Abstract
Rationale: Pulmonary aspergillosis is a lethal mold infection in the immunocompromised host. Understanding initial control of infection and how this is altered in the immunocompromised host are key goals for comprehension of the pathogenesis of pulmonary aspergillosis.
Objectives: To characterize the outcome of human macrophage infection with Aspergillus fumigatus and how this is altered in transplant recipients on calcineurin inhibitor immunosuppressants.
Methods: We defined the outcome of human macrophage infection with A. fumigatus, as well as the impact of calcineurin inhibitors, through a combination of single-cell fluorescence imaging, transcriptomics, proteomics, and in vivo studies.
Measurements and Main Results: Macrophage phagocytosis of A. fumigatus enabled control of 90% of fungal germination. However, fungal germination in the late phagosome led to macrophage necrosis. During programmed necroptosis, we observed frequent cell–cell transfer of A. fumigatus between macrophages, which assists subsequent control of germination in recipient macrophages. Lateral transfer occurred through actin-dependent exocytosis of the late endosome in a vasodilator-stimulated phosphoprotein envelope. Its relevance to the control of fungal germination was also shown by direct visualization in our zebrafish aspergillosis model in vivo. The calcineurin inhibitor FK506 (tacrolimus) reduced cell death and lateral transfer in vitro by 50%. This resulted in uncontrolled fungal germination in macrophages and also resulted in hyphal escape.
Conclusions: These observations identify programmed, necrosis-dependent lateral transfer of A. fumigatus between macrophages as an important host strategy for controlling fungal germination. This process is critically dependent on calcineurin. Our studies provide fundamental insights into the pathogenesis of pulmonary aspergillosis in the immunocompromised host.
Keywords: pulmonary fungal diseases, macrophage, necrosis, Aspergillus, calcineurin
At a Glance Commentary
Scientific Knowledge on the Subject
Macrophages are the frontline defense against pulmonary infection with the mold pathogen Aspergillus fumigatus. Phagocytosis and generation of reactive oxygen species are known to be crucial for control of infection. However, the cellular outcome from progressive macrophage infection with A. fumigatus has not been studied systematically.
What This Study Adds to the Field
We present a systematic analysis of human macrophage infection with A. fumigatus. We show that successful fungal germination in the phagosome leads to necrotic cell death and can result in cell–cell transfer of germinating A. fumigatus between macrophages. Ultimately, this process assists control of fungal germination and is orchestrated through the calcium-responsive serine-threonine phosphatase calcineurin. Our systematic analysis of the macrophage response to A. fumigatus defines necroptosis as a key early event in the pathogenesis of pulmonary aspergillosis.
Aspergillus fumigatus (Af) is a mold with small airborne conidia that are a ubiquitous component of the aerial mycobiota (1). Normally inhaled conidia are cleared by alveolar macrophages to prevent fungal germination, tissue invasion, and destructive lung disease (2, 3). Our previous work with murine and zebrafish models of invasive aspergillosis showed the importance of macrophages in control of Af early postinfection, with neutrophil influx as a later event (4, 5). Previous studies have highlighted the importance of inflammatory monocytes in murine invasive aspergillosis (6).
A spectrum of immunocompromised states predisposes individuals to high-mortality invasive or chronic forms of pulmonary aspergillosis (PA) (7). Organ transplant recipients are at high risk of PA, with lung transplant recipients being particularly susceptible and having a mortality greater than 40% (8, 9). There are an estimated 3 million individuals with chronic PA and 4.5 million with allergic bronchopulmonary aspergillosis globally (10, 11). Af has also been implicated in the pathogenesis of asthma, chronic obstructive airways disease, and bronchiectasis (11–13). Treatment options remain limited, and high mortality rates persist (14). Better understanding of susceptibility to PA in the nonneutropenic host is a key clinical research priority.
Calcineurin–nuclear factor of activated T cells (NFAT) signaling is the target of the calcineurin inhibitor immunosuppressants used in organ transplantation (15). We have previously shown that calcineurin inhibitor immunosuppressants (FK506 [tacrolimus]) increase susceptibility to murine PA through inhibition of the macrophage Toll-like receptor 9–calcineurin–NFAT pathway (4, 5). This pathway is crucial for activation of inflammatory responses to Af and for recruitment of fungicidal neutrophils to the site of infection. Calcineurin-NFAT signaling has also been shown to be critical for the innate immune response to Candida albicans and Escherichia coli through endocytic mechanisms convergent on phospholipase C-γ–dependent calcium flux (16, 17). These observations underscore the importance of calcineurin-NFAT signaling for myeloid immunity, and they reveal a novel relationship between innate sensing and calcineurin-NFAT signaling in the lung that requires further clinical definition.
Here we show that host programmed necrosis is an important outcome of Af germination in the human macrophage. Cell death occurred in response to phagosomal germination, with necroptosis-associated lateral transfer of Af between macrophages. Cell death–dependent transfer was calcineurin dependent and enabled control of germination in recipient macrophages. Crucially, inhibition of either calcineurin-dependent cell death or lateral transfer enabled hyphal escape from the macrophage. In this study, we identify programmed necrosis as a key cellular response in PA and show that it is likely to be critically impaired in organ transplant recipients taking calcineurin inhibitors. Our studies reveal cell–cell transfer as a novel and important cell death–associated defense mechanism that enables control of fungal germination in the myeloid compartment.
Methods
Ethics Statement
The present study was approved by the Biomedical Research Unit (National Research Ethics Service reference 10/H0504/9), Royal Brompton and Harefield NHS Trust (AS1). All experiments conformed to the World Medical Association Declaration of Helsinki. Written informed consent was obtained from all participants.
Zebrafish Care and Maintenance
Our experiments were approved by the U.K. Home Office in accordance with project license PPL 70/7446. Full details are given in the Experimental Procedures section of the online supplement.
Fungal Strains and Culture
A. fumigatus strain CEA10 was used for Western blotting, Luminex Multiplex (Luminex, Austin, TX), fungal burden, ImageStream (EMD Millipore, Billerica, MA), phosphoproteomic, and RNA sequencing experiments. ATCC 46645 enhanced green fluorescent protein (American Type Culture Collection, Manassas, VA), a gift from Frank Ebel, was used for microscopy experiments. See Experimental Procedures section in the online supplement for details.
Isolation of Human Macrophages
Human alveolar macrophages (hAMs) were isolated from bronchoalveolar lavage by adherence. Purified hAMs were rested for 3 days. Monocytes were isolated from peripheral blood mononuclear cells from healthy volunteers by Ficoll-Paque gradient centrifugation and negative magnetic bead isolation (Pan Monocyte Isolation Kit; Miltenyi Biotec, Auburn, CA). Human monocyte-derived macrophages (hMDMs) were differentiated using 5 ng/ml granulocyte-macrophage colony–stimulating factor (GM-CSF) and 10% human serum for 7 days. See Experimental Procedures section in the online supplement for details.
RNA-seq Analysis
Illumina RNA-seq libraries (Illumina, San Diego, CA) were prepared from hMDMs (n = 6 healthy donors) pretreated with 10 ng/ml FK506 for 1 hour or vehicle and stimulated with live Af swollen conidia (SC) (multiplicity of infection, 1) for 1 hour and 6 hours. Illumina paired-end reads were mapped to the human reference genome (hg19) using TopHat2 (18) (Gene Expression Omnibus database accession number GSE74924), and differential expression analyses were performed using Cufflinks v2.2.1 (19). Gene analyses were performed using DAVID v6.7 (20). Human protein–protein interaction network modules were obtained using MCODE (21) analysis in Cytoscape. See Experimental Procedures section in the online supplement for details.
Phosphoproteomic Analysis
Analysis with the Phospho Explorer Antibody Microarray was conducted by Full Moon BioSystems Inc. (Sunnyvale, CA). hMDMs were differentiated at 1 × 107 cells in 75-mm2 tissue culture flasks. At Day 7, cells were stimulated with live Af SC (multiplicity of infection, 1) following pretreatment for 1 hour with FK506 (10 ng/ml; Calbiochem, San Diego, CA) or vehicle (dimethyl sulfoxide diluted in RPMI 1640 medium). At 1 hour postinfection, cells were washed five times with 10 ml of ice-cold phosphate-buffered saline containing protease inhibitors (Cell Signaling Technology, Danvers, MA) and collected by centrifugation (250 × g for 10 min at 4°C). Cells were frozen at −80°C and transferred to Full Moon BioSystems on dry ice. The array consisted of 1,318 phospho-specific antibodies. Proteins were labeled with biotin and adhered to preblocked microarray slides. After a washing step, detection of total and phosphorylated proteins was conducted using cyanine 3–conjugated streptavidin. Expression of phosphorylated proteins was normalized to the corresponding total protein abundance. Fold change was calculated as the phosphoprotein-to-nonphosphoprotein ratio of FK506-treated cells divided by the phosphoprotein-to-nonphosphoprotein ratio of untreated cells.
Statistical Analysis
The results are presented as mean ± SEM and were analyzed using Prism software (version 6.0; GraphPad Software, La Jolla, CA). Significance was determined using Student’s t test for unpaired observations. When three or more groups were compared, we used one-way analysis of variance with the Bonferroni correction. Full details of the experimental procedures are available in the online supplement.
Results
A. fumigatus Activates Calcineurin-NFAT–Dependent Inflammatory Responses in Human Macrophages
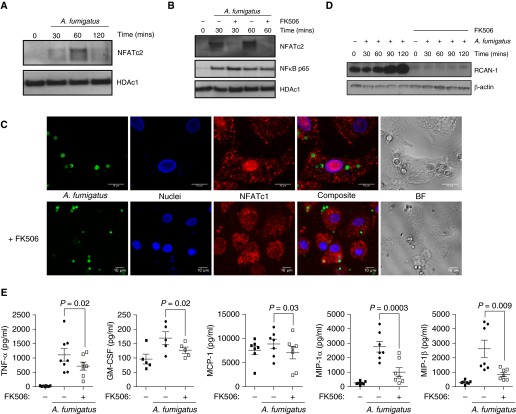
Af phagocytosis activates NFAT nuclear translocation in murine macrophages (4). We therefore characterized the role of NFAT in hMDMs. hMDMs were infected with live Af SC or resting conidia (RC), and NFAT translocation was determined by Western blot analysis and confocal microscopy. SC phagocytosis led to sustained NFAT translocation after 30 minutes (Figures 1A and 1B; see also Figure E1A in the online supplement). RC induced a weak, transient NFAT activation response (Figures E1B and E1C). NFAT translocation in response to SC was confirmed in hAMs from lung transplant recipients (Figure E1D). Maximum translocation of NFATc2 to the nucleus was seen in response to Af at 60 minutes postinfection. NFAT translocation was blocked by the calcineurin inhibitor FK506 (Figures 1B, 1C, and E1E), but nuclear factor-κB Rel65 translocation was unaffected (Figure 1B).
Figure 1.
Aspergillus fumigatus (Af) activates calcineurin–nuclear factor of activated T cells (NFAT)-dependent inflammatory responses in human macrophages. (A) Human monocyte-derived macrophages (hMDMs) were stimulated with live Af swollen conidia (SC) (multiplicity of infection [MOI], 1). hMDM nuclear extracts were collected at 30, 60, and 120 minutes postinfection and probed by Western blotting for NFAT, cytoplasmic, calcineurin-dependent 2 (NFATc2). The maximum translocation of NFATc2 to the nucleus was observed at 60 minutes postinfection. Western blots show representative data from four experiments. (B and C) hMDMs were pretreated with FK506 (10 ng/ml) and stimulated with live Af SC (MOI, 1). Nuclear extracts were probed by Western blotting for NFATc2 and whole-cell extracts for regulator of calcineurin 1 (RCAN-1). NFAT translocation was assessed by confocal microscopy. NFAT is shown in red, nuclei in blue, and Af conidia in green. Representative images of live conidia stimulating NFAT nuclear translocation are shown. Western blots show representative data from four experiments. (D) hMDMs were pretreated with FK506 (10 ng/ml) and stimulated with live Af SC (MOI, 1). FK506 completely inhibited RCAN-1 production. Western blot shows representative data from four experiments. (E) hMDMs were pretreated with FK506 (10 ng/ml) and stimulated with live Af SC (MOI, 1). Culture supernatant cytokines were measured at 6 hours postinfection by Luminex assay (n = 7 biological replicates). Scale bars = 10 μm. BF = brightfield; GM-CSF = granulocyte-macrophage colony–stimulating factor; HDAc1 = histone deacetylase 1; MCP-1 = monocyte chemoattractant protein 1; MIP-1 = macrophage inflammatory protein 1; NF-κB = nuclear factor-κB; TNF-α = tumor necrosis factor-α.
NFAT transcriptional activity was defined by measuring expression of the NFAT-specific target regulator of calcineurin 1 (RCAN1). SC infection increased RCAN1 expression, which was abrogated by FK506 (Figures 1D and E1F). To determine the role of the calcineurin-NFAT pathway in inflammatory responses, we quantified chemokine and cytokine production in hMDMs during SC infection. Expression of tumor necrosis factor-α (TNF-α), GM-CSF, monocyte chemoattractant protein 1 (MCP-1), MCP-1α, and MCP-1β was attenuated by calcineurin inhibition (P = 0.02, P = 0.02, P = 0.03, P = 0.0003, and P = 0.009, respectively) (Figures 1E and E1G). Flow cytometric characterization of hMDMs and lung transplant hAMs revealed similar inflammatory activation status with CD11c+/major histocompatibility complex class II+/CD206+ surface expression. There were significant differences in surface CD11b (P ≤ 0.001) and CD86 (P ≤ 0.001) expression (Figure E1H). Blockade of the fungal C-type lectin receptor Dectin-1 (P = 0.001) or inhibition of downstream Syk signaling (P = 0.001) impaired TNF-α responses, but it had no effect on NFAT translocation (Figures E1I and E1J). These results indicate that the calcineurin-NFAT signaling pathway is critical for early macrophage inflammatory responses to Af, independent of Dectin-1 and Syk, in humans.
Human Macrophage Phagocytosis, Reactive Oxygen Species Production, and Killing of A. fumigatus Are Calcineurin Dependent
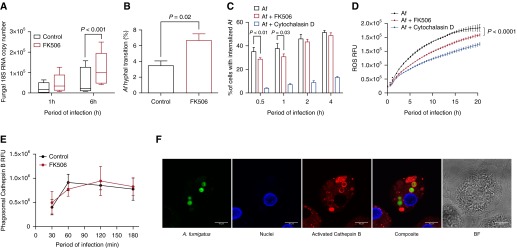
Next, we characterized the role of calcineurin-NFAT in killing of Af. hMDMs were infected with SC following treatment with FK506 or vehicle, and fungal growth was analyzed at 1 and 6 hours postinfection. Both hMDMs and hAMs pretreated with FK506 exhibited impaired control of fungal growth (P < 0.001 and P < 0.0001, respectively) (Figures 2A and E2A). Time-lapse video microscopy demonstrated increased Af hyphal transition in FK506-treated macrophages (P = 0.02) (Figure 2B). To identify the calcineurin-dependent mechanism of fungal growth inhibition, we assessed the efficiency of Af phagocytosis. Calcineurin inhibition delayed phagocytosis of SC in hMDMs (P < 0.01) and hAMs (P = 0.04) early postinfection, but phagocytosis of RC was not affected (Figures 2C, E2B, and E2C). We measured reactive oxygen species (ROS) production and cathepsin B (a lysosomal cysteine protease) activation, which are critical for Af killing (22, 23). ROS production was partially calcineurin dependent (P < 0.0001) but had no effect on mitochondrial ROS (Figures 2D, E2D, and E2E) or activated cathepsin B production (Figures 2E and 2F). Calcineurin-dependent effects on phagosomal maturation were analyzed by phagosomal acidification and recruitment of the late phagosomal maturation marker lysosomal-associated membrane protein 1 (LAMP-1) to Af-containing phagosomes. Calcineurin inhibition increased LAMP-1 recruitment at early time points after infection (P < 0.05) (Figures E2F and E2G). Phagosomal acidification was not calcineurin dependent (Figures E2H and E2I). These results indicate that inhibition of the calcineurin-NFAT pathway impairs the ability of human macrophages to control fungal hyphal transition, delays fungal phagocytosis, and reduces ROS production.
Figure 2.
Human macrophage phagocytosis, reactive oxygen species (ROS) production, and control of Aspergillus fumigatus (Af) growth are calcineurin dependent. (A) Human monocyte-derived macrophages (hMDMs) pretreated with FK506 (10 ng/ml) or vehicle were stimulated with live Af swollen conidia (SC) (multiplicity of infection [MOI], 1), and total RNA was isolated at 1 and 6 hours postinfection. Fungal growth was assayed by reverse transcription–polymerase chain reaction of fungal RNA (n = 5 biological replicates). (B) hMDMs pretreated with FK506 (10 ng/ml) or vehicle were infected with live enhanced green fluorescent protein–expressing Af SC (MOI, 1), and hyphal transition was assessed using time-lapse video microscopy over a 10-hour period (n = 4 biological replicates). (C) hMDMs pretreated with FK506 (10 ng/ml) or cytochalasin D (5 nM) or vehicle were stimulated with live Af SC (MOI, 1) prestained with calcofluor white. Phagocytosis was quantified by using an ImageStream flow cytometer postinfection (n = 4 biological replicates). (D) hMDMs pretreated with FK506 (10 ng/ml) or vehicle were stimulated with live Af SC (MOI, 1), and ROS production was assayed by luminescence (n = 4 biological replicates). (E) hMDMs pretreated with FK506 (10 ng/ml) or vehicle were stimulated with live Af SC (MOI, 1), and cathepsin B activation was assayed by live confocal time-lapse microscopy. Phagosomal cathepsin B activation was quantified by using ImageJ software (National Institutes of Health, Bethesda, MD) (n = 4 biological replicates). (F) Representative confocal microscopic image showing cathepsin B activation in Af conidia–containing phagosomes in hMDMs. Activated cathepsin B is shown in red, nuclei in blue, and Af in green. Data are presented as mean ± SEM. Scale bars = 10 μm. BF = brightfield; RFU = relative fluorescence units.
Macrophages Traffic Af-Containing Endosomes to Neighboring Cells through Calcineurin-Dependent Lateral Transfer
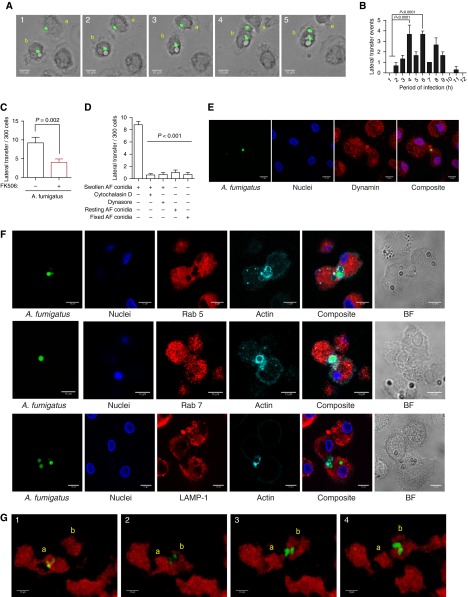
During live time-lapse confocal imaging, we observed lateral transfer of germinating Af between hMDMs (Figure 3A and Video 1). This was confirmed in alveolar macrophages from lung transplant recipients (Video 2). Lateral transfer events occurred in 3.0 ± 0.4% of cells infected with SC, peaking at 4–6 hours postinfection (Figure 3B). Calcineurin inhibition markedly reduced lateral transfer (53.9± 10.3%) (P = 0.002) (Figure 3C).
Figure 3.
Macrophages infected with swollen Aspergillus fumigatus (Af) undergo calcineurin-dependent lateral transfer. (A) Representative time-lapse widefield microscopic images of Af lateral transfer in human monocyte-derived macrophages (hMDMs) infected with live Af swollen conidia (SC) (multiplicity of infection [MOI], 1), shown in green. Transferring macrophage is labeled a, and receiving macrophage is labeled b. Images were taken at 10-minute intervals. (B) hMDMs were stimulated with live enhanced green fluorescent protein (eGFP)-Af SC (MOI, 1), and time-lapse widefield imaging was performed at 10-minute intervals for 12 hours postinfection. The timing of lateral transfer events per hour in macrophages infected with live Af SC is shown (n = 3 biological replicates). (C) hMDMs pretreated with FK506 (10 ng/ml) or vehicle were stimulated with live eGFP-Af SC (MOI, 1), and lateral transfer events were quantified by time-lapse widefield imaging at 10-minute intervals for 12 hours postinfection (n = 5 biological replicates). Total lateral transfer events over the 12-hour period were quantified for each biological replicate. (D) hMDMs were stimulated with live or fixed Af SC or resting conidia (MOI, 1), and lateral transfer events were quantified by time-lapse widefield microscopy. Cytochalasin D (5 nM) and dynasore (100 μM) were added at 90 minutes postinfection, and unbound conidia were washed away 45 minutes postinfection (n = 4 biological replicates). (E) Representative confocal microscopic image of dynamin-dependent lateral transfer in hMDMs stimulated with live Af SC. Dynamin is shown in red, nuclei in blue, and Af in green. (F) hMDMs were stimulated with live Af SC (MOI, 1), and nonphagocytosed conidia were washed away at 45 minutes postinfection. Lateral transfer events were captured by fixing cells every 10 minutes from 120 to 180 minutes postinfection. Characterization of Af lateral transfer was performed by staining for Ras-related protein 5 (Rab 5), Rab 7, and lysosomal-associated membrane protein 1 (LAMP-1) (shown in red) alongside actin (shown in cyan). Nuclei are shown in blue and live Af SC in green. Representative images show positive Rab 7 localization to Af-containing endosomes during lateral transfer, with little Rab 5 localization and no LAMP-1 localization. (G) mpeg:mCherry zebrafish larvae were infected with approximately 50 eGFP-expressing conidia of Af, and time-lapse confocal microscopy was performed. Representative images show Af lateral transfer between macrophages. The transferring macrophage is labeled a, and the receiving macrophage is labeled b. Images shown were taken at 7-minute intervals. Data are represented as mean ± SEM. Scale bars = 10 μm. BF = brightfield.
Video 1.
Human monocyte-derived macrophages infected with swollen Aspergillus fumigatus (Af) undergo lateral transfer. Representative time-lapse widefield microscopy images show Af lateral transfer in human monocyte-derived macrophages infected with live Af swollen conidia (multiplicity of infection, 1; shown in green). Lateral transfer is highlighted with a black arrow. The images were taken at 10-minute intervals.
Video 2.
Human alveolar macrophages (AMs) infected with swollen Aspergillus fumigatus (Af) undergo lateral transfer. Representative time-lapse widefield microscopy images show Af lateral transfer in human AMs infected with live Af swollen conidia (multiplicity of infection, 1; shown in green). Lateral transfer is highlighted with a black arrow. The images were taken at 10-minute intervals. Scale bar = 10 μm.
We assessed the role of fungal germination in lateral transfer of Af by time-lapse microscopy of metabolically inactive RC or dead, fixed SC. Lateral transfer was dependent on both live and swollen Af (P < 0.001) (Figure 3D). We observed colocalization of actin and dynamin to conidia undergoing lateral transfer (Figures 3E and 3F), suggesting an actin- and dynamin-dependent process. This was confirmed by treating hMDMs with actin polymerization or dynamin inhibitors (cytochalasin D or dynasore, respectively) following phagocytosis (P < 0.001) (Figure 3D). Fungal burden was increased after inhibition of lateral transfer post-phagocytosis using dynasore, suggesting that transfer enables recipient macrophage control of hyphal germination (P < 0.01) (Figure E3A). Transmission electron microscopy showed that transfer of Af-containing cargo between hMDMs occurred in membrane-bound compartments (Figure E3B). The Af-containing cargo transferred between hMDMs was further characterized by staining for endolysosomal markers (Ras-related protein 5 [Rab 5], Rab 7, and LAMP-1). This showed that Af was transferred between hMDMs in Rab 7–positive and Rab 5– and LAMP-1–negative compartments, suggesting late endosomal trafficking (Figures 3F and E3C).
To determine whether this phenomenon occurred in vivo, we exploited our zebrafish invasive aspergillosis model (4). Using an mpeg:mCherry transgenic zebrafish line and enhanced green fluorescent protein–expressing Af, we examined the macrophage–Af interaction in vivo using time-lapse high-resolution confocal microscopy. We observed similar macrophage Af lateral transfer in zebrafish larvae, consistent with an evolutionarily conserved macrophage response to Af infection that occurs in vivo (Figure 3G and Video 3). Taken together, these observations indicate that successful fungal germination in the late endosome triggers exocytic transfer of Af between macrophages.
Video 3.
In vivo confirmation of macrophage lateral transfer in zebrafish macrophages infected with swollen Aspergillus fumigatus (Af). MPEG:mCherry Zebrafish larvae were infected with ∼50 enhanced green fluorescent protein–expressing conidia of Af, and time-lapse confocal microscopy was performed. Representative three-dimensional reconstructed images show Af lateral transfer between macrophages. The transfer process is highlighted with a yellow arrow. The images shown are at 7-minute intervals. Scale bar = 10 μm.
Macrophages Undergoing Calcineurin-Dependent Programmed Necrosis Transfer Af Conidia to Neighboring Cells to Control Fungal Germination
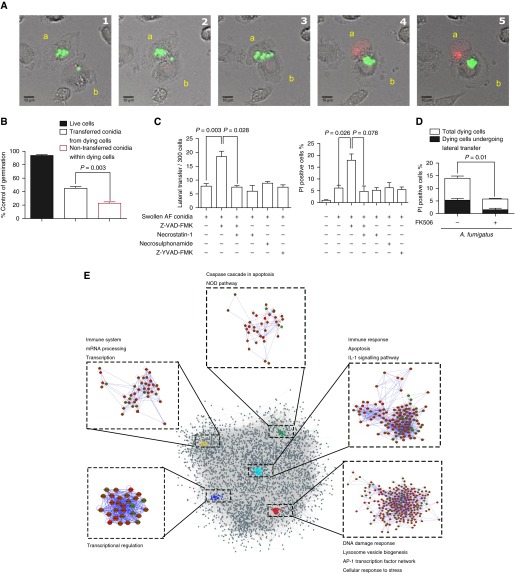
hMDMs transferring Af had increasingly translucent cytoplasm, organelle swelling, and increased cell volume, suggesting programmed cell death during transfer. This was confirmed by confocal microscopy using propidium iodide (Figure 4A and Video 4). Around 90% of macrophages successfully control fungal germination and do not die (Figure 4B). Propidium iodide staining revealed that, in dying macrophages (13 ± 2%), lateral transfer occurred in 37 ± 5% (Figure 4D). This represents approximately 3–4% of total macrophages, as previously described for those infected with Af SC. Transfer of Af conidia from dying hMDMs enhanced control of germination as compared with dying hMDMs, where conidia were not transferred (P = 0.003) (Figure 4B). Germination of Af in live hMDMs was well controlled compared with cells undergoing programmed necrosis (Figure E3D), further supporting a model where programmed cell death and lateral transfer occur in response to successful fungal germination in macrophages to enable containment of infection in recipient macrophages (Figure 4B).
Figure 4.
Macrophages undergoing programmed necrosis laterally transfer germinating Aspergillus fumigatus (Af) conidia in endosomes through an actin-dependent process. (A) Representative time-lapse confocal microscopic images of lateral transfer of live Af swollen conidia (SC) (shown in green) between human monocyte-derived macrophages (hMDMs). Propidium iodide (PI) staining (red) shows cell death of macrophage transferring Af conidia (labeled a) to a neighboring macrophage (labeled b). Images were taken at 10-minute intervals. (B) Time-lapse confocal microscopy was used to quantify the fate of transferred versus nontransferred conidia from dying hMDMs and to conidia within live macrophages. Germination was assessed over a 12-hour period. Control of fungal germination was significantly increased in dying hMDMs that transferred conidia to neighboring cells compared with those that did not (n = 4 biological replicates). (C) hMDMs pretreated with a pan-caspase inhibitor (50 μM Z-VAD-FMK), a receptor-interacting serine/threonine-protein kinase (RIPK-1) inhibitor (10 μM necrostatin-1), a caspase 1 inhibitor (50 μM Z-YVAD-FMK), 10 ng/ml FK506, or vehicle were stimulated with live Af SC (multiplicity of infection [MOI], 1), and lateral transfer events and cell death were quantified by PI fluorescence–based time-lapse confocal microscopy. Treatment with Z-VAD-FMK induced necroptotic cell death coupled to increased Af lateral transfer, which was inhibited by the addition of a RIPK-1 inhibitor (necrostatin-1) (n = 3 biological replicates). (D) hMDMs pretreated FK506 (10 ng/ml) or vehicle were stimulated with live Af SC (MOI, 1), and cell death was assayed by PI fluorescence and quantified by time-lapse confocal microscopy over a 6-hour period. The graph also shows the proportion of lateral transfer events in dying cells (approximately 30% of dying cells) (n = 4 biological replicates). (E) The network modules of the significantly expressed genes in FK506-pretreated macrophages stimulated with live Af SC (MOI, 1) for 6 hours. Significantly expressed genes were mapped onto a protein–protein interaction network, and network modules were identified using the MCODE plugin application of Cytoscape. Overrepresented pathways involving these network modules were identified using the Kyoto Encyclopedia of Genes and Genomes (“KEGG”) and WikiPathways databases (n = 6 biological replicates). Data are presented as mean ± SEM. Scale bars = 10 μm. AP-1 = activator protein 1; NOD = nucleotide-binding oligomerization domain.
Video 4.
Macrophages infected with germinating Aspergillus fumigatus (Af) conidia undergo lateral transfer during programmed cell death. Representative time-lapse confocal microscopy images show lateral transfer of live Af swollen conidia (shown in green) between monocyte-derived macrophages. Propidium iodide staining (red) shows cell death of a macrophage transferring Af conidia to a neighboring macrophage. The transfer process is highlighted by a black arrow. The images were taken at 10-minute intervals.
We observed massive vacuole formation adjacent to the Af-containing compartment consistent with programmed cell death (Figure E3E and Video 5) (24). Transmission electron microscopy also showed conidia residing within large vacuoles (Figure E3F). Vacuole formation was inhibited by pretreatment with the vacuolar H+-ATPase inhibitor bafilomycin (P = 0.003), with a trend toward decreased transfer (P = 0.06) (Figure E3G).
Video 5.
Massive vacuole formation occurs during Aspergillus fumigatus (Af) germination in human monocyte-derived macrophages (hMDMs). Representative time-lapse widefield microscopy images show vacuole formation in hMDMs infected with live Af swollen conidia (multiplicity of infection, 1; shown in green). The images were taken at 10-minute intervals.
To define the relationship between cell death and transfer, induction of necroptosis with the pan-caspase inhibitor Z-VAD-FMK (an inhibitor of caspase-dependent apoptosis that increases necroptosis) was performed (25, 26). This increased lateral transfer (P = 0.003) (Figure 4C). Addition of necrostatin-1, an inhibitor of receptor-interacting serine/threonine-protein kinase 1–dependent necroptosis, to Z-VAD-FMK inhibited both cell death (P = 0.078) and lateral transfer (P = 0.028) induced by Z-VAD-FMK alone (Figure 4C). However, induction of necroptosis with Z-VAD-FMK led to increased fungal burden due to recipient macrophage death (P < 0.01) (Figure E3H). Necrostatin-1 alone or necrosulfonamide could not inhibit macrophage programmed cell death or lateral transfer (Figure 4C). As pyroptosis following macrophage infection with C. albicans was recently shown, we blocked pyroptosis with an irreversible cell-permeable caspase 1 inhibitor (Z-YVAD-FMK), which did not affect cell death or lateral transfer (Figure 4C) (27). These observations are consistent with transfer of Af between macrophages during a programmed cell death process with hallmarks of necroptosis.
As calcineurin regulates cell death, we assessed its role in macrophage necrosis following infection (28). Calcineurin inhibition reduced macrophage programmed necrosis by 57 ± 13.7% (P = 0.01) (Figure 4D) and was associated with accelerated fungal germination at late time points in macrophages that failed to undergo necrosis-dependent transfer (P < 0.001) (Figures E3I and E3J). This is consistent with a calcineurin-dependent checkpoint for programmed necrosis during Af germination.
Together, these results indicate that hMDMs infected with live Af SC undergo calcineurin-dependent cell death with hallmarks of programmed necroptosis and transfer Af-containing endosomes to neighboring cells to facilitate control of fungal germination.
Calcineurin Regulates Cell Death and the Mitogen-activated Protein Kinase Pathway during Af Infection
To further define the macrophage response to Af, we undertook next-generation transcript profiling of hMDMs during phagocytosis. hMDMs pretreated with FK506 were infected with SC for 1 hour or 6 hours, and genes differentially expressed compared with baseline and untreated infection controls were identified (Figure E4A). The major gene sets regulated by calcineurin at 6 hours postinfection were associated with cell death, inflammatory responses, and transcriptional regulation (Figure E4B). Fifty-one genes associated with the cell death pathway were calcineurin dependent at 6 hours postinfection (Table E1).
In overrepresentation analysis using ConsensusPathDB, we identified calcineurin-dependent control of the programmed cell death, mitogen-activated protein kinase (MAPK) signaling, and cytokine–cytokine interaction pathways at 6 hours postinfection (Figure E4C). To assess the interaction between the pathways regulated by calcineurin, a protein–protein interaction network was created. By use of this method, we identified significant interactions and clustering of transcriptional regulation pathways, immune response pathways, apoptosis, and the activator protein 1 (AP-1) transcription factor network (Figure 4E). Other studies have suggested a critical role for the MAPK–AP-1 pathway and dynamin-related protein 1 (Drp1)–mediated mitochondrial fission in necroptosis (25, 29). As mitochondrial translocation of Drp1 and MAPK–AP-1 pathway regulation are thought to be calcineurin dependent, this was explored in Af-infected hMDMs (30–32). Significant modulation of MAPK–AP-1 pathway activation and Ser637 P-Drp1 phosphorylation was observed (Figure E4D). Thus, RNA- and protein-level analyses further support a role for calcineurin in the macrophage programmed cell death response to Af.
Af-Containing Endosomes Transferred from Macrophages to Macrophage Are Enveloped within Actin–Vasodilator-stimulated Phosphoprotein–Rich Cargo
Calcineurin phosphatase activity has multiple NFAT-independent effects on cytoskeletal reorganization and regulation of MAPK pathways (31, 33, 34). To identify calcineurin phosphatase targets, we performed a comparative phosphoproteomic array on FK506- versus vehicle-pretreated hMDMs infected with SC (Figure 5A). There were differences in phosphorylation of proteins within the MAPK pathway, including c-Jun and the cell-cycle regulatory protein M-phase inducer phosphatase 1. This is consistent with our transcriptomic findings of cross-control by calcineurin of the MAPK pathways and cell death pathways.
Figure 5.
Lateral transfer of Aspergillus fumigatus (Af) occurs through vasodilator-stimulated phosphoprotein (VASP) tunnel-like structures. (A) A phosphoprotein array analysis was performed with human monocyte-derived macrophages (hMDMs) pretreated with FK506 and stimulated with live Af swollen conidia (SC) (multiplicity of infection [MOI], 1) for 1 hour postinfection. Proteins with significant fold change differences in the phosphoprotein-to-nonphosphoprotein ratio between FK506 and control macrophages stimulated with live Af SC are shown. (B) hMDMs were pretreated with a cell-permeable cyclic guanosine monophosphate analog (25 mM 8-[4-chlorophenylthio]-guanosine 3′,5′-cyclic monophosphate [8-pCPT-cGMP]) to induce Ser238 VASP phosphorylation and with FK506 (10 ng/ml) or vehicle. They were then stimulated with a calcium ionophore (2 μg/ml ionomycin) to activate calcineurin. Macrophage whole-cell extracts were probed by Western blotting for Ser238 VASP and total VASP. (C) Representative confocal microscopic images of hMDM phagocytosis and lateral transfer of live Af SC (MOI, 1). VASP is shown in red, nuclei in blue, and Af in green. (1) Uninfected hMDMs. (2) Initial phagocytosis of Af by hMDMs. (3) Late phagocytosis of Af by hMDMs. (4) Lateral transfer of Af between hMDMs. (D) Three-dimensional reconstruction of confocal microscopic images of phagocytosis and lateral transfer of live Af SC (MOI, 1) between hMDMs. Af phagocytosis at 30 minutes postinfection with VASP is shown in red, Af in green, and nuclei in blue. (E) Three-dimensional reconstruction of confocal microscopic image of Af lateral transfer with VASP shown in yellow, actin in magenta, Af in green, and nuclei in blue. (F and G) Representative z-slice confocal microscopic image (F) and three-dimensional reconstruction (G) of Af lateral transfer. VASP is shown in red, and Af is shown in green. (H) THP-1 macrophages treated with either control or VASP small interfering RNA (siRNA) (50 µM) were infected with live Af SC and stained with Calcofluor White (Sigma-Aldrich, St. Louis, MO) (MOI, 1). Phagocytosis was quantified by flow cytometry (n = 3 biological replicates). Data are presented as mean ± SEM. Scale bars = 10 μm. AKT1 = RAC-α serine/threonine protein kinase; CDC25A = M-phase inducer phosphatase 1; HDAC1 = histone deacetylase 1; MAPK = mitogen-activated protein kinase; MEK1 = mitogen-activated protein kinase kinase 1; NFAT = nuclear factor of activated T cells; P-VASP = phospho-VASP; SMC1=structural maintenance of chromosomes protein 1; UT = untreated; VEGFR2 = vascular endothelial growth factor receptor 2.
The analysis revealed a 26-fold increase in Ser238 phosphorylated vasodilator-stimulated phosphoprotein (VASP) levels in FK506-treated hMDMs (Figure 5A). VASP is an actin polymerase that promotes assembly of actin networks, and it is important for directional motility and phagocytosis (35–37). Its function is tightly regulated, with Ser238 dephosphorylation required for actin filament formation. We therefore postulated that VASP is a direct calcineurin target. As calcineurin is the only Ca2+-activated serine-threonine phosphatase, we measured Ser238 P-VASP following the addition of the calcium ionophore ionomycin (to selectively activate calcineurin) to hMDMs with phosphorylated VASP (induced using a cell-permeable cyclic guanosine monophosphate analog) in the presence or absence of FK506 (Figure 5B). This confirmed that VASP is a major dephosphorylation target of calcineurin.
As VASP is an important actin cytoskeletal regulator and calcineurin is required for optimal phagocytosis and transfer of Af, we hypothesized that VASP is involved in actin-dependent phagocytosis and lateral transfer of Af. Using high-resolution confocal microscopy, we observed colocalization of VASP to Af at phagocytic cups, which disappeared following internalization (Figures 5C and 5D and Video 6). Localization of VASP to Af was not calcineurin dependent. During Af lateral transfer at late time points, high-intensity colocalization of VASP and actin staining to Af-containing endosomes was observed (Figure 5E). High-resolution three-dimensional reconstruction revealed that Af-containing endosomes trafficked between macrophages are enveloped within actin-VASP–rich cargo (Figures 5C–5G and Video 7).
Video 6.
Vasodilator-stimulated phosphoprotein (VASP) colocalizes to the human monocyte-derived macrophage (hMDM) Aspergillus fumigatus (Af) phagocytic cup. Three-dimensional reconstruction of confocal microscopy image shows phagocytosis of live Af swollen conidia (multiplicity of infection, 1) by hMDMs. The image is of Af phagocytosis at 30 minutes after infection with VASP shown in red, Af in green, and nuclei in blue. Scale bar = 10 μm.
Video 7.
Lateral transfer of germinating Aspergillus fumigatus (Af) conidia between human monocyte-derived macrophages (hMDMs) occurs within vasodilator-stimulated phosphoprotein (VASP)-enveloped endosomes. Three-dimensional reconstruction of confocal microscopy image shows hMDM Af lateral transfer with VASP shown in green and Af in magenta. Scale bar = 10 μm.
To determine the role of VASP in macrophage Af phagocytosis, small interfering RNA knockdown of VASP was performed in human macrophages, followed by analysis of uptake of calcofluor white–stained live Af SC compared with control. This showed that phagocytosis of live Af SC at early time points is VASP dependent (P < 0.01) (Figure 5H), consistent with the defect seen for calcineurin inhibition (Figure 2B). Taken together, these results indicate that calcineurin, by direct dephosphorylation and activation of the actin polymerase VASP, has a key role in macrophage actin cytoskeleton reorganization during fungal infection. This enables optimal actin-dependent Af phagocytosis and lateral transfer of Af-containing endosomes during programmed necrosis, which are critical to achieving control of germinating conidia.
Discussion
PA has emerged as a serious infectious complication of a range of immunocompromised states and chronic respiratory diseases. Overt infection is typically characterized by hyperinflammatory tissue destruction, with more complex relationships between airway colonization and progression of chronic respiratory diseases (38). Here we report human macrophage programmed necrosis as an important response to germination of Af. While necrosis has been recognized as a form of cell death since the mid-19th century, it has recently become clear that programmed necrosis may occur through receptor-interacting serine/threonine-protein kinase 3 necroptotic cell death (39). Necroptosis occurs as a firstline defense against intracellular pathogens and is central to the pathogenesis of a number of chronic inflammatory conditions, including emphysematous change (40, 41).
Remarkably, we observed frequent lateral transfer of Af during necroptosis, which ultimately enabled control of hyphal escape from the macrophage. Transfer occurred through a late endosomal compartment, suggesting that this process may have similarities to exocytosis (42). While lateral transfer has previously been observed for Cryptococcus neoformans at low frequency, the molecular basis for transfer and its relationship to cell death or control of infection have not previously been defined (43). The pathogen escape and transfer processes described to date are pathogen mediated and enhance either tissue dissemination or evasion from immune attack. However, our observations are consistent with host-dependent lateral transfer of Af to limit hyphal escape during macrophage death. The observation of cell–cell transfer of Af supports a model whereby inhaled conidia may persist for prolonged periods in the myeloid compartment as spores. This has potentially important implications for individuals who subsequently undergo immunosuppression.
Notably, we observed that both fungal germination–driven programmed necrosis and lateral transfer were calcineurin dependent, indicating that necroptotic control of infection is likely to be impaired in organ transplant patients. Calcineurin has been postulated to have a role in the regulation of programmed cell death through interaction with Bcl-2 family proteins (25, 26). Consistent with this, we found significant calcineurin-dependent expression of myeloid cell leukemia 1, a Bcl-2 protein that inhibits apoptosis, and Bcl2-modifying factor, a member of the prodeath BH3-only subgroup of Bcl-2 family proteins required for TNF-α–induced necroptosis (27). In transcriptomic and phosphoproteomic analysis, we defined calcineurin as a key phosphatase mediating cross-control of cell death, the MAPK–AP-1 innate pathway, and VASP-dependent cytoskeletal remodeling during macrophage infection. Calcineurin inhibition impaired these cellular processes, leading to enhanced fungal germination in the late phagosome, impaired lateral transfer, and ultimately hyphal escape. Interestingly, virulent Burkholderia spp. have recently been shown to mimic VASP actin polymerases to enable cell fusion and spread (44). Whether fungi are able to directly manipulate host actin polymerases remains to be determined.
In summary, we have identified macrophage programmed necrosis as an important response to progressive germination of Af in the human macrophage, and we report a previously undescribed phenomenon of cell death–dependent lateral transfer as a cooperative macrophage behavior that assists in limiting hyphal escape. We show that calcineurin is a key orchestrator of this process, further defining the importance of this pathway in innate immunity. Our findings yield novel insights into host innate immunity to Af in the lung, extend current understanding of the pathogenesis of PA in organ transplantation, and have broader implications for transplant immunity and chronic lung disease.
Acknowledgments
Acknowledgment
The authors thank the patients of the Royal Brompton and Harefield Hospitals UK who participated in this study. This project was supported by the National Institute for Health Research Respiratory Disease Biomedical Research Unit and the Imperial College Academic Health Science Centre. We thank Avinash Shenoy and Jason Mercer for helpful discussions and the Imperial College London Facility for Imaging by Light Microscopy and Amelia Shoemark for technical support.
Footnotes
This work was supported by Medical Research Council (MRC) Clinician Scientist Fellowship G0902260/1 (D.P.A.-J.) and MRC Clinical Research Fellowship MR/K002708/1 (A.S.). S.M. is supported by a Wellcome Trust Research Career Development Fellowship (WT097411MA) and by the Lister Institute for Preventative Medicine.
Author Contributions: D.P.A.-J., A.S., S.K., S. Shaunak, and S.M. designed the experiments; D.P.A.-J., A.S., S.K., S.H., and A. Rogers conducted the experiments; S. Soresi, A. Reed, and M.C. obtained consent from the patients and undertook the bronchoscopies; and A.S. and S.K. wrote the first draft of the manuscript. All authors contributed to the data analysis and the writing of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
The uncompressed videos are accessible from this article’s supplementary material page.
Originally Published in Press as DOI: 10.1164/rccm.201601-0070OC on May 10, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Latgé JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia S, Fei M, Yarlagadda M, Qi Z, Akira S, Saijo S, Iwakura Y, van Rooijen N, Gibson GA, St Croix CM, et al. Rapid host defense against Aspergillus fumigatus involves alveolar macrophages with a predominance of alternatively activated phenotype. PLoS One. 2011;6:e15943. doi: 10.1371/journal.pone.0015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen GH, Teitz-Tennenbaum S, Neal LM, Murdock BJ, Malachowski AN, Dils AJ, Olszewski MA, Osterholzer JJ. Local GM-CSF-dependent differentiation and activation of pulmonary dendritic cells and macrophages protect against progressive cryptococcal lung infection in mice. J Immunol. 2016;196:1810–1821. doi: 10.4049/jimmunol.1501512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbst S, Shah A, Mazon Moya M, Marzola V, Jensen B, Reed A, Birrell MA, Saijo S, Mostowy S, Shaunak S, et al. Phagocytosis-dependent activation of a TLR9-BTK-calcineurin-NFAT pathway co-ordinates innate immunity to Aspergillus fumigatus. EMBO Mol Med. 2015;7:240–258. doi: 10.15252/emmm.201404556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbst S, Shah A, Carby M, Chusney G, Kikkeri N, Dorling A, Bignell E, Shaunak S, Armstrong-James D. A new and clinically relevant murine model of solid-organ transplant aspergillosis. Dis Model Mech. 2013;6:643–651. doi: 10.1242/dmm.010330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinosa V, Jhingran A, Dutta O, Kasahara S, Donnelly R, Du P, Rosenfeld J, Leiner I, Chen CC, Ron Y, et al. Inflammatory monocytes orchestrate innate antifungal immunity in the lung. PLoS Pathog. 2014;10:e1003940. doi: 10.1371/journal.ppat.1003940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70:270–277. doi: 10.1136/thoraxjnl-2014-206291. [DOI] [PubMed] [Google Scholar]
- 8.Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, Anaissie EJ, Brumble LM, Herwaldt L, Ito J, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin Infect Dis. 2010;50:1101–1111. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong-James D, Teo I, Herbst S, Petrou M, Shiu KY, McLean A, Taube D, Dorling A, Shaunak S. Renal allograft recipients fail to increase interferon-γ during invasive fungal diseases. Am J Transplant. 2012;12:3437–3440. doi: 10.1111/j.1600-6143.2012.04254.x. [DOI] [PubMed] [Google Scholar]
- 10.Hogan C, Denning DW. Allergic bronchopulmonary aspergillosis and related allergic syndromes. Semin Respir Crit Care Med. 2011;32:682–692. doi: 10.1055/s-0031-1295716. [DOI] [PubMed] [Google Scholar]
- 11.Denning DW, Pleuvry A, Cole DC. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol. 2013;51:361–370. doi: 10.3109/13693786.2012.738312. [DOI] [PubMed] [Google Scholar]
- 12.Ader F, Nseir S, Le Berre R, Leroy S, Tillie-Leblond I, Marquette CH, Durocher A. Invasive pulmonary aspergillosis in chronic obstructive pulmonary disease: an emerging fungal pathogen. Clin Microbiol Infect. 2005;11:427–429. doi: 10.1111/j.1469-0691.2005.01143.x. [DOI] [PubMed] [Google Scholar]
- 13.Maturu VN, Agarwal R. Prevalence of Aspergillus sensitization and allergic bronchopulmonary aspergillosis in cystic fibrosis: systematic review and meta-analysis. Clin Exp Allergy. 2015;45:1765–1778. doi: 10.1111/cea.12595. [DOI] [PubMed] [Google Scholar]
- 14.Taccone FS, Van den Abeele AM, Bulpa P, Misset B, Meersseman W, Cardoso T, Paiva JA, Blasco-Navalpotro M, De Laere E, Dimopoulos G, et al. AspICU Study Investigators. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care. 2015;19:7. doi: 10.1186/s13054-014-0722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor AL, Watson CJ, Bradley JA. Immunosuppressive agents in solid organ transplantation: mechanisms of action and therapeutic efficacy. Crit Rev Oncol Hematol. 2005;56:23–46. doi: 10.1016/j.critrevonc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol. 2007;178:3107–3115. doi: 10.4049/jimmunol.178.5.3107. [DOI] [PubMed] [Google Scholar]
- 17.Tourneur E, Ben Mkaddem S, Chassin C, Bens M, Goujon JM, Charles N, Pellefigues C, Aloulou M, Hertig A, Monteiro RC, et al. Cyclosporine A impairs nucleotide binding oligomerization domain (Nod1)-mediated innate antibacterial renal defenses in mice and human transplant recipients. PLoS Pathog. 2013;9:e1003152. doi: 10.1371/journal.ppat.1003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 21.Bader GD, Hogue CW. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics. 2003;4:2. doi: 10.1186/1471-2105-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Philippe B, Ibrahim-Granet O, Prévost MC, Gougerot-Pocidalo MA, Sanchez Perez M, Van der Meeren A, Latgé JP. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect Immun. 2003;71:3034–3042. doi: 10.1128/IAI.71.6.3034-3042.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mittal A, Gahlaut A, Sharma GL, Dabur R. Antifungal treatments delineate a correlation between cathepsins and cytokines in murine model of invasive aspergillosis. Indian J Pharm Sci. 2013;75:688–699. [PMC free article] [PubMed] [Google Scholar]
- 24.Chan FKM. Fueling the flames: mammalian programmed necrosis in inflammatory diseases. Cold Spring Harb Perspect Biol. 2012;4(11) doi: 10.1101/cshperspect.a008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu YT, Tan HL, Huang Q, Sun XJ, Zhu X, Shen HM. zVAD-induced necroptosis in L929 cells depends on autocrine production of TNFα mediated by the PKC-MAPKs-AP-1 pathway. Cell Death Differ. 2011;18:26–37. doi: 10.1038/cdd.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SJ, Li J. Caspase blockade induces RIP3-mediated programmed necrosis in Toll-like receptor-activated microglia. Cell Death Dis. 2013;4:e716. doi: 10.1038/cddis.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krysan DJ, Sutterwala FS, Wellington M. Catching fire: Candida albicans, macrophages, and pyroptosis. PLoS Pathog. 2014;10:e1004139. doi: 10.1371/journal.ppat.1004139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erin N, Bronson SK, Billingsley ML. Calcium-dependent interaction of calcineurin with Bcl-2 in neuronal tissue. Neuroscience. 2003;117:541–555. doi: 10.1016/s0306-4522(02)00933-8. [DOI] [PubMed] [Google Scholar]
- 29.Kanamaru Y, Sekine S, Ichijo H, Takeda K. The phosphorylation-dependent regulation of mitochondrial proteins in stress responses. J Signal Transduct. 2012;2012:931215. doi: 10.1155/2012/931215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, Scorrano L. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA. 2008;105:15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang CC, Wang JM, Kikkawa U, Mukai H, Shen MR, Morita I, Chen BK, Chang WC. Calcineurin-mediated dephosphorylation of c-Jun Ser-243 is required for c-Jun protein stability and cell transformation. Oncogene. 2008;27:2422–2429. doi: 10.1038/sj.onc.1210888. [DOI] [PubMed] [Google Scholar]
- 32.Wu CC, Hsu SC, Shih HM, Lai MZ. Nuclear factor of activated T cells c is a target of p38 mitogen-activated protein kinase in T cells. Mol Cell Biol. 2003;23:6442–6454. doi: 10.1128/MCB.23.18.6442-6454.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao JW, Gao ZL, Ji QY, Wang H, Zhang HY, Yang YD, Xing FJ, Meng LJ, Wang Y. Regulation of cofilin activity by CaMKII and calcineurin. Am J Med Sci. 2012;344:462–472. doi: 10.1097/MAJ.0b013e318244745b. [DOI] [PubMed] [Google Scholar]
- 34.Escolano A, Martínez-Martínez S, Alfranca A, Urso K, Izquierdo HM, Delgado M, Martín F, Sabio G, Sancho D, Gómez-del Arco P, et al. Specific calcineurin targeting in macrophages confers resistance to inflammation via MKP-1 and p38. EMBO J. 2014;33:1117–1133. doi: 10.1002/embj.201386369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen SD, Mullins RD. VASP is a processive actin polymerase that requires monomeric actin for barbed end association. J Cell Biol. 2010;191:571–584. doi: 10.1083/jcb.201003014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- 37.Coppolino MG, Krause M, Hagendorff P, Monner DA, Trimble W, Grinstein S, Wehland J, Sechi AS. Evidence for a molecular complex consisting of Fyb/SLAP, SLP-76, Nck, VASP and WASP that links the actin cytoskeleton to Fcγ receptor signalling during phagocytosis. J Cell Sci. 2001;114:4307–4318. doi: 10.1242/jcs.114.23.4307. [DOI] [PubMed] [Google Scholar]
- 38.Warris A. The biology of pulmonary aspergillus infections. J Infect. 2014;69(Suppl 1):S36–S41. doi: 10.1016/j.jinf.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;370:455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silke J, Rickard JA, Gerlic M. The diverse role of RIP kinases in necroptosis and inflammation. Nat Immunol. 2015;16:689–697. doi: 10.1038/ni.3206. [DOI] [PubMed] [Google Scholar]
- 41.Mizumura K, Cloonan SM, Nakahira K, Bhashyam AR, Cervo M, Kitada T, Glass K, Owen CA, Mahmood A, Washko GR, et al. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J Clin Invest. 2014;124:3987–4003. doi: 10.1172/JCI74985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szumowski SC, Estes KA, Popovich JJ, Botts MR, Sek G, Troemel ER. Small GTPases promote actin coat formation on microsporidian pathogens traversing the apical membrane of Caenorhabditis elegans intestinal cells. Cell Microbiol. 2016;18:30–45. doi: 10.1111/cmi.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnston SA, May RC. The human fungal pathogen Cryptococcus neoformans escapes macrophages by a phagosome emptying mechanism that is inhibited by Arp2/3 complex-mediated actin polymerisation. PLoS Pathog. 2010;6:e1001041. doi: 10.1371/journal.ppat.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benanti EL, Nguyen CM, Welch MD. Virulent Burkholderia species mimic host actin polymerases to drive actin-based motility. Cell. 2015;161:348–360. doi: 10.1016/j.cell.2015.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]