;188:521–522. doi: 10.1164/rccm.201307-1203ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prosperi MC, Sahiner UM, Belgrave D, Sackesen C, Buchan IE, Simpson A, Yavuz TS, Kalayci O, Custovic A. Challenges in identifying asthma subgroups using unsupervised statistical learning techniques. Am J Respir Crit Care Med. 2013;188:1303–1312. doi: 10.1164/rccm.201304-0694OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rubner Y, Tomasi C, Guibas LJ. Presented at the 1998 IEEE Sixth International Conference on Computer Vision. Bombay, India: January 1998. A metric for distributions with applications to image databases; pp. 59–66. [Google Scholar]
- 85.Depner M, Fuchs O, Genuneit J, Karvonen AM, Hyvärinen A, Kaulek V, Roduit C, Weber J, Schaub B, Lauener R, et al. PASTURE Study Group. Clinical and epidemiologic phenotypes of childhood asthma. Am J Respir Crit Care Med. 2014;189:129–138. doi: 10.1164/rccm.201307-1198OC. [DOI] [PubMed] [Google Scholar]
- 86.Detsky AS, Stricker SC, Mulley AG, Thibault GE. Prognosis, survival, and the expenditure of hospital resources for patients in an intensive-care unit. N Engl J Med. 1981;305:667–672. doi: 10.1056/NEJM198109173051204. [DOI] [PubMed] [Google Scholar]
- 87.Esteban A, Alía I, Tobin MJ, Gil A, Gordo F, Vallverdú I, Blanch L, Bonet A, Vázquez A, de Pablo R, et al. Spanish Lung Failure Collaborative Group. Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Am J Respir Crit Care Med. 1999;159:512–518. doi: 10.1164/ajrccm.159.2.9803106. [DOI] [PubMed] [Google Scholar]
- 88.Esteban A, Frutos F, Tobin MJ, Alía I, Solsona JF, Valverdú I, Fernández R, de la Cal MA, Benito S, Tomás R, et al. Spanish Lung Failure Collaborative Group. A comparison of four methods of weaning patients from mechanical ventilation. N Engl J Med. 1995;332:345–350. doi: 10.1056/NEJM199502093320601. [DOI] [PubMed] [Google Scholar]
- 89.Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. 1991;324:1445–1450. doi: 10.1056/NEJM199105233242101. [DOI] [PubMed] [Google Scholar]
- 90.Bravi A, Longtin A, Seely AJ. Review and classification of variability analysis techniques with clinical applications. Biomed Eng Online. 2011;10:90. doi: 10.1186/1475-925X-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ahmad S, Ramsay T, Huebsch L, Flanagan S, McDiarmid S, Batkin I, McIntyre L, Sundaresan SR, Maziak DE, Shamji FM, et al. Continuous multi-parameter heart rate variability analysis heralds onset of sepsis in adults. Plos One. 2009;4:e6642. doi: 10.1371/journal.pone.0006642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bravi A, Green G, Longtin A, Seely AJ. Monitoring and identification of sepsis development through a composite measure of heart rate variability. Plos One. 2012;7:e45666. doi: 10.1371/journal.pone.0045666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bradley BD, Green G, Ramsay T, Seely AJ. Impact of sedation and organ failure on continuous heart and respiratory rate variability monitoring in critically ill patients: a pilot study. Crit Care Med. 2013;41:433–444. doi: 10.1097/CCM.0b013e31826a47de. [DOI] [PubMed] [Google Scholar]
- 94.Green GC, Bradley B, Bravi A, Seely AJ. Continuous multiorgan variability analysis to track severity of organ failure in critically ill patients. J Crit Care. 2013;28:879.e1–11. doi: 10.1016/j.jcrc.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 95.Brack T, Jubran A, Tobin MJ. Dyspnea and decreased variability of breathing in patients with restrictive lung disease. Am J Respir Crit Care Med. 2002;165:1260–1264. doi: 10.1164/rccm.2201018. [DOI] [PubMed] [Google Scholar]
- 96.Bien MY, Hseu SS, Yien HW, Kuo BI, Lin YT, Wang JH, Kou YR. Breathing pattern variability: a weaning predictor in postoperative patients recovering from systemic inflammatory response syndrome. Intensive Care Med. 2004;30:241–247. doi: 10.1007/s00134-003-2073-8. [DOI] [PubMed] [Google Scholar]
- 97.El-Khatib M, Jamaleddine G, Soubra R, Muallem M. Pattern of spontaneous breathing: potential marker for weaning outcome. Spontaneous breathing pattern and weaning from mechanical ventilation. Intensive Care Med. 2001;27:52–58. doi: 10.1007/s001340000758. [DOI] [PubMed] [Google Scholar]
- 98.Seely AJ, Bravi A, Herry C, Green G, Longtin A, Ramsay T, Fergusson D, McIntyre L, Kubelik D, Maziak DE, et al. Canadian Critical Care Trials Group (CCCTG) Do heart and respiratory rate variability improve prediction of extubation outcomes in critically ill patients? Crit Care. 2014;18:R65. doi: 10.1186/cc13822. [DOI] [PMC free article] [PubMed] [Google Scholar]