To the Editor:
Inhaled nitric oxide (NO) is a selective pulmonary vasodilator (1) that is commonly used to perform diagnostic procedures and to treat a spectrum of cardiopulmonary conditions, including pulmonary hypertension (PH) (1–4). The high price of providing NO and its complex delivery system limit access to inhaled NO therapy to in-hospital use at well-equipped medical centers in economically advantaged countries.
We recently developed a novel method of synthesizing NO from air or oxygen (O2)-rich gas mixtures by electric plasma generation (5). Two NO generators were built: an offline device, which continuously produces the gas and maintains a constant concentration of NO throughout the respiratory cycle, and an inline generator, which produces NO for 800 milliseconds beginning 20 milliseconds after the onset of inspiration. Both generators can produce more than 100 parts per million (ppm) NO in air for at least a week. The NO delivery systems include a scavenger to remove nitrogen dioxide (NO2) and a high-efficiency particulate arresting filter. We previously demonstrated that NO produced by an electric plasma generator caused pulmonary vasorelaxation in lambs with pulmonary vasoconstriction induced by infusing U46619, a thromboxane analog. Both the offline and inline plasma NO generators reliably reduced the lamb’s pulmonary vascular resistance (PVR) and pulmonary artery pressure (PAP) (5).
In this exploratory study, we tested both NO generators on human subjects. Each subject received 25 ppm of NO for 10 minutes from each of the two generators. This was a two-part study in adult subjects with predefined objectives. The first objective was to optimize the devices for human use and confirm safety in healthy volunteers (part 1). The second objective was to determine whether electrically generated NO could produce selective pulmonary vasodilation and reduce PAP in adults with chronic PH (part 2). The study was approved by the Massachusetts General Hospital institutional review board and performed at the hospital.
Study: Part 1
Six healthy subjects (four females and two males) completed the study. Vital signs were unchanged and remained stable during both continuous and inspiratory NO delivery. Methemoglobin did not increase after breathing 25 ppm NO for 10 minutes and remained below 1.1%. No adverse events occurred during or after breathing NO.
Study: Part 2
Six patients (two females and four males, aged 59 ± 7 years; all data are mean ± SD) with chronic PH were enrolled in the study. As part of the inclusion criteria, each patient had a positive acute pulmonary vasodilator test to 25 ppm NO diluted from NO/N2 cylinders. Patient characteristics are reported in Table 1. Five of the six subjects were taking one or more medications for pulmonary arterial hypertension (including sildenafil, macitentan, and Iloprost). Five of the six subjects required supplemental O2 at rest (range, 4–6 L/min O2); the average peripheral oxygen saturation before the NO test procedure was 95%.
Table 1.
Characteristics of Patients with PH (n=6) Enrolled in Part 2 of the Study
| Subject | Diagnosis | PH Diagnostic Group (WHO) | Oxygen Therapy (L/min) | PAH Medications |
|---|---|---|---|---|
| 1 | Interstitial lung disease of uncertain etiology | 3 | 5 | Sildenafil |
| 2 | Scleroderma, type CREST and suspected PVOD | 1 | 5 | Sildenafil, macitentan |
| 3 | Suspected pulmonary capillary hypertension/PVOD | 1 | 6 | Sildenafil |
| 4 | Idiopathic PH | 1 | 4 | Sildenafil, macitentan, inhaled iloprost |
| 5 | Mixed restrictive and obstructive lung disease | 3 | 5 | — |
| 6 | Sarcoidosis | 5 | 0 | Sildenafil |
Definition of abbreviations: CREST = calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia; PAH = pulmonary arterial hypertension; PH = pulmonary hypertension; PVOD = pulmonary veno-occlusive disease; WHO = World Health Organization.
Diagnosis, PH diagnostic group, oxygen requirement at rest, and PAH medications are listed.
The two generators were safely tested in the cardiac catheterization laboratory. The generators did not cause electrical interference with other electronic equipment (including fluoroscope, electrocardiogram, and invasive and noninvasive vital signs monitoring). There were no adverse effects of administering electric plasma–generated NO to subjects with PH, and there were no instances of shocks or burns. The inspiratory NO2 levels did not exceed 0.2 ppm in any subject, and the maximum measured blood methemoglobin was 1.2%. The NO concentrations ranged from a minimum of 20 ppm to a maximum of 25 ppm NO during the 10-minute test period with both the inline and offline devices.
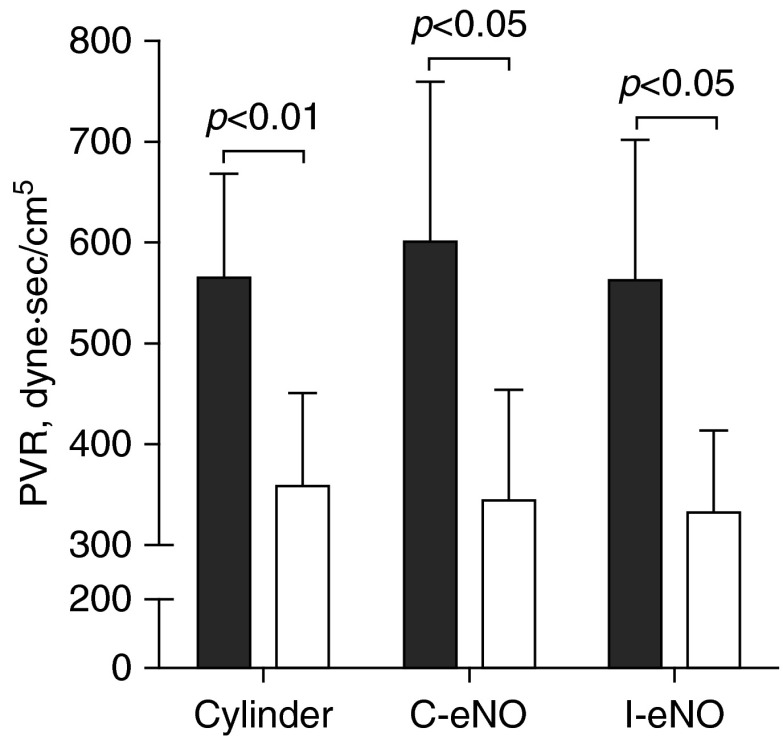
Mean systemic arterial pressure was unchanged while breathing NO generated by each method, confirming that inhaled NO does not alter systemic vascular resistance, as previously reported (1). The fraction of inspired oxygen was maintained at the same concentration as during baseline breathing before testing. Peripheral oxygen saturation increased during 25 ppm NO breathing delivered from a standard NO cylinder (95 ± 2% to 99 ± 1%; P < 0.01) and showed a trend toward increasing during NO breathing with either the continuous, offline (95 ± 2% to 98 ± 1%; P = 0.056), or inspiratory, inline (94 ± 2% to 98 ± 2%; P = 0.053), electrical NO generator. Electric plasma NO generation by the offline generator reduced mean PAP (mPAP) from 46 ± 5 to 32 ± 5 mm Hg (P < 0.01); NO produced by the inline generator reduced mPAP from 43 ± 4 to 32 ± 4 mm Hg (P < 0.01). Standard cylinder-derived 25 ppm NO decreased mPAP from 48 ± 4 to 35 ± 5 mm Hg (P < 0.01). Pulmonary capillary wedge pressure and cardiac index did not change with any of the treatments. As a result of the mPAP decrease and unchanged pulmonary capillary wedge pressure and cardiac index, PVR markedly decreased during NO breathing when delivered via each of the three NO delivery systems (Figure 1). There was a similar percentage reduction in PVR from the resting pretreatment PVR when subjects breathed 25 ppm NO, irrespective of the method used to produce NO. The offline NO generator reduced PVR by 41 ± 9% compared with the PVR before breathing NO, the inline generator reduced PVR by 40 ± 3%, and cylinder-derived NO reduced PVR by 39 ± 7%. The acute pulmonary vasodilator response to NO occurred despite the fact that five of six patients were receiving other pulmonary arterial hypertension therapies, including Sildenafil, a type 5 phosphodiesterase inhibitor. Inadequate endothelial NO production may limit the effect of type 5 phosphodiesterase inhibitors in these patients; providing chronic, supplemental inhaled NO might therefore be clinically useful in patients with pulmonary arterial hypertension.
Figure 1.
Pulmonary vascular resistance (PVR) of six patients with pulmonary hypertension before (solid bars) and during 25 parts per million of nitric oxide (NO) breathing (open bars) produced by cylinder-derived NO (Cylinder), or continuous offline (C-eNO) and inspiratory inline (I-eNO) electric plasma–generated NO.
In conclusion, the synthesis and testing of electric generation of NO in a hospital setting was safe and led to acute pulmonary hemodynamic effects equivalent to NO obtained from commercially available cylinders. Electric generation of NO from air offers the potential for delivering NO gas for inhalation for prolonged periods and may augment the effects of other chronic therapies. The devices could also expand the delivery of NO to hospitals and clinics around the world because electric plasma generation is economical, easy to use, and safe.
Footnotes
This project was funded in part by the Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital. L.B. was awarded a “Mentored Research Training Grant - Clinical or Translational” by the Foundation for Anesthesia Education and Research. J.R.-L. receives research support from Actelion Pharmaceuticals. D.B.B.’s research is supported by National Institutes of Health grant R01DK082971 and the Leducq Foundation. M.J.S.’s research is supported, in part, by R01HL105562. R.N.C. receives support for his research from Actelion, Bayer, Gilead, and United Therapeutics.
Author Contributions: L.B., J.R.-L., E.R., B.Y., D.F.F., M.J.S., D.B.B., R.N.C., and W.M.Z. made substantial contributions to the conception and design of the work and the acquisition, analysis, and interpretation of data. L.B. and W.M.Z. drafted the manuscript, and all authors critically revised the manuscript for important intellectual content.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation. 1991;83:2038–2047. doi: 10.1161/01.cir.83.6.2038. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JD, Polaner DM, Lang P, Zapol WM. Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet. 1992;340:818–819. doi: 10.1016/0140-6736(92)92686-a. [DOI] [PubMed] [Google Scholar]
- 3.Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, Walsh MC, Durand DJ, Mayock DE, Eichenwald EC, et al. NO CLD Study Group. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med. 2006;355:343–353. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 4.Malhotra R, Hess D, Lewis GD, Bloch KD, Waxman AB, Semigran MJ. Vasoreactivity to inhaled nitric oxide with oxygen predicts long-term survival in pulmonary arterial hypertension. Pulm Circ. 2011;1:250–258. doi: 10.4103/2045-8932.83449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu B, Muenster S, Blaesi AH, Bloch DB, Zapol WM. Producing nitric oxide by pulsed electrical discharge in air for portable inhalation therapy. Sci Transl Med. 2015;7:294ra107. doi: 10.1126/scitranslmed.aaa3097. [DOI] [PubMed] [Google Scholar]