This study is the first to explore if moderate heart rate elevations could be therapeutically employed to induce beneficial structural changes in the concentrically hypertrophied left ventricle. The result that heart rate elevations can induce structural changes that improve diastolic filling challenges the canonical thought that they are harmful.
Keywords: heart rate, hypertrophy, pacemaker, remodeling
Abstract
Lowering the heart rate is considered to be beneficial in heart failure (HF) with reduced ejection fraction (HFrEF). In a dilated left ventricle (LV), pharmacological heart rate lowering is associated with a reduction in LV chamber size. In patients with HFrEF, this structural change is associated with better survival. HF with preserved ejection fraction (HFpEF) is increasingly prevalent but, so far, without any evidence-based treatment. HFpEF is typically associated with LV concentric remodeling and hypertrophy. The effects of heart rate on this structural phenotype are not known. Analogous with the benefits of a low heart rate on a dilated heart, we hypothesized that increased heart rates could lead to potentially beneficial remodeling of a concentrically hypertrophied LV. This was explored in an established porcine model of concentric LV hypertrophy and fibrosis. Our results suggest that a moderate increase in heart rate can be used to reduce wall thickness, normalize LV chamber volumes, decrease myocardial fibrosis, and improve LV compliance. Our results also indicate that the effects of heart rate can be titrated, are reversible, and do not induce HF. These findings may provide the rationale for a novel therapeutic approach for HFpEF and its antecedent disease substrate.
Listen to this article’s corresponding podcast at http://ajpheart.podbean.com/e/heart-rate-modeling/.
NEW & NOTEWORTHY
This study is the first to explore if moderate heart rate elevations could be therapeutically employed to induce beneficial structural changes in the concentrically hypertrophied left ventricle. The result that heart rate elevations can induce structural changes that improve diastolic filling challenges the canonical thought that they are harmful.
heart failure (HF) is a leading cause of hospitalizations and a major public health problem (7). About half of patients with HF have a preserved ejection fraction (HFpEF), and the prevalence of HFpEF is growing relative to HF with reduced ejection fraction (HFrEF) (19, 24). No treatment has been shown to improve survival in patients with HFpEF, and few interventions improve symptoms or quality of life. Thus, identification of novel treatment targets for the underlying myocardial substrate is an important goal.
At a structural level, HFpEF is typically associated with concentric remodeling with an increased left ventricular (LV) mass-to-volume ratio or overt LV hypertrophy and fibrosis (29, 30). A high prevalence of this structural phenotype in HFpEF was recently confirmed in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial (21). Despite the inclusion of patients with LV chamber dilation in this trial, about a quarter of patients had below-normal chamber volumes. A 20–50% reduction in LV chamber size has also been documented with normal aging in population studies (1, 12). Small chamber volumes are associated with a poor exercise capacity and contribute to an increase in the LV mass-to-volume ratio, which is a strong independent predictor of the future development of HF (12, 18).
In line with the established beneficial structural effects of lower heart rates in dilated cardiomyopathy, we hypothesized that a moderate increase in heart rate could be used to favorably alter the basic structure of the concentrically remodeled LV. This would be accomplished by a reduction in LV wall thickness, normalization of LV chamber volumes, and reorganization of the interstitial matrix that, together, may improve diastolic LV filling. In a first attempt to explore the potential and safety of this approach, we studied the effects of continuous atrial pacing in a well-characterized porcine model of concentric LV hypertrophy and fibrosis induced by unilateral renal artery stenosis (RAS) (10, 27). Because no previous work provides guidance on the heart rates needed to achieve these goals, we used an exploratory study design that would allow us to first find and then confirm a target heart rate.
MATERIALS AND METHODS
Animal Studies
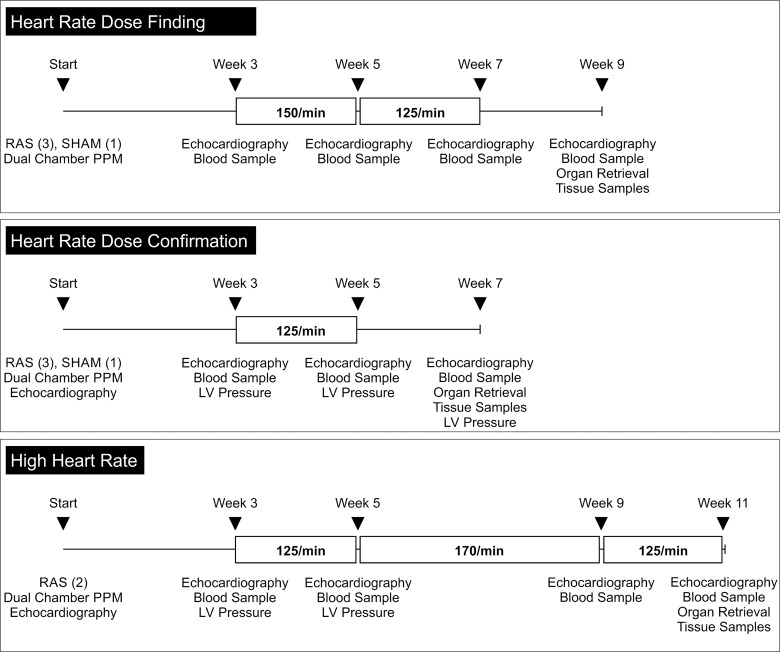
All aspects of animal care were in accordance with National Institutes of Health guidelines and the standards of the Animal Care and Use Committee of the University of Vermont, which approved the protocol used in this study. Because the effects of moderately elevated heart rates on the structural (volume) and functional [ejection fraction (EF)] effects are unknown, a “heart rate finding study” allowed us to determine a target heart rate that would be associated with a structural change without affecting the EF. This was followed by a “heart rate confirmatory study” and a “high heart rate study.” A schematic outline of the experimental protocols is provided in Fig. 1.
Fig. 1.
Schematic of the experimental approach. RAS, renal artery stenosis; PPM, permanent pacemaker; LV, left ventricle.
A total of 10 adult female Yucatán miniature swine (9 mo old, 40–45 kg body wt; Sinclair Bio Resources, Windham, ME) were used for these studies. A pacemaker was implanted in all pigs. At the same time, a unilateral RAS was established in eight pigs to induce LV hypertrophy. Two pigs underwent a sham procedure without RAS. At each study time point, meloxicam (0.2 mg/kg po) was administered as a preoperative analgesic followed by ketamine (20 mg/kg im) and atropine (0.024 mg/kg im). Anesthesia was induced with 5% isoflurane to allow endotracheal intubation and maintained at 1–3%. Thermal support was provided by a heated water blanket. Oxygen saturation, expired carbon dioxide levels, limb lead ECG, and rectal temperature were recorded throughout the surgical and diagnostic procedures. At each time point, a blood sample was collected and centrifuged (10 min at 3,000 rpm). The resulting supernatant was transferred to a −80°C freezer for later analyses.
During the entire course of the study, the animals were assessed on a daily basis for signs of discomfort, the incision site was evaluated, and respiratory abnormalities were assessed. The lungs were auscultated once a week, and the pacemaker was interrogated intermittently to ensure proper function. Upon study termination, the heart and both kidneys were removed. The atria were removed, and the right ventricle and LV were weighed. Tibia length and body weight were measured and used to index LV mass. Transmural samples were excised from the LV anterior wall. One sample was frozen in liquid nitrogen and stored in a −80°C freezer, and another sample was preserved in 10% formaldehyde solution.
Unilateral RAS and Dual-Chamber Pacemaker Implantation
RAS.
Unilateral RAS in pigs rapidly induces LV hypertrophy and tissue fibrosis (17, 27). A right dorsolateral skin incision was made under sterile conditions, and the fasciae and musculature were dissected to obtain access to the right renal hilum. The renal artery was isolated and ligated with an 18-gauge placeholder. The placeholder was then removed to immediately restore perfusion, which was confirmed by visual inspection of the artery. High-grade RAS was fluoroscopically confirmed in the first experimental group. For the first two experimental groups (heart rate dose finding and dose confirmation), one of four animals underwent a sham procedure and served as the control.
Pacemaker insertion.
The right external jugular vein was dissected and ligated under sterile conditions. The vein was accessed, and ventricular and atrial leads (Medtronic Silicon 5076 leads, 65 cm) were advanced under fluoroscopic guidance. The leads were positioned in the right ventricular apex and the right atrial appendage to avoid phrenic stimulation. The pacing leads were tunneled subcutaneously and connected to a clinical dual-chamber pacemaker (Sensia DR, Medtronic), which was secured in a pocket behind the right shoulder. Stimulation thresholds were determined, and the surgical sites were closed. For determination of the intrinsic heart rates over the first 3 wk, all pacemakers were initially programmed to sense without pacing.
Pacing Protocols
At 3 wk after the initial procedure, the animals were sedated and intubated to document the cardiac effect of RAS. In the first experimental group (heart rate dose finding), the pacemaker was programmed to pace the atrium at 150/min for the following 2 wk (weeks 3–5 after the initial surgeries). Ventricular pacing was limited by selection of a rate-adaptive atrioventricular algorithm that prioritizes physiological atrioventricular conduction. All rates are well below those reported to result in tachycardia-induced dilated cardiomyopathy in large-animal models (10).
Echocardiography
Transthoracic and transesophageal echocardiography (Sequoia C256, Siemens Acuson) was performed to quantify LV volume and assess diastolic function. All measurements were obtained in sinus rhythm without pacing. Optimized two-dimensional transthoracic echocardiographic views that maximize endocardial definition were obtained from the parasternal long- and short-axis views, and two transesophageal LV chamber views were obtained at 0° and 90°. Echocardiographic images were recorded over at least two cardiac cycles. LV end-diastolic and end-systolic volumes were obtained using the method of disks (modified Simpson's analysis) according to clinical guideline recommendations (13). LV end-diastolic and end-systolic volumes and EF were calculated by averaging the data from the three echocardiographic windows. LV mass was calculated from the parasternal short-axis dimensions (3). Doppler-based measurements of peak early mitral inflow (E) and the early displacement of the mitral annulus tissue (E′) were obtained to calculate E/E′ as a marker of diastolic function.
End-Diastolic Pressure and Volume Measurements
We also assessed the effect of pacing-induced structural changes after pacing at 125/min on LV end-diastolic pressures (EDP) at prespecified time points in six animals (heart rate dose confirmation and high heart rate studies). The right carotid artery was accessed via a surgical cutdown. A catheter was advanced into the LV cavity to allow for pressure measurements accompanied by simultaneous echocardiographic volume assessments. After the first pressure-volume measurement, three sequential 250-ml infusions of normal saline were administered over 5–10 min. Pressure-volume measurements were made after each infusion. LV compliance was calculated as the ratio of LV EDP change to LV end-diastolic volume (EDV) change (ΔEDV/ΔEDP) from baseline to the completion of the last saline infusion.
Blood Samples
Blood samples were collected from each pig at baseline and at each time point. The following biomarkers were assayed: B-type natriuretic peptide (BNP) and norepinephrine (NE) for neurohumoral activation and galectin-3, a cardiac fibrosis marker (2, 9). BNP and NE have been reported to be elevated in HF models of tachycardia-induced cardiomyopathy (10, 22, 23). Porcine-specific BNP, NE, and porcine-specific galectin-3 levels were determined using commercial competitive ELISAs: Phoenix Pharmaceuticals (Burlingame, CA) for BNP, LDN Labor Diagnostika Nord (Nordhorn, Germany) for NE, and BlueGene Biotech (Shanghai, China) for galectin-3. In addition to the samples provided to establish the standard curve, all kits provided for negative and positive controls.
PicroSirius Red Stain
The LV anterior wall samples preserved in formaldehyde were embedded in paraffin. The paraffin blocks were cut in 5-μm sections with a standard low-profile steel microtome blade on a Leica paraffin microtome (Buffalo Grove, IL). Sections on glass slides were air-dried overnight and then baked at 70°C for 30 min. PicroSirius red staining was performed as previously described (11). Tissue sections were deparaffinized, rehydrated, and stained with filtered 0.1% Sirius red F3BA (Pfaltz & Bauer, Waterbury, CT) in saturated aqueous picric acid, pH 2, for 90 min at room temperature, as previously described by Dolber and Spach (4). An upright light microscope (model BX50, Olympus) was used to electronically overlay a grid on the slide, and each box was assigned a number. Ten grid squares were selected for imaging via a random number generator. Images were acquired at ×10 magnification with an UPLANFL ×10 (numerical aperture 0.3) objective lens. Polarized light images were captured as eight-bit gray-scale images with a digital charge-coupled device camera (Retiga 2000R, QImaging, Burnaby, BC, Canada). The images were analyzed using MetaMorph Imaging Series 7.1 image analysis software (Molecular Devices, Downington, PA). Each image was opened and calibrated to ×10 magnification. A threshold overlay was applied to the image to detect PicroSirius red staining. Values for threshold area and threshold area percentage were recorded and automatically logged to a spreadsheet. Ten random areas were analyzed, and the individual percent areas of fibrosis were averaged for each sample. This randomized systematic uniform analysis avoids selection bias, as it does not focus on perivascular connective tissue accumulations.
For comparison, myocardial sections from five additional nonpaced female pigs with unilateral RAS were analyzed to provide porcine reference values (unstained slides were provided by L. O. Lerman). The animals had similar weights and were matched for RAS duration and level of LV hypertrophy. The same automated and blinded procedures were used to process and analyze the tissue slides.
Protein Slot Blot
LV myocardial samples from all animals were homogenized using a glass tissue grinder followed by ultrasonication. A total of 3 μg of the homogenate from each animal were bound to a nitrocellulose membrane using a slot-blot apparatus (Bio-Dot SF, Bio-Rad, Hercules, CA). After it was blocked, the membrane was incubated with a collagen I/III-specific polyclonal rabbit antibody raised against pig collagen (Bio-Rad) followed by a secondary horseradish peroxidase anti-rabbit antibody and detection by chemiluminescence. Signal linearity was confirmed in a dilution series using two additional samples. The densitometric analysis was performed using ImageJ 1.50b software (National Institutes of Health, Bethesda, MD).
Data Presentation and Statistical Methods
Individual data items were examined using a linear mixed model for within-animal repeated-measure observations with a decomposition of the overall repeated-measure effect into single degree-of-freedom polynomial contrasts followed by pair-wise exploratory contrasts using paired t-tests. Sham and RAS group comparisons were conducted using two-sample tests and confirmed using the nonparametric Kruskal-Wallis rank-sum test when small sample sizes were involved. All rate-mediated postintervention and recovery effects were compared with the baseline measurements made 3 wk after surgery. Values are means ± SD if not otherwise indicated. No adjustments for multiple testing were made in this exploratory study; P ≤ 0.05 was used for significance testing. The data analysis was conducted using SYSTAT version 11 (SYSTAT Software, Richmond, CA). Box-and-whisker plots are used to present the biomarker results. A linear regression analysis was performed to evaluate the interaction between change in chamber volume and degree of hypertrophy.
RESULTS
RAS-Induced LV Concentric Hypertrophy
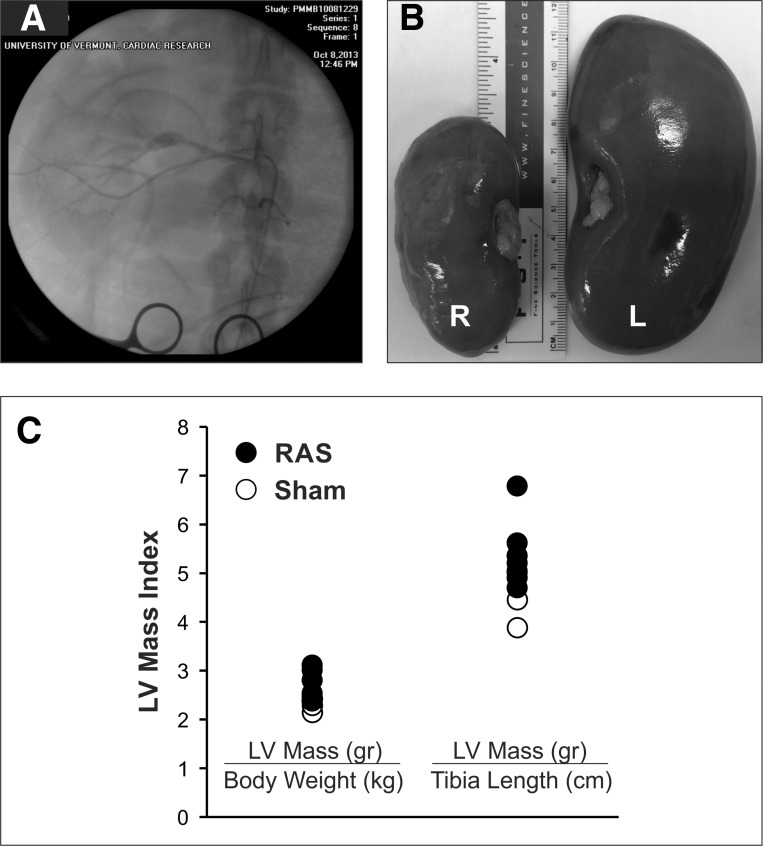
In the eight animals with high-grade unilateral RAS, the right kidney size was markedly smaller (Fig. 2B). In RAS animals, the LV septal and posterior wall thickness increased from 7.7 ± 0.1 to 10.6 ± 0.1 mm (P < 0.01) and from 7.1 ± 0.1 to 9.4 ± 0.1 mm (P < 0.01), respectively, at 3 wk after surgery prior to initiation of pacing. The average LV EDV was 28 ± 6 ml at baseline and 21 ± 2 ml after 3 wk (P = 0.18). The LV mass-to-volume ratio was substantially higher in RAS than sham-operated animals (4.0 ± 0.5 vs. 2.9 ± 0.3 g/ml, P < 0.05).
Fig. 2.
Unilateral RAS and LV hypertrophy. A: radiographic contrast image of the right renal artery with a high-grade RAS and poststenosis dilation. B: reduction of right (R) kidney size after 11 wk of RAS. L, left. C: LV mass measured at the end of the study indexed to body weight and tibia length.
Heart Rate Effects on LV Structure and Function
Heart rate finding study.
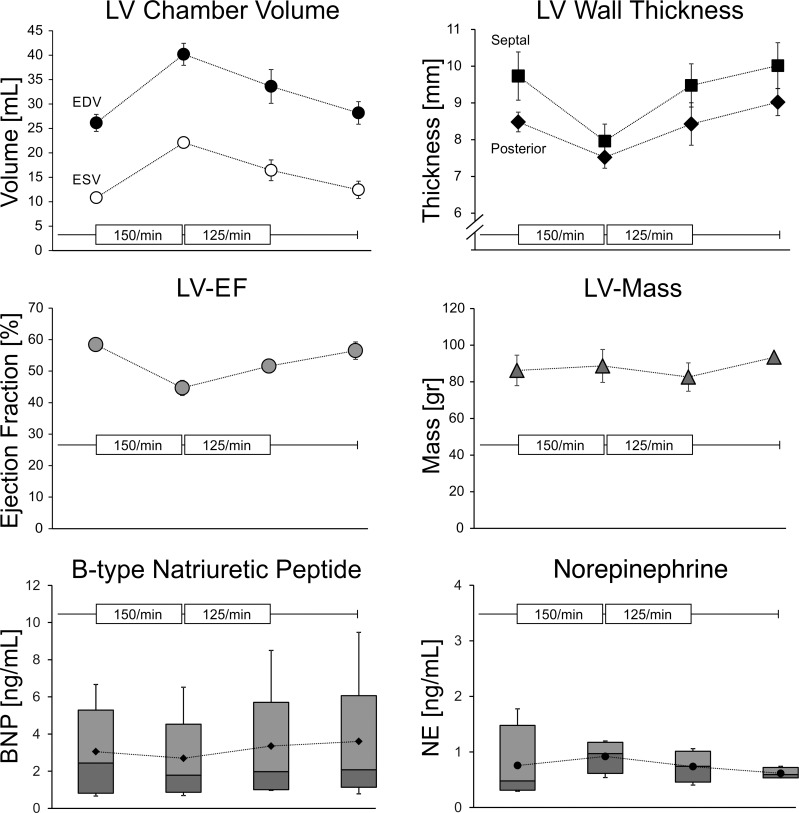
The average intrinsic heart rate prior to the pacing intervention was 93 ± 6/min. After 2 wk of predominant atrial pacing at 150/min, LV volumes markedly increased (Fig. 3). LV end-diastolic and systolic volumes assessed in sinus rhythm increased by 54 ± 3% and 106 ± 23%, respectively (both P < 0.01), whereas the EF fell from 58 ± 1% to 45 ± 1% (P < 0.05). LV mass was unchanged due to wall thinning. Because of the substantial increase in LV volume and the decrease in EF after 2 wk, the atrial pacing rate was subsequently decreased to 125/min. After 2 wk, the LV cavity size was smaller but still enlarged compared with baseline. LV end-diastolic and systolic volumes were increased by 29 ± 24% (P = 0.09) and 50 ± 19% (P < 0.01), respectively. Importantly, the EF normalized to 52 ± 1%. Thereafter, the pacing was stopped. After 2 wk, the LV chamber size had returned to baseline levels prior to the pacing (Fig. 3).
Fig. 3.
Pacing-induced changes in LV dimensions and biomarkers in the heart rate finding study. LV dimensions and plasma biomarkers were assessed 3 wk after the initial surgery. Thereafter, right atrial pacing was started at a rate of 150/min. After 2 wk, pacing was stopped to obtain all measurements before atrial pacing was restarted at a rate of 125/min. After 2 wk, pacing was stopped to assess LV dimensions, and a final assessment was carried out after 2 wk of sinus rhythm. Error bars represent SE. Biomarker [B-type natriuretic peptide (BNP) and norepinephrine (NE)] results are presented as box-and-whisker plots. EDV, end-diastolic volume; ESV, end-systolic volume; EF, ejection fraction.
Heart rate confirmation study.
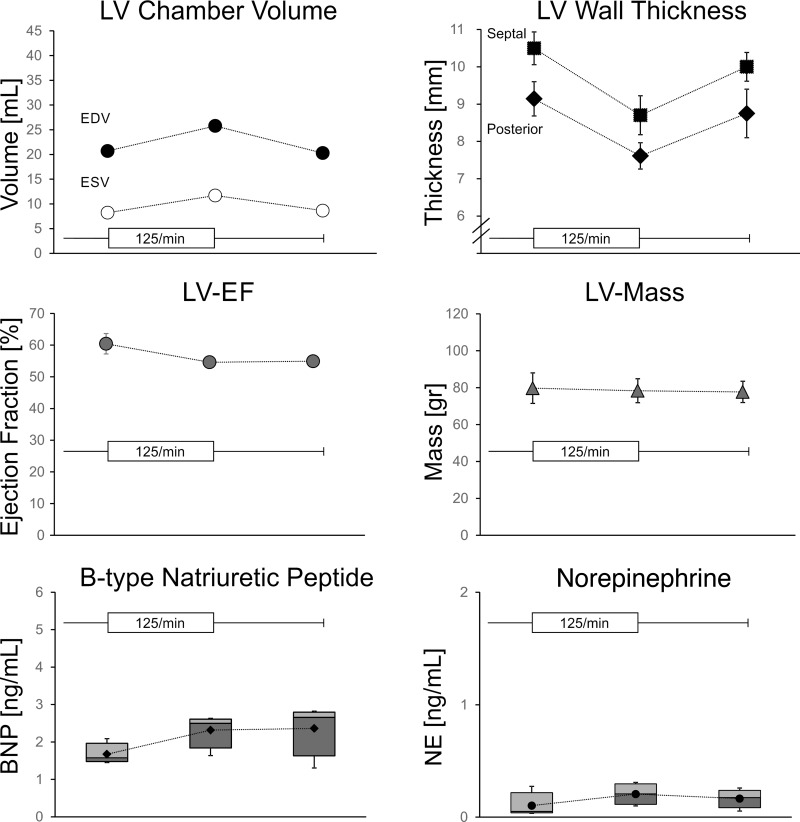
In another four animals, we confirmed the effects of 125/min pacing in the absence of the initial period of pacing at 150/min (Fig. 4). The heart rate in the first 3 wk prior to pacing was 88 ± 4/min. After 2 wk of pacing at 125/min, the LV EDV in sinus rhythm increased by 25 ± 14% (P < 0.05), with a trend toward an increased EDV (P = 0.09). The EF was 55 ± 1%, not different from baseline. The LV mass remained unchanged as well. After cessation of pacing, LV volumes returned to baseline values within 2 wk.
Fig. 4.
Pacing-induced changes in LV dimensions and biomarkers in the heart rate confirmation study. LV dimensions and plasma biomarkers were assessed 3 wk after the initial surgery. Thereafter, right atrial pacing was started at a rate of 125/min. After 2 wk, pacing was stopped to obtain all measurements. A final assessment was made after 2 wk of sinus rhythm. Error bars represent SE. Biomarker results are presented as box-and-whisker plots.
High heart rate study.
In two additional animals, we set out to induce pathological LV dilation at a rate of 170/min. The goal was to explore if a pronounced dilation would regress once the rate was lowered to 125/min. After 4 wk at 170/min, the animals did not develop any signs of HF, despite a 141% increase in the average LV end-diastolic and a 246% increase in average end-systolic chamber volume. EF was reduced from 51% to 37% and from 59% to 33%. Within 2 wk of lowering the rate from 170/min to 125/min, the cavity size regressed to previously established 125/min levels and the EF was restored to 54% and 59%, respectively.
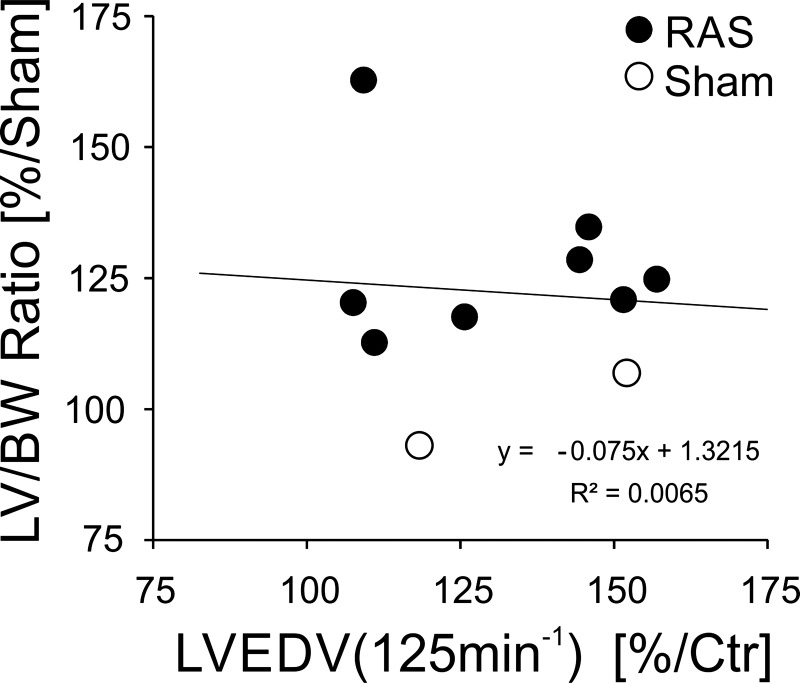
Table 1 summarizes the cumulative 125/min data under the following conditions: prior to pacing (baseline), immediately after 2 wk of pacing at 125/min, and 2 wk after pacing cessation (recovery). This recapitulates the significant increase in LV chamber dimensions and thinning of the LV wall, which in effect reduced the average mass-to-volume ratio by ∼27% to normal levels. The two animals without RAS were included, reproducing the natural distribution of hypertrophy in HFpE. As shown in Fig. 5, there was no correlation between the degree of hypertrophy and the heart rate-induced change in LV dimension.
Table 1.
Cumulative data prior to pacing, after 2 wk of pacing, and after 2 wk without pacing
| Collective Data |
P Values |
||||
|---|---|---|---|---|---|
| Baseline (n = 10) | 125/min (n = 10) | Recovery (n = 8) |
Baseline vs. 125/min | Baseline vs. Recovery | |
| Body wt/heart rate | |||||
| Body wt, kg | 42 ± 3 | 44 ± 2 | 46 ± 2 | 0.01* | <0.01* |
| Heart rate, min−1 | 90 ± 5 | 125 | 81 ± 4 | <0.01* | 0.01* |
| Atrial pacing, % | 75 ± 20 | ||||
| Gross anatomy | |||||
| LV mass, g | 118 ± 14 | ||||
| RV mass, g | 35 ± 3 | ||||
| LV echocardiography | |||||
| EDD, mm | 31.9 ± 2.8 | 35.2 ± 2.8 | 32.9 ± 2.8 | 0.03* | 0.37 |
| ESD, mm | 20.7 ± 3.8 | 25.6 ± 3.2 | 21.7 ± 2.6 | <0.01* | 0.26 |
| A2-D, cm2 | 9.7 ± 1.7 | 11.0 ± 2.4 | 9.3 ± 1.9 | 0.08 | 0.95 |
| A2-S, cm2 | 5.5 ± 1.6 | 7.6 ± 1.5 | 5.3 ± 1.6 | 0.01* | 0.85 |
| LV EDV, ml | 25.4 ± 5.4 | 34.2 ± 11.1 | 24.2 ± 5.3 | <0.01* | 0.37 |
| LV ESV, ml | 10.5 ± 2.7 | 18.0 ± 9.2 | 10.8 ± 3.0 | <0.01* | 0.14 |
| EF, % | 58 ± 6 | 54 ± 4 | 55 ± 4 | 0.16 | 0.30 |
| Mass-to-volume ratio, g/ml | 3.4 ± 0.8 | 2.5 ± 0.8 | 3.6 ± 0.7 | <0.01* | 0.84 |
| E/E′ lat | 7.6 ± 2.0 | 7.0 ± 2.9 | 5.4 ± 1.7 | 0.56 | 0.07 |
| Heart failure assessment | |||||
| Tachypnea, % | 0 (0) | 0 (0) | 0 (0) | ||
| Crackles, % | 0 (0) | 0 (0) | 0 (0) | ||
| BNP, ng/ml | 2.9 ± 2.5 | 3.4 ± 2.9 | 3.0 ± 2.7 | 0.09 | 0.19 |
| NE, ng/ml | 0.4 ± 0.5 | 0.4 ± 0.3 | 0.4 ± 0.3 | 0.84 | 0.80 |
| Galectin-3, ng/ml | 23 ± 8 | 23 ± 6 | 24 ± 8 | 0.98 | 0.49 |
Values are means ± SD. Baseline, prior to pacing; 125/min, after 2 wk of pacing; Recovery, after 2 wk without pacing; LV, left ventricle; RV, right ventricle; EDD, end-diastolic diameter; ESD, end-systolic diameter; A2-D, parasternal short-axis LV area at end diastole; A2-S, parasternal short-axis LV area at systole; EDV, end-diastolic volume; ESV, systolic volume; EF, ejection fraction; E/E′ lat, ratio of early ventricular filling peak velocity to lateral early diastolic mitral annular velocity; BNP, B-type natriuretic peptide; NE, norepinephrine.
Significant difference (P < 0.05).
Fig. 5.
Comparison of 125/min pacing-induced change in chamber volume and degree of hypertrophy: (LV mass/tibia length)/mean sham (LV mass/tibia length). There is no significant relationship between pacing-mediated LV volume change and degree of hypertrophy. BW, body weight; Ctr, control.
Heart Rate Effects on Biomarkers
None of the animals developed HF, and the biomarkers did not change (Figs. 3 and 4). Galectin-3 levels were also unchanged at 150/min compared with baseline (27.2 ± 4.9 vs. 27.6 ± 6.0 ng/ml). In addition, BNP and NE levels remained in the normal range throughout the high heart rate study at all time points (BNP <8 ng/ml and NE <0.5 ng/ml). The cumulative biomarker results at 125/min are presented in Table 1.
EDP-EDV Relationships
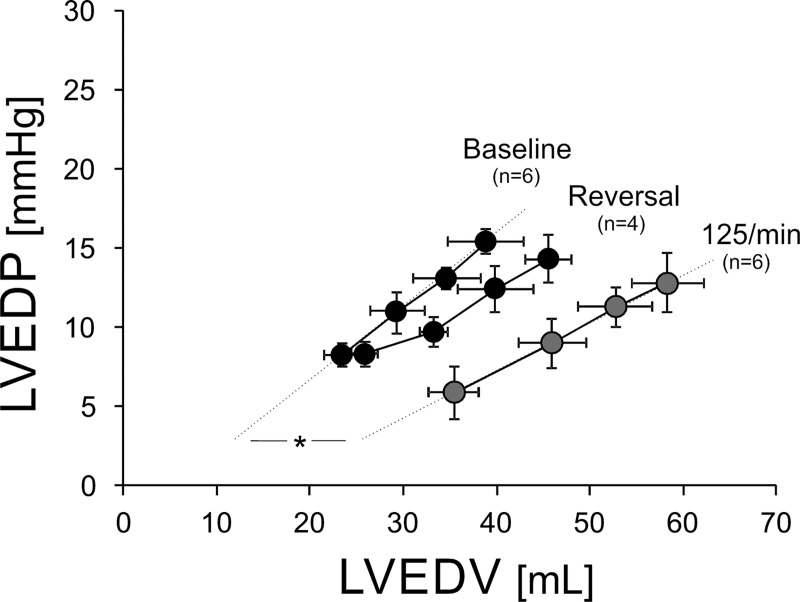
In six animals, we were able to serially assess the LV EDP-EDV relationship before and after the heart rate-induced structural change (Fig. 6). After 2 wk of pacing at 125/min, we found a rightward shift of the pressure-volume relationship due to the increase in chamber volume. Despite nominally lower baseline filling pressures after 2 wk of pacing at 125/min, this did not reach significance (P = 0.24). However, diastolic compliance, expressed as ΔEDV/ΔEDP with the volume challenge, increased from 1.95 ± 0.72 ml/mmHg at baseline to 3.18 ± 0.57 ml/mmHg (P < 0.05) after 2 wk of pacing at 125/min.
Fig. 6.
Pressure-volume relationships. Serial assessment of LV EDP-EDV relationship prior to pacing (baseline), after 2 wk of pacing at 125/min, and 2 wk after pacing cessation (reversal). All time points include a baseline measurement followed by measurements after three 250-ml normal saline infusions. Error bars represent SE. Slope of lines represents LV compliance. *P < 0.05.
Tissue Fibrosis Levels
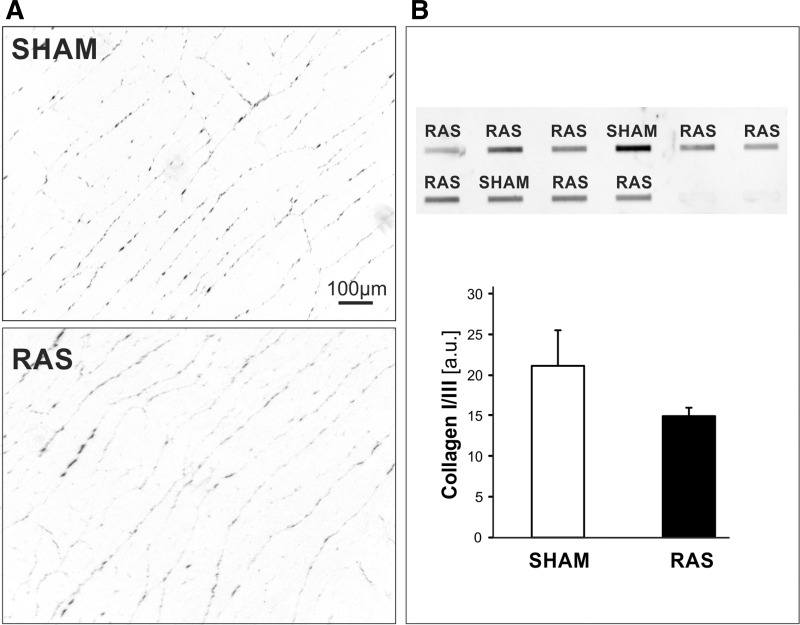
Analysis of the myocardial connective tissue levels at the end of the study protocol revealed very low levels of interstitial connective tissue in all paced animals. Importantly, there was no difference between RAS animals and sham animals (Fig. 7). The relative area of total myocardial interstitial fibrosis was 0.69 ± 0.08% in sham and 0.52 ± 0.16% in RAS animals. Levels of myocardial connective tissue were significantly higher in referent control sections from five RAS animals that were not paced than in the paced RAS animals (1.45 ± 0.98%, P < 0.05).
Fig. 7.
A: PicroSirius-stained myocardial section from a RAS animal and a sham animal after pacing demonstrating similar amounts of interstitial connective tissue. B: slot-blot analysis of collagen I/III protein levels in myocardial tissue homogenates. Collagen levels were not different in RAS and sham animals. au, Arbitrary units.
There was no significant difference in collagen I and III levels in homogenates from RAS and sham animals (Fig. 7).
DISCUSSION
In this study we explored whether moderately increased heart rates could be used to favorably modify the LV structure in pigs with concentric LV hypertrophy. This myocardial substrate is very common and is frequently encountered in patients with concentric remodeling and HFpEF.
The following novel observations were made: 1) a moderate elevation in heart rate can increase LV volumes and lower mass-to-volume ratios in concentrically hypertrophied LVs without reducing the EF, and 2) this change in LV structure is reversible, can be titrated, and is not associated with HF. Our results also suggest that rate-induced (re)modeling reduces myocardial fibrosis and improves diastolic compliance.
The rationale for heart rate as a treatment target originates from several physiological and clinical observations. Heart rate, through its high variability, plays a central role in cardiac workload. It is believed to play a key part in many physiological adaptations such as athlete's heart and in pregnancy (8). Heart rate is an established treatment target in dilated cardiomyopathy, where a lower heart rate reduces LV chamber size (25). In contrast, extreme tachycardia is known to severely dilate the LV, as evident in tachycardia-induced cardiomyopathy. Accordingly, we speculated that modest heart rate elevations could be used to restore chamber volumes, reduce LV wall thickness, and trigger an interstitial remodeling process, which in aggregate could improve LV filling. If so, patients with concentric hypertrophy and impaired LV filling might potentially benefit from modest heart rate elevations, if the structural remodeling can be safely accomplished.
The unilateral RAS model is well characterized and recapitulates concentric LV hypertrophy and myocardial fibrosis (14, 15, 27). Although the lack of overt HF and only moderate levels of fibrosis are clear limitations of this model, they allowed us to establish the safety of moderately elevated heart rates in a common antecedent disease substrate of HFpEF. Because there is no established large-animal model that recapitulates HFpEF, this limitation cannot be overcome (10).
The potential to titrate the effects of heart rate was first apparent after the rate was lowered from 150/min to 125/min. The second experimental series confirmed that this reduction in LV chamber size was not merely a result of an ongoing regression of chamber volumes after pacing at 150/min but was specific to pacing at 125/min. We also determined that even a very pronounced chamber dilation induced by a heart rate of 170/min was not pathological, as determined by the lack of HF and biomarker elevations, and could be reversed with a rate of 125/min. Within 2 wk, chamber dimensions regressed and EF normalized. These rapid structural changes demonstrate that the swiftness of the physiological cardiac remodeling process is preserved in concentric hypertrophy, even when pushed to levels that exceed the physiological adaptations reported in human subjects exposed to extreme bed rest, spaceflight, or reconditioning (16, 20).
Although the results after pacing at >125/min suggest a gradual rate-dependent decrease in EF, no clinical signs or biomarker changes suggestive of HF were seen at any of the studied heart rates. This suggests that the observed structural changes are within the limits of physiological adaptations without causing decompensation. This is an important finding, as it is well established that pigs and other similar-sized animals develop overt HF with increased BNP and NE levels when exposed to pacing at >200/min (10).
Our analysis of the EDP-EDV relationship after pacing at 125/min demonstrates a rightward shift with a trend toward lower LV EDP with a substantial improvement in diastolic compliance. This may be primarily facilitated by wall thinning, but a pacing-induced reduction in myocardial connective tissue could contribute to this finding. Compared with nonpaced RAS animals, the level of myocardial connective tissue was low in all paced animals. The overall very low levels of connective tissue in all paced animals were very likely the result of a concurrent interstitial matrix remodeling process that reduces collagen levels to support the elongation and thinning of individual myocytes, as shown in animal models of tachycardia-induced cardiomyopathy (5, 22). Because the analyzed tissues originate from hearts that were allowed to return to their baseline geometry, it is conceivable that intermittent increases in heart rate may suffice to reduce tissue fibrosis without a permanent chamber enlargement.
It is important to acknowledge that it is not known if a reversal of concentric remodeling would be beneficial in patients. However, it is equally possible that a lack of structural remodeling in clinical HFpEF trials may also have contributed to their failure. It is also unclear if a moderate increase in heart rate would be feasible and safe in patients, as it could result in symptoms and tachycardia-induced cardiomyopathy. It is also possible that older age, advanced myocardial fibrosis, and other changes in the composition of the myocardium may reduce myocardial plasticity (28). High resting heart rates are generally viewed as an unfavorable prognostic sign (6). This may be the reason why only very few clinical studies have investigated interventions that allow increased heart rates. An important trial in this regard is the guideline-influencing Rate Efficacy in Permanent Atrial Fibrillation (RACE-2) study (26). This trial compared two heart rate control strategies in patients with atrial fibrillation and included patients with HFpEF. The strict rate control group had a target heart rate of <80/min at rest. The lenient rate control allowed heart rates of up to 110/min. This study established the noninferiority of higher heart rates with a numerical signal toward better outcomes. Similar to our own preliminary experience of nocturnal pacing at comparable rates, no untoward safety signals were observed. This may also provide some reassurance for older patient populations with a high prevalence of coronary artery disease.
Despite the discussed limitations of the experimental model and the exploratory approach, our proof-of-concept study suggests that a heart rate-mediated innate cardiac adaptation mechanism could be employed to safely induce potentially beneficial myocardial remodeling. This may present a therapeutic opportunity in symptomatic patients with concentric remodeling and increased LV mass-to-volume ratios, as commonly encountered in HFpEF. Furthermore, it appears possible that such an intervention could achieve favorable effects, even if applied intermittently and at lower rates.
GRANTS
This research was supported by Medtronic and National Institutes of Health Grants R01 DK-102325 (L. O. Lerman), R01 HL-118524 (M. M. LeWinter), and R01 HL-122744 (M. Meyer).
DISCLOSURES
Medtronic supported this research. The University of Vermont has received intellectual property protection for the use of pacemakers to prevent and treat heart failure with preserved ejection fraction and related conditions.
AUTHOR CONTRIBUTIONS
F.J.K., S.B., K.E.R., R.L., and M.M. performed the experiments; F.J.K. and M.M. analyzed the data; F.J.K., T.A., L.O.L., M.M.L., and M.M. interpreted the results of the experiments; F.J.K., S.B., K.E.R., R.L., L.O.L., M.M.L., and M.M. edited and revised the manuscript; F.K., S.B., K.E.R., R.L., T.A., M.M.L., and M.M. approved the final version of the manuscript; K.E.R., M.M.L., and M.M. drafted the manuscript; M.M.L. and M.M. developed the concept and designed the research; M.M. prepared the figures.
ACKNOWLEDGMENTS
We thank Trina Brand and Evan Liberman (Medtronic) for expert advice and support. We also gratefully acknowledge Richard Lachapelle and Yuan Wang for providing assistance with the animal studies. The histological analysis of the myocardial tissue samples was performed by the University of Vermont College of Medicine Imaging Core facility.
REFERENCES
- 1.Cheng S, Fernandes VR, Bluemke DA, McClelland RL, Kronmal RA, Lima JA. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging 2: 191–198, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Boer RA, Edelmann F, Cohen-Solal A, Mamas MA, Maisel A, Pieske B. Galectin-3 in heart failure with preserved ejection fraction. Eur J Heart Fail 15: 1095–1101, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 57: 450–458, 1986. [DOI] [PubMed] [Google Scholar]
- 4.Dolber PC, Spach MS. Conventional and confocal fluorescence microscopy of collagen fibers in the heart. J Histochem Cytochem 41: 465–469, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Eble DM, Spinale FG. Contractile and cytoskeletal content, structure, and mRNA levels with tachycardia-induced cardiomyopathy. Am J Physiol Heart Circ Physiol 268: H2426–H2439, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, Steg PG, Tardif JC, Tavazzi L, Tendera M. Resting heart rate in cardiovascular disease. J Am Coll Cardiol 50: 823–830, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 6: 606–619, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med 358: 1370–1380, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, Larson MG, Levy D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol 60: 1249–1256, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS, Prabhu SD, Rockman HA, Kass DA, Molkentin JD, Sussman MA, Koch WJ. Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res 111: 131–150, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11: 447–455, 1979. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28: 1–39, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Lerman LO, Chade AR, Sica V, Napoli C. Animal models of hypertension: an overview. J Lab Clin Med 146: 160–173, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Lerman LO, Schwartz RS, Grande JP, Sheedy PF, Romero JC. Noninvasive evaluation of a novel swine model of renal artery stenosis. J Am Soc Nephrol 10: 1455–1465, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation 96: 517–525, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Lin J, Zhu X, Chade AR, Jordan KL, Lavi R, Daghini E, Gibson ME, Guglielmotti A, Lerman A, Lerman LO. Monocyte chemoattractant proteins mediate myocardial microvascular dysfunction in swine renovascular hypertension. Arterioscler Thromb Vasc Biol 29: 1810–1816, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer M, McEntee RK, Nyotowidjojo I, Chu G, LeWinter MM. Relationship of exercise capacity and left ventricular dimensions in patients with a normal ejection fraction. An exploratory study. PLos One 10: e0119432, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355: 251–259, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Perhonen MA, Franco F, Lane LD, Buckey JC, Blomqvist CG, Zerwekh JE, Peshock RM, Weatherall PT, Levine BD. Cardiac atrophy after bed rest and spaceflight. J Appl Physiol 91: 645–653, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O'Meara E, Desai AS, Heitner JF, Li G, Fang J, Rouleau J, Zile MR, Markov V, Ryabov V, Reis G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail 7: 740–751, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinbane JS, Wood MA, Jensen DN, Ellenbogen KA, Fitzpatrick AP, Scheinman MM. Tachycardia-induced cardiomyopathy: a review of animal models and clinical studies. J Am Coll Cardiol 29: 709–715, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Spinale FG, de Gasparo M, Whitebread S, Hebbar L, Clair MJ, Melton DM, Krombach RS, Mukherjee R, Iannini JP, O SJ. Modulation of the renin-angiotensin pathway through enzyme inhibition and specific receptor blockade in pacing-induced heart failure. I. Effects on left ventricular performance and neurohormonal systems. Circulation 96: 2385–2396, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 126: 65–75, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Tardif JC, O'Meara E, Komajda M, Bohm M, Borer JS, Ford I, Tavazzi L, Swedberg K. Effects of selective heart rate reduction with ivabradine on left ventricular remodelling and function: results from the SHIFT echocardiography substudy. Eur Heart J 32: 2507–2515, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM, Hillege HL, Bergsma-Kadijk JA, Cornel JH, Kamp O, Tukkie R, Bosker HA, Van Veldhuisen DJ, Van den Berg MP. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med 362: 1363–1373, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 83: 1849–1865, 1991. [DOI] [PubMed] [Google Scholar]
- 28.Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, Van Buren P, Meyer M, Redfield MM, Bull DA, Granzier HL, LeWinter MM. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 131: 1247–1259, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zile MR, Gaasch WH, Patel K, Aban IB, Ahmed A. Adverse left ventricular remodeling in community-dwelling older adults predicts incident heart failure and mortality. Heart Fail 2: 512–522, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation 124: 2491–2501, 2011. [DOI] [PubMed] [Google Scholar]