Abstract
AIM
To study the differences in immune response and cytokine profile between acute liver failure and self-limited acute hepatitis.
METHODS
Forty-six patients with self-limited acute hepatitis (AH), sixteen patients with acute liver failure (ALF), and twenty-two healthy subjects were involved in this study. The inflammatory and anti-inflammatory products in plasma samples were quantified using commercial enzyme-linked immunoassays and quantitative real-time PCR. The cellular immune responses were measured by proliferation assay using flow cytometry. The groups were divided into viral- and non-viral-induced self-limited AH and ALF. Thus, we worked with five groups: Hepatitis A virus (HAV)-induced self-limited acute hepatitis (HAV-AH), HAV-induced ALF (HAV-ALF), non-viral-induced self-limited acute hepatitis (non-viral AH), non-viral-induced acute liver failure (non-viral ALF), and healthy subjects (HC). Comparisons among HAV and non-viral-induced AH and ALF were performed.
RESULTS
The levels of mitochondrial DNA (mtDNA) and the cytokines investigated [interleukin (IL)-6, IL-8, IL-10, interferon gamma, and tumor necrosis factor] were significantly increased in ALF patients, independently of etiology (P < 0.05). High plasma mtDNA and IL-10 were the best markers associated with ALF [mtDNA: OR = 320.5 (95%CI: 14.42-7123.33), P < 0.0001; and IL-10: OR = 18.8 (95%CI: 1.38-257.94), P = 0.028] and death [mtDNA: OR = 12.1 (95%CI: 2.57-57.07), P = 0.002; and IL-10: OR = 8.01 (95%CI: 1.26-50.97), P = 0.027]. In the cellular proliferation assay, NKbright, NKT and regulatory T cells (TReg) predominated in virus-specific stimulation in HAV-induced ALF patients with an anergic behavior in the cellular response to mitotic stimulation. Therefore, in non-viral-induced ALF, anergic behavior of activated T cells was not observed after mitotic stimulation, as expected and as described by the literature.
CONCLUSION
mtDNA and IL-10 may be predictors of ALF and death. TReg cells are involved in immunological disturbance in HAV-induced ALF.
Keywords: Acute liver failure, Cytokines, Mitochondrial DNA, Cellular immune response, Hepatitis A virus
Core tip: Acute liver diseases induced by viral infections are considered major causes of liver failure and death in Brazil. To better understand this pathogenesis, we investigated in a pioneering way the cellular immune response, inflammatory mediators and mitochondrial products in patients with hepatitis A virus (HAV)-induced acute liver failure (ALF) in comparison to patients with non-virus-induced ALF in a cross-sectional study. The results showed that non-invasive samples could be helpful to assay early prognostic markers that would indicate the necessity for liver transplantation. The contribution of in vitro immune response involved in ALF can be helpful to show the necessity of mass vaccination against HAV.
INTRODUCTION
Acute liver failure (ALF) is a rare (0.5%-1% of the acute hepatitis cases) and devastating clinical syndrome resulting from an acute insult that occurs when a high percentage of liver cells are rapidly lost. Liver transplantation is the only effective therapy[1-4]. Non-invasive methods have been proposed to evaluate the liver damage[5-7] and predict the worst outcome (death)[8-10], with little success. Nevertheless, there are few studies on the systemically released inflammatory products that indicate liver failure or regeneration before liver transplantation, such as cytokine profile or mitochondrial DNA[11-15]. Additional early prognostic markers are urgently requested to evaluate the necessity of liver transplantation therapy.
The causes of ALF involve a variety of toxic, viral, metabolic, and vascular liver injuries. The etiology of ALF varies with geography[16], and the hepatitis A virus (HAV) is the major cause of acute hepatitis in Brazil[17,18] due to absence of an effective hepatitis A vaccination program. Recent studies have shown high counts of natural killer (NK) cells (NKbright and NKdim) during self-limited hepatitis A[19]. Functionally, NK cells are important components of liver immunology, mediating pro-inflammatory functions, such as IFNγ secretion by NKbright (CD3-CD56+CD16-) cells, as well as the lysis of target cells by a subset of NKdim (CD3-CD56low CD16+) cells[7,19-22].
Perrella et al[23] (2008) showed that regulatory T cells (TReg) (CD4+CD25+) are important factors in acute hepatitis A resolution. Trujillo-Ochoa et al[14] showed that serum IL-17 is elevated in children with acute hepatitis A infection; however, the involvement of TReg and helper T cells in ALF caused by hepatitis A is unknown.
The goal of our study was to evaluate plasma levels of inflammatory and anti-inflammatory cytokines and mtDNA in a pilot study with a case series of liver injury patients and their association with ALF and occurrence of death. We quantified the mechanism of viral (HAV) and non-viral liver dysfunction by phenotypically characterizing cytotoxic, helper, and TReg and analyzed the cytokine secretion in a peripheral blood mononuclear cell (PBMC) clonal proliferation assay.
MATERIALS AND METHODS
Patients and samples
Eighty-four subjects agreed in participate in this study in Rio de Janeiro, Brazil, from 2009 to 2012: 46 (54.76%) were consecutive outpatients with self-limited acute hepatitis (AH) that were referred to the Viral Hepatitis Clinic of Oswaldo Cruz Institute - Fiocruz; 16 (19.05%) inpatients were admitted to the Bonsucesso Federal Hospital, a referral hospital for patients with ALF requiring transplantation; and 22 (26.19%) were healthy donors.
All samples were assayed for HAV, hepatitis B virus (HBV), hepatitis C virus (HCV), and hepatitis E virus (HEV) serological markers using commercially available enzyme-linked immunoassays (ELISAs): Anti-HAV IgM (Abbott, United States), Vikia HBsAg (Biomerieux, France), Murex anti-HCV version 4.0 (Diasorin, South Africa), and bioELISA HEV IgM version 3.0 (Biokit, Spain). Blood samples were also assayed using rapid tests for syphilis (DPP®, Bio-manguinhos, Brazil), HIV-1/2 (DPP®, Bio-manguinhos, Brazil), dengue (SD BIOLINE, Standard Diagnostics, South Korea), and leptospirosis (SD BIOLINE, Standard Diagnostics, South Korea). Other current infections and autoimmune diseases were analyzed with a chemiluminescent ELISA for Epstein-Barr, cytomegalovirus and antinuclear antibodies (ANA). The respective reference levels of ≥ 20U/mL, ≥ 30 UA/mL, and ≥ 1.5 UI/mL were considered positive. Herpes virus type 1 (HSV-1) and herpes virus type 2 (HSV-2) were investigated using a TaqMan-based multiplex assay as previously described[24]. Metabolic disorders were also investigated whether the routine exams (biochemical, hematological, etc.) presented alterations or whether the patient had a family history of metabolic disorders.
AH cases were defined by aminotransferase levels of at least 10 × the upper normal limit and the onset of jaundice in a previously healthy individual[25]. The cases were further categorized according to international normalized ratio (INR) and hepatic encephalopathy grade (HE). Cases with INR < 1.5 and no HE were classified as self-limited AH and those with INR ≥ 1.5 and an HE score above II as ALF[4].
The timing of sample collection was based on the onset of jaundice and liver enzyme levels for self-limited AH patients. In ALF patients, the timing of sample collection was based on ALF diagnosis and hospital admission. In healthy subjects, the sample collection was based on the lack of infection found in their routine exams.
The study population was divided into five groups according to etiology and clinical condition: Group 1: Virus-induced self-limited hepatitis, of which all cases were caused by HAV-AH; group 2: Non-viral-induced self-limited hepatitis, which included drug and indeterminate causes (non-viral AH); group 3: Virus-induced ALF, of which all cases were caused by HAV-ALF; group 4: Non-viral-induced ALF, which included drug and indeterminate causes (non-viral ALF); and group 5: Healthy subjects, as the control group (HC).
To assess the PBMCs, blood samples were collected in the anticoagulant citrate-dextrose solution-A (Greiner Bio-one, Kremsmünster, Austria) and stored at -70 °C (plasma) or in liquid nitrogen (peripheral blood mononuclear cells, PBMCs) until assay. Plasma and PBMC samples used were thawed only once for the different assays.
The study protocol was approved by the National Commission on Ethics in Research (CONEP), and by the institutional review board of the Oswaldo Cruz Foundation, FIOCRUZ (222/03). Signed informed consent was obtained from all participants. The study was performed in compliance with the relevant laws and institutional guidelines and in accord with the ethical standards of the Declaration of Helsinki.
Quantitative detection of cytokines and mitochondrial-derived DNA in ALF, AH and healthy control subjects
To assess the liver inflammatory/anti-inflammatory status, plasma levels of the cytokines IL-6, IL-8, IL-10, IFNγ and tumor necrosis factor alpha (TNFα) were quantified using commercially available Standard ELISA Development kits (Peprotech, United States). To assess hepatocellular damage, the total DNA was purified from the plasma samples using the QIAamp DNA Blood Mini Kit (Qiagen, United States) according to the manufacturer’s instructions[26]. The mitochondrial DNA (mtDNA) was quantified by real-time PCR as previously reported[26] using 3 pairs of primers specific for human cytochrome B (sense 5’atgaccccaatacgcaaaat-3’ and antisense 5’cgaagtttcatcatgcggag3’), human cytochrome C oxidase subunit III (sense 5’atgacccaccaatcacatgc3’ and antisense 5’atcacatggctaggccggag3’), and human NADH dehydrogenase (sense 5’atacccatggccaacctcct3’ and antisense 5’gggcctttgcgtagttgtat3’). The total mtDNA value corresponds to the sum of the individual values from each test. Colorimetric commercial kits were used to assess the levels of liver enzymes and total bilirubin.
Quantitative evaluation of the clonal proliferation response and cell phenotypes of proliferated PBMCs from ALF and AH patients
Twenty-nine PBMC samples from 62 patients were evaluated for the proliferative cellular immune response: 16 samples from patients with self-limited AH (8 patients diagnosed with HAV-induced hepatitis and 8 with non-viral hepatitis) and 13 samples from patients with ALF (8 patients diagnosed with HAV-induced hepatitis and 5 with non-viral hepatitis). Ten of twenty-two healthy subject samples were included in the cellular response assay.
The PBMCs from each patient were separated on a Ficoll density gradient by centrifugation (30 min at 400 g at 18 °C). The concentration of viable cells was determined by trypan blue exclusion. Samples with less than 80% of viable cells at this stage were excluded. In the proliferation assay, the PBMCs were suspended in RPMI 1640 (Sigma Aldrich, United States) medium at a concentration of 5 × 106 cells/mL and mixed with an equal volume of 10 mmol/L carboxyfluorescein succinimidyl ester working solution (CFSE-FITC) (Molecular Probes, Invitrogen, United States) that was diluted 1/1000 for all analyses. Cells that were not labeled with CFSE were used as a negative control for the flow cytometry analysis. The mitogen inducers phytohemagglutinin (PHA) and lipopolysaccharide (LPS) (Sigma Aldrich, United States) were used at final concentrations of 10 μg/mL and 1 ng/mL, respectively, for non-viral proliferation. The HAF-203 strain of HAV was propagated in FRhK-4 cells[27] and was used for viral-antigen-specific (HAV Ag) proliferation (viral titer of 106 HAV-RNA/mL). Duplicate proliferation cultures were performed with 5 × 105 cells/well in 96-well flat bottom culture plates. The plates were incubated at 37 °C in a 5% CO2 incubator for 72 h with PHA, 24 h with LPS and 96 h with HAV Ag. After incubation, the cells were harvested for the flow cytometry assay.
To assess the cell phenotypes and proliferative response, 20000 live cells were collected from each sample using a Cyan flow cytometer (BD Biosciences, United States) and analyzed using the off-line software Summit version 6.0 (Dako Cytomation, United States) (Figures 1 and 2). PBMCs were labeled and quantified with αCD8-PerCP (clone DK25), αCD25-PE (clone ACT1), αCD56-PE (clone CM55B), αCD16-FITC (clone DJ130c) (all from Dako Cytomation, United States), αCD3-APC (clone OKT3), αCD29-FITC (clone MEM101a), αCD44-PECy7 (clone IM7), αFoxP3-FITC (clone PCH101) and isotypes (eBiosciences, San Diego, CA, United States). The intracellular staining for FoxP3 expression was performed with a Cytofix/Cytoperm® kit (BD Biosciences, United States). Total mononuclear cells were electronically gated in R1 plus R2 using forward (FSC) and side (SSC) properties; cellular debris and granular cells were excluded (Figure 1A and B). The proliferating cells (R1 + R2) were defined based on their FSC and SSC properties[28]. The proliferation index (PI) was determined by the software program; this index is a measure of the frequency of cells that have gone through more than three divisions (positive proliferation, CFSElow) (Figure 1C and D)[28-30]. The final PI was determined by calculating the ratio of the average PI for mitogen- or antigen-stimulated cells divided by the average PI of unstimulated cells (Figure 1). The highly expressed surface markers on the T, NK and NKT cell subsets that were activated by antigenic stimulation (R1 + R2) were considered in the off-line software analysis (e.g., Figure 1A and B, and Figure 2). The cell culture supernatants were assayed to quantify IL-6, IL-8, IL-10, IFNγ and TNFα using commercially available Standard ELISA Development kits (Peprotech, United States). Human cytokine IL-17/17A was quantified with the commercially available Mini ELISA Development kit (Peprotech, United States).
Figure 1.
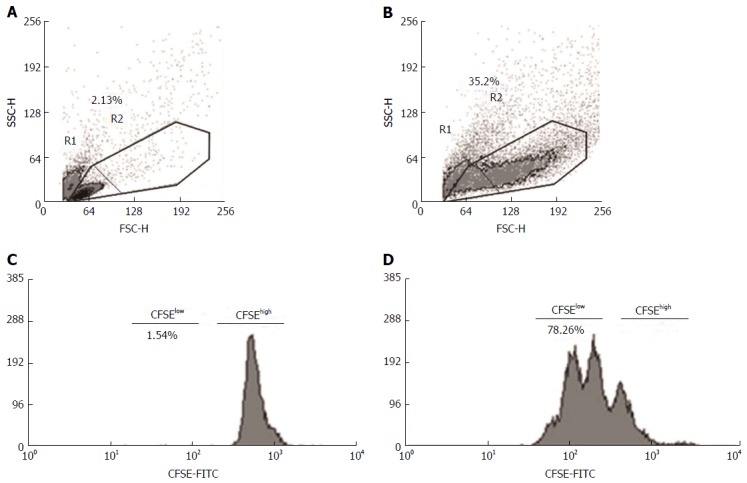
Flow cytometry analysis of proliferating mononuclear cells in antigenic stimulation. Mononuclear cell populations were gated using forward (FSC) and side (SSC) scatter, and the dot plot identifies the total cells (R1 + R2), resting cells (R1) and blasts (R2). Peripheral blood mononuclear cells, either unstimulated (A and C) or stimulated with antigens (PHA, LPS or HAV Ag) (B and D), were labeled with CFSE. The histograms show the proportion of total (CFSElow + CFSEhigh), resting (CFSEhigh) and proliferating cells (CFSElow) observed using the Cyan flow cytometer and analyzed using the off-line software Summit version 6.0. CFSE: Carboxyfluorescein succinimidyl ester; PHA: Phytohemagglutinin; LPS: Lipopolysaccharide.
Figure 2.
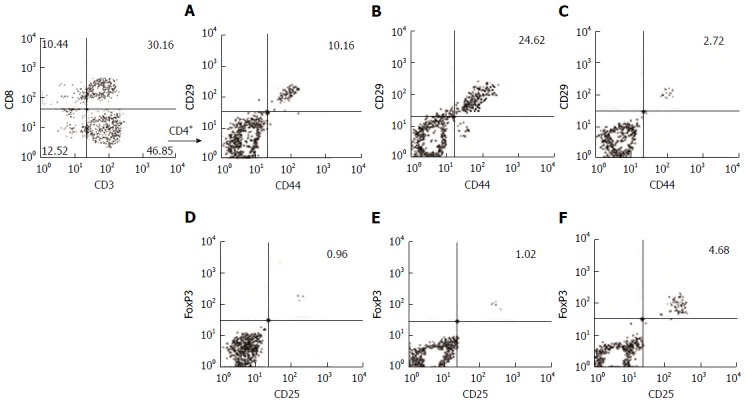
Hepatitis A virus Ag-activated CD4+ T cells in acute liver disease caused by hepatitis A virus. The data from three subjects selected from our study groups were used to represent the gating strategy to select CD29+CD44+ and CD25+FoxP3+ on CD4+ cells (CD3+CD8-). Representative contour plots of the frequency of migratory T helper cells (%) in HAV Ag-activated mononuclear cells from healthy subjects (A), patients with acute hepatitis A (B), patients with acute liver failure with HAV infection (C) and in HAV Ag-activated regulatory T cells from healthy subjects (D), patients with acute hepatitis (E), and patients with acute liver failure (F). HAV: Hepatitis A virus.
Statistical analysis
The data are expressed as the mean ± standard deviation (SD) at a 95%CI. The distribution of the data in the groups was initially evaluated by the Kolmogorov-Smirnov test. The correlations were evaluated using the Spearman rank correlation test (R project for Statistical Computing (http://www.r-project.org/). The differences between self-limited AH, ALF, and healthy subjects were evaluated by intergroup comparisons using the Kruskal-Wallis test. If a significant difference was found, a pair of variables in the three groups was assessed with the Mann-Whitney U-test. For the plasma samples, receiver operating characteristic (ROC) curve analysis was used to compare the predictive strength of markers with chance. The area under the curve was used as a measure of the ability of the test to distinguish between the two groups. The software GraphPad Prism 5 for Windows, version 5.01 (San Diego, CA, United States), was used to perform statistical ROC curve analysis. Multivariate logistic regression was applied to select the independent predictors in plasma samples associated with ALF based on cut-off points (90% specificity and with the highest likelihood ratio value) obtained from ROC curve analysis. In the initial logistic model, all variables were tested for predictive strength. The variables showing statistically significant differences were kept in the final model. The logistic regression analyses were performed using SPSS software version 17.0 for Windows (SPSS Inc., Chicago, IL, United States). The significance for all statistical analyses was defined as P < 0.05.
RESULTS
Characterization of the AH and ALF patients
Non-viral ALF cases were caused by α-methyldopa (1 patient), rifampicin (1), and cryptogenic disease (3). The self-limited AH were caused by NSAIDs (2) and cryptogenic disease (6). HAV infection was the viral etiology found in self-limited AH (38) and ALF (11). The mean ± SD of viral load for the HAV was 1.4 × 106 ± 8.6 × 105 HAV-RNA/mL in plasma samples from ALF patients and 3.6 × 103 ± 1.8 × 103 HAV-RNA/mL in samples from AH self-limited patients (AH).
The time of blood collection in self-limited AH was 1-4 wk in the HAV-AH group and 2-6 wk in the non-viral AH group. In the ALF patient group it was 1-3 wk for HAV-ALF and non-viral ALF. Three patients with acute HAV infection, INR < 1.5 and no coma grade (HE < I) had their samples collected before the evolution to liver failure. They progressed to death before transplant, according to medical records, so they were included in the ALF group. Table 1 shows more information about the study population, including age, gender, coma grade, coagulopathy, liver enzymes, total bilirubin, and outcome.
Table 1.
Clinical characteristics of the studied population n (%)
| Acute liver failure (n = 16) | Acute hepatitis (n = 46) | Healthy control (n = 22) | |
| Age (yr) | |||
| Mean ± SD | 24.88 ± 21.52 | 21.21 ± 10.32 | 24.64 ± 8.79 |
| 25%, 75% | 9.25, 49 | 9.1, 29.75 | 15.2, 47 |
| Gender | |||
| Male | 6 (37.50) | 25 (54.34) | 9 (40.9) |
| Diagnosis | |||
| Hepatitis A | 11 (68.75) | 38 (82.60) | 0 |
| Drug toxicity | 2 (12.50) | 2 (4.34) | 0 |
| Indeterminate | 3 (18.75) | 6 (13.04) | 0 |
| Liver enzymes | |||
| AST (UI/L) | 1095.5 ± 1460 | 344.5 ± 444.9 | 21.68 ± 4.87 |
| ALT (UI/L) | 806.12 ± 639.11 | 517.90 ± 884.30 | 14.36 ± 4.50 |
| Total bilirubin (mg/dL) | 21.47 ± 10.48 | 10.01 ± 6.88 | 0.85 ± 0.09 |
| Coma grade | |||
| 0-I | 3 (18.75) | 0 | 0 |
| II-IV | 13 (81.25) | 0 | 0 |
| Coagulopathy | |||
| INR (mean ± SD) | 4.88 ± 0.99 | 1.16 ± 0.04 | 0.98 ± 0.06 |
| Outcome | |||
| Survived | 6 (46.15) | 46 (100.00) | 22 (100) |
| Died | 10 (53.84) | 0 | 0 |
INR: International normalized ratio; SD: Standard deviation; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase.
Elevated plasma cytokines and mtDNA are seen in AH and ALF patients compared to healthy controls
The intensity of the inflammatory status was not associated with etiology (P > 0.05). Table 2 compares the systemic inflammatory parameters between clinical conditions. The cytokines IL-6, IL-8, IL-10 and IFNγ were significantly raised in the AH and ALF patients compared to the healthy subjects. TNFα was also elevated in the ALF patients compared to the healthy subjects (Table 2). Similarly, total mtDNA was significantly higher in both the AH and ALF groups than in the healthy controls. ALF patients showed a significant elevation in IL-6, IL-10, IFNγ and TNFα as well as high levels of mtDNA compared to the AH patients (Table 2).
Table 2.
Systemic inflammatory products in the plasma samples from patients with acute hepatitis or acute liver failure and healthy subjects
| Plasma variables | HC (n = 22) | AH (n = 49) | ALF (n = 13) | HC vs AHa | HC vs ALF | AH vs ALF |
| IL-6 (pg/mL) | 15.07 ± 25.92 (3.58-26.57)1 | 68.93 ± 109.7 (38.39-99.46) | 509.30 ± 678.70 (147.6-870.9) | 0.0009 | < 0.0001 | < 0.0001 |
| IL-8 (pg/mL)2 | ND | 10.50 ± 20.05 (4.92-16.09) | 144.70 ± 437.6 (-88.45-377.9) | < 0.001 | < 0.0001 | ns |
| IL-10 (pg/mL) | 1.81 ± 5.58 (-0.66-4.28) | 17.28 ± 51.97 (2.81-31.75) | 249.60 ± 379.60 (47.35-451.9) | 0.0006 | < 0.0001 | < 0.0001 |
| IFNγ (pg/mL) | 4.80 ± 18.00 (-3.18-12.79) | 113.0 ± 265.33 (39.1-186.8) | 229.70 ± 342.20 (47.37-412.1) | 0.0075 | < 0.0001 | 0.0016 |
| TNFα (pg/mL) | 1.08 ± 2.38 (0.02-2.13) | 27.25 ± 64.05 (9.42-45.08) | 179.40 ± 161.40 (93.42-265.4) | ns | < 0.0001 | < 0.0001 |
| mtDNA (ng/100 μL plasma) | 81.79 ± 121.6 (27.88-135.7) | 159.6 ± 202.2 (64.99-254.3) | 4228.00 ± 4286.0 (1944-6512) | 0.0131 | < 0.0001 | 0.0008 |
Mean ± standard deviation (95%CI);
IL-8 levels in the plasma samples were evaluated only by the Kruskal-Wallis test.
P < 0.05. The differences between the acute liver failure patients, the self-limited acute hepatitis patients and the healthy controls were evaluated by intergroup comparisons using the Mann-Whitney U-test. IL: Interleukin; IFNγ: Interferon gamma; TNFα: Tumor necrosis factor alpha; mtDNA: Total mitochondrial DNA; ND: Not detectable; ns: Not significant; HC: Healthy control; AH: Acute hepatitis (viral plus non-viral etiologies); ALF: Acute liver failure (viral plus non-viral etiologies).
Elevated plasma cytokines and mtDNA are positively correlated with the degree of liver damage, as represented by the presence of HE or coagulopathy
When we evaluated the correlations between INR and HE and the plasma cytokine and mtDNA levels, the HE grade showed significant positive correlations with IL-6 (P < 0.0001), IL-10 (P < 0.0001), TNFα (P = 0.0001), and IL-8 (P = 0.0034) (Supplementary Figure 1A, C, E and G). The elevated INR values showed significant positive correlations with IL-6 (P < 0.0001), IL-10 (P = 0.0002), TNFα (P = 0.0004) and IFNγ (P = 0.0057) (Supplementary Figure 1B, D, F and H). A positive correlation was observed between mtDNA and HE (P = 0.0002; Supplementary Figure 1I) as well as INR (P = 0.0043; Supplementary Figure 1J).
Elevated cytokines and mtDNA are correlated with outcome in ALF
To determine whether the plasma concentrations of the inflammatory or anti-inflammatory cytokines could be used as indicators of liver dysfunction, we used ROC curve analysis, which showed that IL-6 (P < 0.0001), IL-10 (P < 0.0001), TNFα (P < 0.0001), and IFNγ (P < 0.00104) had the highest diagnostic accuracy for ALF. When we evaluated hepatocyte damage, the ROC curve showed that mtDNA (P = 0.0046) had the highest diagnostic accuracy for ALF.
Among the cytokines, elevated IL-10 was the best indicator of ALF (P = 0.028). Although the IL-6, IL-8, IFNγ and TNFα levels had a positive correlation with hepatic encephalopathy, the association with ALF was not significant (Table 3). Elevated mtDNA (P < 0.0001) was associated with ALF diagnosis.
Table 3.
Potential clinical and inflammatory parameters as indicators of acute liver failure syndrome and death
| Cut-off | Adjusted OR | 95%CI | P value | |
| Plasma variables1 | ||||
| IL-6 (pg/mL) | > 197.6 | 1.36 | 0.04-40.27 | 0.856 |
| IL-10 (pg/mL) | > 55.77 | 18.86 | 1.38-257.94 | 0.028 |
| TNFα (pg/mL) | > 122.6 | 4.42 | 0.185-105.93 | 0.359 |
| mtDNA (ng/100 μL plasma) | > 174 | 320.54 | 14.42-7123.33 | 0.000 |
| Plasma variables2 | ||||
| IL-6 (pg/mL) | > 473.2 | 2.27 | 0.19-26.92 | 0.515 |
| IL-8 (pg/mL) | > 66.30 | 10.42 | 1.54-70.45 | 0.016 |
| IL-10 (pg/mL) | > 95.71 | 8.01 | 1.26-50.97 | 0.027 |
| TNFα (pg/mL) | > 313.7 | 0.27 | 0.03-2.17 | 0.220 |
| mtDNA (ng/100 μL plasma) | > 405.3 | 12.11 | 2.57-57.07 | 0.002 |
| INR | > 2.12 | 29.88 | 5.44-164.19 | 0.000 |
Multivariate analysis from clinical and inflammatory parameters associated with ALF;
Multivariate analysis from clinical and inflammatory parameters associated with death. OR: Odds ratio; IL: Interleukin; IFNγ: Interferon gamma; TNFα: Tumor necrosis factor alpha; mtDNA: Total mitochondrial DNA; ALF: Acute liver failure; INR: International normalized ratio.
Subsequently, the indicators that were associated with death were investigated in all 62 acute liver disease patients: 52 survived (AH and ALF patients) and 10 died (ALF patients). Figure 3 shows that the mtDNA (P < 0.01) and all investigated cytokines were significantly elevated in the non-surviving patients (P < 0.01). The ROC curve analysis showed that elevated INR, IL-6, IL-8, IL-10, TNFα, IFNγ and mtDNA in the plasma samples were able to discriminate survivors from non-survivors with a sensitivity and specificity above 70%. The high plasma levels of mtDNA, IL-8, IL-10 and INR were considered predictive factors for poor outcome (death) in patients with acute liver disease (Table 3). Despite the high levels of IL-6, and TNFα, these factors did not predict death (Figure 3 and Table 3).
Figure 3.
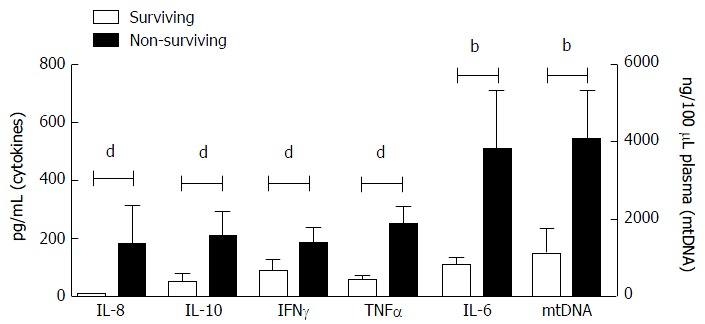
Differences in inflammatory cytokines (interleukin 6, 8 and 10, interferon gamma, and tumor necrosis factor α) and hepatocyte damage (mitochondrial DNA) parameters between the surviving and non-surviving patients. bP < 0.01; dP < 0.001. IL-6: Interleukin 6; IFNγ: Interferon gamma; TNFα: Tumor necrosis factor alpha; mtDNA: Mitochondrial DNA.
Changes in the frequency of mononuclear cell phenotypes and cytokine secretion after the clonal proliferation assay are associated with virus (HAV)- induced AH and ALF syndrome
The panel of phenotypic analyses for PBMC clonal proliferation was composed of activated and migratory T helper cells (CD4+CD29+CD44+), activated and migratory cytotoxic T cells (CD8+CD29+CD44+), activated NK cells [CD3-CD56lowCD16+ (NKdim), CD3-CD56+CD16- (NKbright)], and NKT cells (CD3+CD16+CD56+). Mitogens (PHA and LPS) and virus particles (HAV Ag) were used for non-specific and specific PBMC proliferation, respectively. The mitogen stimulation showed a reduced frequency (anergic behavior) in all investigated phenotypes from HAV-induced hepatitis (ALF and AH patients) (Table 4). The same patients, when stimulated with HAV Ag, exhibited positive proliferation of the regulatory (CD4+CD25+FoxP3+), NKT (CD3+CD16+CD56+), and NKbright (CD3-CD56+CD16-) phenotypes, and only the helper phenotype (CD4+CD29+CD44+) frequency was reduced in HAV-induced ALF patients (Figure 2). In general, the PBMCs from HAV-induced AH showed a tendency toward negative proliferation after mitogen stimulation in all analyzed phenotypes. A significant decrease was detected in in the T helper and NKT cells (AH vs HC) (Table 4). The PBMCs showed a significant positive proliferation of the T helper, cytotoxic (CD8+CD29+CD44+), and NKT cells with HAV-specific stimulation.
Table 4.
Variables from the mitogen-stimulated peripheral blood mononuclear cell phenotypes from patients with acute hepatitis A infection and healthy subjects
| Phenotypes/cytokines (PHA/LPS) | HC (n = 10) | AH (n = 8) | ALF (n = 8) | HC vs AH | HC vs ALF | AH vs ALF |
| PI of CD3+ | 133.1 ± 71.12 | 44.4 ± 25.83 | 17.48 ± 5.94 | 0.0155 | 0.0021 | 0.0426 |
| (95.19-171.0) | (22.80-65.99) | (11.24-23.72) | ||||
| CD4+CD25+FoxP3+ (%) | 17.23 ± 9.74 | 17.08 ± 5.37 | 5.99 ± 2.80 | ns | 0.0009 | 0.0003 |
| (12.03-22.42) | (12.59-21.57) | (3.65-8.33) | ||||
| CD4+CD29+CD44+ (%) | 38.63 ± 18.37 | 20.75 ± 7.82 | 10.22 ± 4.74 | 0.0062 | < 0.0001 | 0.0047 |
| (28.84-48.42) | (14.21-27.29) | (6.25-14.18) | ||||
| CD8+CD29+CD44+ (%) | 39.76 ± 19.91 | 37.56 ± 25.01 | 9.03 ± 4.59 | ns | 0.0002 | 0.0104 |
| (29.15-50.36) | (16.74-58.57) | (5.18-12.88) | ||||
| CD3-CD56+CD16- (%) | 8.31 ± 6.75 | 4.19 ± 2.28 | 0.50 ± 0.37 | ns | 0.0006 | 0.0009 |
| (2.07-14.56) | (2.08-6.30) | (0.15-0.84) | ||||
| CD3-CD56lowCD16+ (%) | 12.70 ± 8.93 | 8.52 ± 5.68 | 1.11 ± 0.66 | ns | 0.0012 | 0.0018 |
| (4.44-20.96) | (3.26-13.78) | (0.50-1.72) | ||||
| CD3+CD56+CD16+ (%) | 13.66 ± 3.54 | 7.20 ± 5.28 | 1.83 ± 1.06 | 0.0117 | < 0.0001 | 0.0070 |
| (11.77-15.55) | (2.79-11.63) | (0.94-2.72) | ||||
| IL-6 (pg/mL) | 2625.33 ± 3320 | 565.7 ± 313.3 | 156.8 ± 173.9 | 0.0155 | < 0.0001 | 0.0070 |
| (856.5-4394) | (303.8-827.6) | (11.40-302.2) | ||||
| TNFα (pg/mL) | 1675.20 ± 623.4 | 1405.0 ± 324.3 | 145.3 ± 107.9 | ns | < 0.0001 | 0.0002 |
| (1343-2007) | (1134-1676) | (55.09-235.5) | ||||
| IL-10 (pg/mL) | 528.86 ± 755.1 | 269.5 ± 145.8 | 188.4 ± 267.1 | ns | 0.0454 | ns |
| (126.5-931.2) | (147.6-391.5) | (-16.88-393.7) | ||||
| IFNγ (pg/mL) | 3379.1 ± 1869 | 2467.0 ± 2787 | 1249.0 ± 2067 | ns | 0.0205 | ns |
| (2383-4375) | (137.1-4798) | (-479.2-2977) | ||||
| IL-8 (pg/mL) | 273.9 ± 116.3 | 274.2 ± 148.5 | 151.0 ± 156.4 | ns | 0.0205 | 0.0379 |
| (211.9-335.9) | (150.0-398.3) | (20.21-281.8) | ||||
| IL-17 (pg/mL) | 73.81 ± 107.0 | 31.55 ± 35.58 | 4.16 ± 3.20 | ns | 0.0029 | 0.0116 |
| (16.81-130.8) | (1.80-61.30) | (1.49-6.84) |
The differences between the hepatitis A-induced acute liver failure (ALF) patients, the self-limited acute hepatitis A (AH) patients, and the healthy control (HC) subjects were evaluated by intergroup comparisons using the Mann-Whitney U-test. The significance cutoff for all statistical analyses was defined as P < 0.05. IL: Interleukin; IFNγ: Interferon gamma; TNFα: Tumor necrosis factor alpha; ns: Not significant; PI: Proliferation index.
The secreted cytokines, IL-6, TNFα, IL-8 and IL-17, were reduced in the supernatant of HAV-induced hepatitis PBMCs from ALF patients compared to AH patients during mitogen stimulation. Additionally, IL-10 and IFNγ were reduced in ALF patients vs the HC subjects. In patients with AH A, we observed a significant reduction in IL-6 secretion and a general tendency toward a reduced secretion of all cytokines, but there were no significant differences (Table 4).
The analysis of the secreted cytokines after HAV Ag stimulation of PBMCs from HAV-induced hepatitis patients showed reduced levels of TNFα and IL-17 when comparing the ALF and AH patients. Reduced levels of secreted TNFα were also observed in the ALF patients compared to the HC subjects. Additionally, we observed elevated levels of secreted IL-10 and IFNγ. The ALF patients presented elevated levels of secreted IL-8 compared to the HC subjects. The levels of secreted IL-10, IFNγ, IL-8 and IL-17 were elevated in cultures from the AH patients (Table 5).
Table 5.
Variables from hepatitis A virus Ag-stimulated peripheral blood mononuclear cell phenotypes from patients with acute hepatitis A infection and healthy subjects
| Phenotypes/cytokines (HAVAg) | HC (n = 10) | AH (n = 8) | ALF (n = 8) | HC vs AH | HC vs ALF | AH vs ALF |
| PI of CD3+ | 1.09 ± 0.85 | 3.15 ± 1.92 | 3.34 ± 2.29 | 0.0053 | 0.0044 | ns |
| (0.64-1.55) | (1.54-4.76) | (1.42-5.25) | ||||
| CD4+CD25+FoxP3+ (%) | 0.85 ± 0.96 | 1.0 ± 0.97 | 3.19 ± 1.49 | ns | 0.0011 | 0.0070 |
| (0.34-1.37) | (0.18-1.81) | (1.95-4.44) | ||||
| CD4+CD29+CD44+ (%) | 11.99 ± 6.43 | 27.93 ± 8.16 | 5.46 ± 5.92 | 0.0008 | 0.0077 | 0.0006 |
| (8.55-15.42) | (21.1-34.76) | (0.50-10.42) | ||||
| CD8+CD29+CD44+ (%) | 12.65 ± 4.31 | 30.13 ± 6.74 | 36.05 ± 10.59 | 0.0001 | 0.0001 | ns |
| (10.35-14.95) | (24.49-35.77) | (27.19-44.90) | ||||
| CD3-CD56+CD16- (%) | 0.19 ± 0.20 | 0.30 ± 0.19 | 1.33 ± 0.85 | ns | 0.0009 | 0.0005 |
| (0.05-0.34) | (0.13-0.46) | (0.62-2.04) | ||||
| CD3-CD56lowCD16+ (%) | 4.28 ± 2.22 | 10.09 ± 8.94 | 14.24 ± 11.81 | ns | ns | ns |
| (2.70-5.87) | (2.61-17.5) | (4.36-24.12) | ||||
| CD3+CD56+CD16+ (%) | 1.67 ± 2.71 | 4.25 ± 4.06 | 15.06 ± 7.74 | 0.0110 | 0.0003 | 0.0019 |
| (0.22-3.12) | (0.85-7.65) | (8.58-21.53) | ||||
| IL-6 (pg/mL) | 50.49 ± 76.14 | 76.41 ± 93.18 | 139.7 ± 165.9 | ns | ns | ns |
| (9.92-91.06) | (-1.46-154.3) | (0.98-278.4) | ||||
| TNFα (pg/mL) | 92.49 ± 133.4 | 23.96 ± 28.92 | 1.63 ± 1.01 | ns | 0.0089 | 0.0098 |
| (21.42-163.6) | (-0.21-48.14) | (0.78-2.48) | ||||
| IL-10 (pg/mL) | 10.39 ± 13.97 | 52.78 ± 62.06 | 164.3 ± 75.56 | 0.0297 | 0.0001 | 0.0148 |
| (2.94-17.84) | (0.89-104.7) | (101.1-227.5) | ||||
| IFNγ (pg/mL) | 0.88 ± 1.08 | 106.6 ± 183.9 | 1095 ± 1962 | 0.0035 | 0.0001 | 0.0499 |
| (0.30-1.46) | (-47.14-260.4) | (-546-2735) | ||||
| IL-8 (pg/mL) | 88.64 ± 45.40 | 148.9 ± 54.77 | 150.2 ± 72.19 | 0.0131 | 0.0110 | ns |
| (64.44-112.8) | (103.1-194.7) | (89.84-210.5) | ||||
| IL-17 (pg/mL) | 3.36 ± 3.75 | 32.61 ± 38.30 | 7.58 ± 5.43 | 0.0008 | ns | 0.0499 |
| (1.36-5.36) | (0.59-64.64) | (3.05-12.13) |
The differences between the hepatitis A-induced acute liver failure (ALF) patients, the self-limited acute hepatitis A (AH) patients and the healthy control (HC) subjects were evaluated by intergroup comparisons using the Mann-Whitney U-test. The significance cutoff for all statistical analyses was defined as P < 0.05. IL: Interleukin; IFNγ: Interferon gamma; TNFα: Tumor necrosis factor alpha; ns: Not significant; PI: Proliferation index.
Changes in the frequency of mononuclear cell phenotypes and cytokine secretion after the clonal proliferation assay in non-viral-induced AH and ALF
We observed a tendency toward positive proliferation for the migratory T helper (CD4+CD29+CD44+) and cytotoxic T (CD8+CD29+CD44+) cells for IL-6 and IL-17 release in the ALF patients compared to the AH patients. Significant elevations of NKdim (CD3-CD56lowCD16+) and NKbright (CD3-CD56+CD16-) cell frequencies were associated with high levels of TNFα in the non-viral ALF patients compared to the AH patients and the HC subjects. The IL-8 levels were also significantly elevated in the ALF patients compared to the HC subjects (Table 5). In general, the comparison between the PBMCs from non-viral AH patients and the HC subjects showed a tendency toward a negative proliferation of all phenotypes investigated and the secreted cytokines IL-6, IL-10, IFNγ and IL-17 (Table 6).
Table 6.
Variables from mitogen-stimulated peripheral blood mononuclear cells from non-viral acute hepatitis patients and healthy control subjects
| Phenotypes/cytokines (PHA/LPS) | HC (n = 10) | AH (n = 8) | ALF (n = 5) | HC vs AH | HC vs ALF | AH vs ALF |
| PI of CD3+ | 133.1 ± 71.12 | 173.3 ± 91.84 | 268.4 ± 101.6 | ns | ns | ns |
| (95.19-171.0) | (96.51-250.1) | (142.3-394.5) | ||||
| CD4+CD25+FoxP3+ (%) | 17.23 ± 9.74 | 15.82 ± 8.13 | 6.84 ± 5.12 | ns | ns | ns |
| (12.03-22.42) | (9.02-22.62) | (0.48-13.2) | ||||
| CD4+CD29+CD44+ (%) | 38.63 ± 18.37 | 21.84 ± 7.50 | 36.67 ± 14.54 | ns | ns | ns |
| (28.84-48.42) | (15.56-28.11) | (18.61-54.73) | ||||
| CD8+CD29+CD44+(%) | 39.76 ± 19.91 | 22.26 ± 11.16 | 29.59 ± 15.21 | ns | ns | ns |
| (29.15-50.36) | (12.92-31.59) | (10.71-48.47) | ||||
| CD3-CD56+CD16- (%) | 8.31 ± 6.75 | 6.33 ± 4.13 | 17.22 ± 4.94 | ns | 0.0289 | 0.0030 |
| (2.07-14.56) | (2.88-9.79) | (13.09-21.35) | ||||
| CD3-CD56lowCD16+ (%) | 12.70 ± 8.93 | 11.38 ± 4.67 | 27.66 ± 3.49 | ns | 0.0061 | 0.007 |
| (4.44-20.96) | (7.05-15.71) | (22.11-33.21) | ||||
| CD3+CD56+CD16+ (%) | 13.66 ± 3.54 | 11.63 ± 3.01 | 8.52 ± 4.97 | ns | ns | ns |
| (11.77-15.55) | (9.11-14.15) | (2.35-14.70) | ||||
| IL-6 (pg/mL) | 2625.33 ± 3320 | 966 ± 622.6 | 1309 ± 851.6 | ns | ns | ns |
| (856.5-4394) | (445.5-1486.0) | (251.60-2366) | ||||
| TNFα (pg/mL) | 1675.20 ± 623.4 | 1497 ± 219.8 | 3217 ± 991.5 | ns | 0.0044 | 0.0016 |
| (1343-2007) | (1313-1681) | (1986-4448) | ||||
| IL-10 (pg/mL) | 528.86 ± 755.1 | 217.1 ± 159.1 | 152.1 ± 126.4 | ns | ns | ns |
| (126.5-931.2) | (84.12-350.2) | (-4.89-309.0) | ||||
| IFNγ (pg/mL) | 3379.1 ± 1869 | 2257 ± 2872 | 1378 ± 2533 | ns | ns | ns |
| (2383-4375) | (-143.3-4658) | (-1767-4524) | ||||
| IL-8 (pg/mL) | 273.9 ± 116.3 | 293.9 ± 120.2 | 733.1 ± 404.8 | ns | 0.0267 | ns |
| (211.9-335.9) | (193.4-394.4) | (230.4-1236) | ||||
| IL-17 (pg/mL) | 73.81 ± 107.0 | 36.31 ± 34.62 | 62.73 ± 5.78 | ns | ns | ns |
| (16.81-130.8) | (7.36-65.25) | (55.55-69.91) |
The differences between the non-viral acute liver failure (ALF) patients, the self-limited acute hepatitis (AH) patients, and the healthy control (HC) subjects were evaluated by intergroup comparisons using the Mann-Whitney U-test. The significance cutoff for all statistical analyses was defined as P < 0.05. IL: Interleukin; IFNγ: Interferon gamma; TNFα: Tumor necrosis factor alpha; PI: Proliferation index; HC: Healthy control; ns: Not significant.
Evidence of effects on the TReg and migratory T helper cells obtained from viral and non-viral AH and ALF patients
To understand the influence of the TReg in AH and ALF, we evaluated the balance between the frequency of TReg with the innate and adaptive immune cells studied. Tables 5 and 6 reveal the change in the frequencies of TReg (CD4+CD25+FoxP3+) and migratory T helper frequencies (CD4+CD29+CD44+) in viral ALF after HAV stimulation and in non-viral ALF after mitogen stimulation. Figure 4A shows an elevated TReg-to-Thelper ratio in the HAV-induced ALF patients after HAV stimulation compared to the AH patients and the HC subjects. No changes in the ratios between the TReg and the other phenotypes were observed. After mitogen stimulation, the imbalance between the TReg-to-T helper ratio was significantly reduced in the non-viral ALF patients compared to the AH patients (Figure 4B). For the other investigated phenotypes, the alterations in this ratio were not significant.
Figure 4.
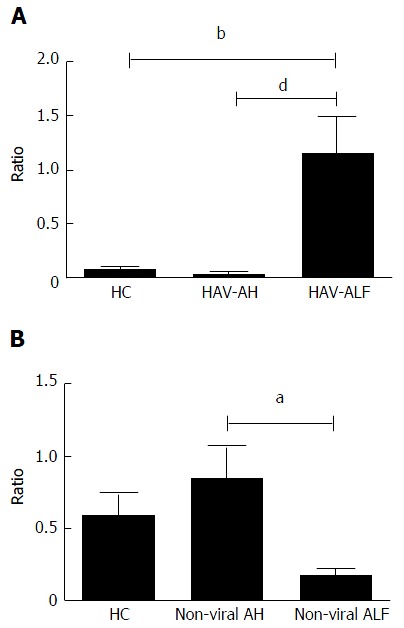
Imbalance between peripheral CD4+ regulatory T cells and migratory T helper cells in viral and non-viral acute hepatitis cases. A: Comparison of the ratio of CD4+ TReg-to-Thelper in HAV-induced acute liver disease (ALF and AH) and healthy controls (HC); B: Comparison of the ratio of CD4+ TReg-to-Thelper cells in non-viral-induced acute liver disease (ALF and AH). aP < 0.05, bP < 0.01; dP < 0.001. HAV: Hepatitis A virus; AH: Acute hepatitis; ALF: Acute liver failure.
DISCUSSION
Acute viral hepatitis was represented by hepatitis A cases in our study. There are a large number of outbreaks of hepatitis A in Brazil; HAV infection is the major etiology of AH and ALF[17,31,32]. Here, we introduced plasma mtDNA level as a new predictor for HAV-induced ALF syndrome. In our opinion, the gross elevation of mtDNA in the ALF patients resulted from massive liver necrosis, as expected. mtDNA, inflammatory and anti-inflammatory cytokines and effector cells are involved in drug-induced liver failure in murine models and in patients[5,8,33].
Increased levels of cytokines and chemokines have been observed in all ALF and non-surviving patients, as described by other authors investigating both drug- and viral-induced ALF[5,34-36]. In our study, the high levels of IL-8 and IL-10 were predictive markers of death in acute liver disease.
Additionally, the imbalance between IL-10 and IL-12 levels has been noted in HBV-induced ALF[37], indicating an ineffective attempt to activate the anti-inflammatory pathway[38-40]. The elevated plasma levels of IL-8 that were detected in all cases of ALF are also described in patients with drug-induced ALF and are correlated with granulocyte migration into the liver parenchyma[5,41]. The elevated levels of circulating IL-6 and TNFα, also described by others[42,43], have been related to attempts at liver regeneration[44,45] and liver injury[46], respectively. Therapeutic approaches targeting the clearance of inflammatory/toxic products (plasmapheresis, hemodiafiltration, and bioartificial livers) from the liver or anti-cytokine therapy are currently being considered[42,47-49] despite contradictory clinical results[50,51].
Even though the profile of monocytes was not explored here, several studies showed the important role of these cells in association with their activation, migration to the liver, and differentiation into hepatic macrophages induced by grow-factor β and IL-10 in humans[52,53] and experimental animal models[54]. Production of the inflammatory cytokines TNF, IL1-β, IL-6, IL-8 and MCP-1 by hepatic macrophages has been associated with cytokine storm in liver injury[52,53]. These findings could explain the biological relevance of high levels of circulating IL-6, IL-8 and IL-10 in ALF patients with the worst outcomes, which were produced by activated monocytes/macrophages, by antigen presentation, and by T cell proliferation.
When we evaluated the linear correlation between coagulopathy/encephalopathy and the plasma variables studied, we observed that the INR and HE scores increased in ALF cases. mtDNA, IL-6, IL-10, IFNγ, TNFα and IL-8 were also significantly elevated and were positively correlated with the elevated INR and/or HE scores observed in severe liver disease. Thus, this study also showed that elevated mtDNA and IL-10 are positively associated with the risk of ALF and mortality. Other authors described IL-10 as an important immunosuppressive cytokine that is released by TReg and is strongly expressed in HBV-induced acute-on-chronic liver failure[38,55,56].
Indeed, the most puzzling fact revealed here was the anergic behavior of the PBMCs from HAV-induced AH and ALF after in vitro mitotic stimulation. This fact may be explained by PBMC clonal exhaustion[57-59] or may suggest that the- TReg influence HAV Ag-primed PBMCs in vivo during AH and ALF syndrome[23]. In addition, when the TReg cells have been previously primed by a specific antigen (e.g., viral antigen), they may develop a non-specific suppressor activity, as described by others[60].
Here, the impairment of the PBMC response was associated with liver dysfunction in patients with AH A. The high TReg cell frequencies in HAV-induced ALF and the increase in IL-10 after HAV Ag stimulation were consistent with the reduced frequency found for the Th17 migratory phenotype (CD4+CD29+CD44+ and IL-17 secretion) and the modulation of the T lymphocyte (CD3+) and cytotoxic T cell (CD8+CD29+CD44+) phenotypes.
Our results suggest that the negative regulation of the TReg cells attempts to control liver inflammation and disease progression by reducing the Th17 migration to the liver tissue in patients with HAV-induced ALF. A similar profile of antigen-specific and unspecific stimulation was observed in patients with HEV-induced AH and ALF[59] and in chronic hepatitis B infection after anti-CD3/CD28 (unspecific) stimulation[56]. Our results did not confirm the TReg influence in non-viral ALF patients, which corroborates other results[61-63]. The expansion of T helper cells (Th17) and the suppression TReg cell production are involved in the mechanisms of liver damage in drug-induced liver disease[61,63].
In HAV-AH, T helper cell proliferation was increased after HAV Ag stimulation and was reduced after mitogen stimulation. The scarce literature available describes defects in cell signaling in CD4+ T cells that are secondary to ALF[59]. Other authors reported that an increase in TReg cells and a decrease in Th17 cells are associated with the survival of HBV-related acute-on-chronic liver failure patients[56], although contradictory opinions have been reported[38,64]. In our study, a similar profile was exhibited by migratory cytotoxic T cells (CD8+CD29+CD44+) for both antigens (viral and mitogen) in HAV-induced AH and ALF. Impaired proliferation was also demonstrated with HEV Ag (pORF3), which was dependent on ERK activation (a member of mitogen-activated protein kinase) and involved in cell proliferation through the TCR/CD3 complex[65].
A linear reduction of NKbright, NKdim and NKT cell reactivity occurred after mitogen stimulation in patients with HAV-induced AH and ALF, which was reversed by HAV Ag-stimulation. The loss of NKdim reactivity in our ALF patients corroborated the suppressor function of the TReg cells, as described above, which appears to modulate the NK-mediated liver injury. A marked elevation in the frequency of NKbright and NKT cells in patients with HAV-induced ALF reinforces the importance of these cells in liver injury[19,66-69].
The significant reduction in the secreted TNFα levels following the HAV Ag-stimulation of patients with HAV-induced AH and ALF shown here was also observed by other authors in HEV-induced AH and ALF[59]. However, TNFα, IL-17 and T helper cell reactivity are positively correlated with the progression to chronic liver disease and acute-on-chronic liver failure in hepatitis B infection[39,64]. In addition, Zhou et al[70] (2012) observed a reduced frequency of the CD4+IL-2+IFNγ+TNF+ population after resolution of hepatitis A, suggesting an increased risk of hepatitis relapse. We observed that the frequency of T cells was not reduced in mitogen stimulated non-viral-induced AH and ALF. The NKbright and NKdim cells with TNFα and IL-8 secretion were significantly elevated in patients with ALF compared to patients with AH and the HC subjects, as expected. The literature describes that the NK cells have an important role in liver damage during non-viral-induced liver diseases and contribute to ALF progression[7,33].
The relative weaknesses of our study included the variance in the plasma cytokine levels, the age of the patients, and the timing of sampling during the evolution of the disease. To minimize the effect of time on our analysis, the blood collection was performed considering the clinical manifestations in self-limited AH and the time of liver failure diagnosis and hospital admission for ALF patients. The sample size was small because the participants who were in the acute symptomatic phase (including pain and malaise) had to agree to the collection of additional samples for cellular immune response investigation; many patients did not return to the ambulatory clinic after resolution of their infection, hindering longitudinal assessment.
In conclusion, The increase of systemically released inflammatory and anti-inflammatory products is associated with AH and ALF. mtDNA and IL-10 may be useful clinical markers as part of a panel to indicate viral (HAV) and non-viral liver disease outcome. These markers, along with IL-8, may be useful to predict death. The anergic behavior of mononuclear cells in fulminant hepatitis A may, in part, be a consequence of the predominant TReg influence that is exclusively detected in HAV infection. Taken together, our results provide additional information to understand the complex immunological disturbances presented during ALF syndrome. Additional efforts are necessary to clarify the anergy mechanism in HAV infection.
ACKNOWLEDGMENTS
The authors thank the blood donors and patients who participated in this study, and Renata Tourinho for standardizing the molecular assay for detecting the HAV-RNA in biological samples.
COMMENTS
Background
The immune response can induce gross inflammation and consequently liver damage in acute liver diseases, independently of etiology. The role of immune cells in inducing acute liver failure (ALF) in hepatitis A infection is still unknown. High levels of systemic inflammatory products and in vitro immune response can be helpful markers to evaluate the necessity for liver transplantation, mainly in hepatitis A patients. Additionally, to minimize the effects of liver failure caused by hepatitis A, universal vaccination should be improved in developing countries such as Brazil.
Research frontiers
Circulating cytokines have been associated with liver failure. Imbalance between peripheral regulatory T cells and helper T cells has been correlated with the worst outcome in hepatitis B-induced liver failure, a disease preventable by vaccination.
Innovations and breakthroughs
This is the first study evaluating biological markers to show the necessity of liver transplantation, particularly in hepatitis A patients. The role of antigen-specific T cells during ALF caused by hepatitis A virus was investigated in a pioneering way in comparison to non-viral etiologies.
Applications
Non-invasive samples as early prognostic markers are urgently needed to determine the necessity of liver transplantation. These findings can be helpful to highlight the development of facilities for laboratory diagnostics in acute liver diseases progression. This study supports the mass vaccination against hepatitis A in developing countries.
Peer-review
The authors describe interesting findings in the circulating cytokines, mitochondrial damage and cell proliferation when comparing different clinical statuses in acute liver diseases (self-limited acute hepatitis and ALF) and healthy controls. The correlation of these factors with the severity of liver disease and outcome is also interesting. This study evaluated accurate markers to predict the necessity for liver transplantation, which is very important for guiding clinical work. Data from T cells in the hepatitis A cohort with liver failure, as the authors note, have not been reported.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study protocol was approved by the National Commission on Ethics in Research (CONEP) and by the institutional review board of the Oswaldo Cruz Foundation, FIOCRUZ (222/03).
Conflict-of-interest statement: The authors declare that they have no competing interests.
Data sharing statement: No additional data are available.
Peer-review started: May 25, 2016
First decision: July 20, 2016
Article in press: September 18, 2016
P- Reviewer: Kulkarni S, Kumar R, Zhao YR S- Editor: Gong ZM L- Editor: A E- Editor: Li D
References
- 1.Lee HS, Choi GH, Joo DJ, Kim MS, Kim SI, Han KH, Ahn SH, Kim DY, Park JY, Choi JS. Prognostic value of model for end-stage liver disease scores in patients with fulminant hepatic failure. Transplant Proc. 2013;45:2992–2994. doi: 10.1016/j.transproceed.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 2.Jayakumar S, Chowdhury R, Ye C, Karvellas CJ. Fulminant viral hepatitis. Crit Care Clin. 2013;29:677–697. doi: 10.1016/j.ccc.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Soundravally R, Narayanan P, Bhat BV, Soundraragavan J, Setia S. Fulminant hepatic failure in an infant with severe dengue infection. Indian J Pediatr. 2010;77:435–437. doi: 10.1007/s12098-010-0027-z. [DOI] [PubMed] [Google Scholar]
- 4.Sugawara K, Nakayama N, Mochida S. Acute liver failure in Japan: definition, classification, and prediction of the outcome. J Gastroenterol. 2012;47:849–861. doi: 10.1007/s00535-012-0624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marques PE, Amaral SS, Pires DA, Nogueira LL, Soriani FM, Lima BH, Lopes GA, Russo RC, Avila TV, Melgaço JG, et al. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology. 2012;56:1971–1982. doi: 10.1002/hep.25801. [DOI] [PubMed] [Google Scholar]
- 6.McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foureau DM, Walling TL, Maddukuri V, Anderson W, Culbreath K, Kleiner DE, Ahrens WA, Jacobs C, Watkins PB, Fontana RJ, et al. Comparative analysis of portal hepatic infiltrating leucocytes in acute drug-induced liver injury, idiopathic autoimmune and viral hepatitis. Clin Exp Immunol. 2015;180:40–51. doi: 10.1111/cei.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGill MR, Staggs VS, Sharpe MR, Lee WM, Jaeschke H. Serum mitochondrial biomarkers and damage-associated molecular patterns are higher in acetaminophen overdose patients with poor outcome. Hepatology. 2014;60:1336–1345. doi: 10.1002/hep.27265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azhar N, Ziraldo C, Barclay D, Rudnick DA, Squires RH, Vodovotz Y. Analysis of serum inflammatory mediators identifies unique dynamic networks associated with death and spontaneous survival in pediatric acute liver failure. PLoS One. 2013;8:e78202. doi: 10.1371/journal.pone.0078202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bucuvalas J, Filipovich L, Yazigi N, Narkewicz MR, Ng V, Belle SH, Zhang S, Squires RH. Immunophenotype predicts outcome in pediatric acute liver failure. J Pediatr Gastroenterol Nutr. 2013;56:311–315. doi: 10.1097/MPG.0b013e31827a78b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llovet JM, Peña CE, Lathia CD, Shan M, Meinhardt G, Bruix J. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2012;18:2290–2300. doi: 10.1158/1078-0432.CCR-11-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasztelan-Szczerbinska B, Surdacka A, Slomka M, Rolinski J, Celinski K, Cichoz-Lach H, Madro A, Szczerbinski M. Angiogenesis-related biomarkers in patients with alcoholic liver disease: their association with liver disease complications and outcome. Mediators Inflamm. 2014;2014:673032. doi: 10.1155/2014/673032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baquerizo A, Anselmo D, Shackleton C, Chen TW, Cao C, Weaver M, Gornbein J, Geevarghese S, Nissen N, Farmer D, et al. Phosphorus ans an early predictive factor in patients with acute liver failure. Transplantation. 2003;75:2007–2014. doi: 10.1097/01.TP.0000063219.21313.32. [DOI] [PubMed] [Google Scholar]
- 14.Trujillo-Ochoa JL, Corral-Jara KF, Escobedo-Meléndez G, Realpe M, Panduro A, Roman S, Fierro NA. T-helper 17-related cytokines and IgE antibodies during hepatitis A virus infection in children. Mem Inst Oswaldo Cruz. 2015;110:263–266. doi: 10.1590/0074-02760140309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castro-García FP, Corral-Jara KF, Escobedo-Melendez G, Sandoval-Hernandez MA, Rosenstein Y, Roman S, Panduro A, Fierro NA. Conjugated bilirubin affects cytokine profiles in hepatitis A virus infection by modulating function of signal transducer and activator of transcription factors. Immunology. 2014;143:578–587. doi: 10.1111/imm.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang DW, Yin YM, Yao YM. Advances in the management of acute liver failure. World J Gastroenterol. 2013;19:7069–7077. doi: 10.3748/wjg.v19.i41.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitral CL, Souto FJ, Gaspar AM. Changing epidemiology of hepatitis A in Brazil: reassessing immunization policy. J Viral Hepat. 2008;15 Suppl 2:22–25. doi: 10.1111/j.1365-2893.2008.01024.x. [DOI] [PubMed] [Google Scholar]
- 18.Vitral CL, da Silva-Nunes M, Pinto MA, de Oliveira JM, Gaspar AM, Pereira RC, Ferreira MU. Hepatitis A and E seroprevalence and associated risk factors: a community-based cross-sectional survey in rural Amazonia. BMC Infect Dis. 2014;14:458. doi: 10.1186/1471-2334-14-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho H. Phenotypic characteristics of natural killer cells in acute hepatitis. J Microbiol. 2013;51:247–251. doi: 10.1007/s12275-013-2522-1. [DOI] [PubMed] [Google Scholar]
- 20.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 21.Björkström NK, Ljunggren HG, Sandberg JK. CD56 negative NK cells: origin, function, and role in chronic viral disease. Trends Immunol. 2010;31:401–406. doi: 10.1016/j.it.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Lugli E, Marcenaro E, Mavilio D. NK Cell Subset Redistribution during the Course of Viral Infections. Front Immunol. 2014;5:390. doi: 10.3389/fimmu.2014.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perrella A, Vitiello L, Atripaldi L, Sbreglia C, Grattacaso S, Bellopede P, Patarino T, Morelli G, Altamura S, Racioppi L, et al. Impaired function of CD4+/CD25+ T regulatory lymphocytes characterizes the self-limited hepatitis A virus infection. J Gastroenterol Hepatol. 2008;23:e105–e110. doi: 10.1111/j.1440-1746.2007.05008.x. [DOI] [PubMed] [Google Scholar]
- 24.Weidmann M, Armbruster K, Hufert FT. Challenges in designing a Taqman-based multiplex assay for the simultaneous detection of Herpes simplex virus types 1 and 2 and Varicella-zoster virus. J Clin Virol. 2008;42:326–334. doi: 10.1016/j.jcv.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Motta-Castro AR, Yoshida CF, Lemos ER, Oliveira JM, Cunha RV, Lewis-Ximenez LL, Cabello PH, Lima KM, Martins RM. Seroprevalence of Hepatitis B virus infection among an Afro-descendant community in Brazil. Mem Inst Oswaldo Cruz. 2003;98:13–17. doi: 10.1590/s0074-02762003000100002. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaspar AM, Vitral CL, Yoshida CF, Schatzmayr HG. Primary isolation of a Brazilian strain of hepatitis A virus (HAF-203) and growth in a primate cell line (FRhK-4) Braz J Med Biol Res. 1992;25:697–705. [PubMed] [Google Scholar]
- 28.Carollo M, Palazzo R, Bianco M, Smits K, Mascart F, Ausiello CM. Antigen-specific responses assessment for the evaluation of Bordetella pertussis T cell immunity in humans. Vaccine. 2012;30:1667–1674. doi: 10.1016/j.vaccine.2011.12.104. [DOI] [PubMed] [Google Scholar]
- 29.Dalgaard TS, Norup LR, Rubbenstroth D, Wattrang E, Juul-Madsen HR. Flow cytometric assessment of antigen-specific proliferation in peripheral chicken T cells by CFSE dilution. Vet Immunol Immunopathol. 2010;138:85–94. doi: 10.1016/j.vetimm.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Moore SM, Wilkerson MJ, Davis RD, Wyatt CR, Briggs DJ. Detection of cellular immunity to rabies antigens in human vaccinees. J Clin Immunol. 2006;26:533–545. doi: 10.1007/s10875-006-9044-0. [DOI] [PubMed] [Google Scholar]
- 31.Santos DC, Martinho JM, Pacheco-Moreira LF, Araújo CC, Oliveira BC, Lago BV, Pinto MA, Paula VS. Fulminant hepatitis failure in adults and children from a Public Hospital in Rio de Janeiro, Brazil. Braz J Infect Dis. 2009;13:323–329. doi: 10.1590/S1413-86702009000500002. [DOI] [PubMed] [Google Scholar]
- 32.Lima LR, De Almeida AJ, Tourinho Rdos S, Hasselmann B, Ximenez LL, De Paula VS. Evidence of hepatitis A virus person-to-person transmission in household outbreaks. PLoS One. 2014;9:e102925. doi: 10.1371/journal.pone.0102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.dos Santos DC, Neves PC, Azeredo EL, Pelajo-Machado M, Martinho JM, Pacheco-Moreira LF, Araújo CC, Cruz OG, de Oliveira JM, Pinto MA. Activated lymphocytes and high liver expression of IFN-γ are associated with fulminant hepatic failure in patients. Liver Int. 2012;32:147–157. doi: 10.1111/j.1478-3231.2011.02654.x. [DOI] [PubMed] [Google Scholar]
- 34.Sekiyama KD, Yoshiba M, Thomson AW. Circulating proinflammatory cytokines (IL-1 beta, TNF-alpha, and IL-6) and IL-1 receptor antagonist (IL-1Ra) in fulminant hepatic failure and acute hepatitis. Clin Exp Immunol. 1994;98:71–77. doi: 10.1111/j.1365-2249.1994.tb06609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steuerwald NM, Foureau DM, Norton HJ, Zhou J, Parsons JC, Chalasani N, Fontana RJ, Watkins PB, Lee WM, Reddy KR, et al. Profiles of serum cytokines in acute drug-induced liver injury and their prognostic significance. PLoS One. 2013;8:e81974. doi: 10.1371/journal.pone.0081974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang JY, Wang XL, Liu P. Detection of serum TNF-alpha,IFN-beta,IL-6 and IL-8 in patients with hepatitis B. World J Gastroenterol. 1999;5:38–40. doi: 10.3748/wjg.v5.i1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leifeld L, Cheng S, Ramakers J, Dumoulin FL, Trautwein C, Sauerbruch T, Spengler U. Imbalanced intrahepatic expression of interleukin 12, interferon gamma, and interleukin 10 in fulminant hepatitis B. Hepatology. 2002;36:1001–1008. doi: 10.1053/jhep.2002.35532. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q, Zheng Y, Huang Z, Tian Y, Zhou J, Mao Q, Wu Y, Ni B. Activated IL-23/IL-17 pathway closely correlates with increased Foxp3 expression in livers of chronic hepatitis B patients. BMC Immunol. 2011;12:25. doi: 10.1186/1471-2172-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye Y, Xie X, Yu J, Zhou L, Xie H, Jiang G, Yu X, Zhang W, Wu J, Zheng S. Involvement of Th17 and Th1 effector responses in patients with Hepatitis B. J Clin Immunol. 2010;30:546–555. doi: 10.1007/s10875-010-9416-3. [DOI] [PubMed] [Google Scholar]
- 40.Xu D, Fu J, Jin L, Zhang H, Zhou C, Zou Z, Zhao JM, Zhang B, Shi M, Ding X, et al. Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol. 2006;177:739–747. doi: 10.4049/jimmunol.177.1.739. [DOI] [PubMed] [Google Scholar]
- 41.dos Santos DC, da Silva Gomes Martinho JM, Pacheco-Moreira LF, Carvalho Viana de Araújo C, Caroli-Bottino A, Pannain VL, Soares Trinta K, Gandini M, da Costa Neves PC, de Souza Matos DC, et al. Eosinophils involved in fulminant hepatic failure are associated with high interleukin-6 expression and absence of interleukin-5 in liver and peripheral blood. Liver Int. 2009;29:544–551. doi: 10.1111/j.1478-3231.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- 42.Topuria D, Kakabadze Z, Lobdjanidze N, Chavchanidze N. Anti-hepato-cytotoxic serum treatment results in acute liver failure. Georgian Med News. 2006;(130):111–115. [PubMed] [Google Scholar]
- 43.Yamada K, Yamamoto Y, Uchiyama A, Ito R, Aoki Y, Uchida Y, Nagasawa H, Kimura H, Ichiyama T, Fukao T, et al. Successful treatment of neonatal herpes simplex-type 1 infection complicated by hemophagocytic lymphohistiocytosis and acute liver failure. Tohoku J Exp Med. 2008;214:1–5. doi: 10.1620/tjem.214.1. [DOI] [PubMed] [Google Scholar]
- 44.Tiberio GA, Tiberio L, Benetti A, Cervi E, Montani N, Dreano M, Garotta G, Cerea K, Steimberg N, Pandolfo G, et al. IL-6 Promotes compensatory liver regeneration in cirrhotic rat after partial hepatectomy. Cytokine. 2008;42:372–378. doi: 10.1016/j.cyto.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Best DH, Butz GM, Coleman WB. Cytokine-dependent activation of small hepatocyte-like progenitor cells in retrorsine-induced rat liver injury. Exp Mol Pathol. 2010;88:7–14. doi: 10.1016/j.yexmp.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Jia B, Guo M, Li G, Yu D, Zhang X, Lan K, Deng Q. Hepatitis B virus core protein sensitizes hepatocytes to tumor necrosis factor-induced apoptosis by suppression of the phosphorylation of mitogen-activated protein kinase kinase 7. J Virol. 2015;89:2041–2051. doi: 10.1128/JVI.03106-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brenndörfer ED, Weiland M, Frelin L, Derk E, Ahlén G, Jiao J, Bode JG, Sällberg M. Anti-tumor necrosis factor α treatment promotes apoptosis and prevents liver regeneration in a transgenic mouse model of chronic hepatitis C. Hepatology. 2010;52:1553–1563. doi: 10.1002/hep.23870. [DOI] [PubMed] [Google Scholar]
- 48.Akdogan M, Camci C, Gurakar A, Gilcher R, Alamian S, Wright H, Nour B, Sebastian A. The effect of total plasma exchange on fulminant hepatic failure. J Clin Apher. 2006;21:96–99. doi: 10.1002/jca.20064. [DOI] [PubMed] [Google Scholar]
- 49.Ash SR. Powdered sorbent liver dialysis and pheresis in treatment of hepatic failure. Ther Apher. 2001;5:404–416. doi: 10.1046/j.1526-0968.2001.00384.x. [DOI] [PubMed] [Google Scholar]
- 50.Laszikova E, Prazak J, Ryska O, Koblihova E, Tyll T, Ryska M. Fractionated plasmatic separation and adsorption does not alter haemodynamic parameters in experimental acute liver failure. Neuro Endocrinol Lett. 2014;35:280–284. [PubMed] [Google Scholar]
- 51.Cisneros-Garza LE, Muñoz-Ramírez Mdel R, Muñoz-Espinoza LE, Ruiz Velasco JA, Moreno-Alcántar R, Marín-López E, Méndez-Sánchez N. The molecular adsorbent recirculating system as a liver support system: summary of Mexican experience. Ann Hepatol. 2014;13:240–247. [PubMed] [Google Scholar]
- 52.Liaskou E, Zimmermann HW, Li KK, Oo YH, Suresh S, Stamataki Z, Qureshi O, Lalor PF, Shaw J, Syn WK, et al. Monocyte subsets in human liver disease show distinct phenotypic and functional characteristics. Hepatology. 2013;57:385–398. doi: 10.1002/hep.26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Antoniades CG, Quaglia A, Taams LS, Mitry RR, Hussain M, Abeles R, Possamai LA, Bruce M, McPhail M, Starling C, et al. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology. 2012;56:735–746. doi: 10.1002/hep.25657. [DOI] [PubMed] [Google Scholar]
- 54.Zimmermann HW, Trautwein C, Tacke F. Functional role of monocytes and macrophages for the inflammatory response in acute liver injury. Front Physiol. 2012;3:56. doi: 10.3389/fphys.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dunn C, Peppa D, Khanna P, Nebbia G, Jones M, Brendish N, Lascar RM, Brown D, Gilson RJ, Tedder RJ, et al. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology. 2009;137:1289–1300. doi: 10.1053/j.gastro.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 56.Liang XS, Li CZ, Zhou Y, Yin W, Liu YY, Fan WH. Changes in circulating Foxp3(+) regulatory T cells and interleukin-17-producing T helper cells during HBV-related acute-on-chronic liver failure. World J Gastroenterol. 2014;20:8558–8571. doi: 10.3748/wjg.v20.i26.8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Azeredo EL, Neves-Souza PC, Alvarenga AR, Reis SR, Torrentes-Carvalho A, Zagne SM, Nogueira RM, Oliveira-Pinto LM, Kubelka CF. Differential regulation of toll-like receptor-2, toll-like receptor-4, CD16 and human leucocyte antigen-DR on peripheral blood monocytes during mild and severe dengue fever. Immunology. 2010;130:202–216. doi: 10.1111/j.1365-2567.2009.03224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Srivastava R, Aggarwal R, Jameel S, Puri P, Gupta VK, Ramesh VS, Bhatia S, Naik S. Cellular immune responses in acute hepatitis E virus infection to the viral open reading frame 2 protein. Viral Immunol. 2007;20:56–65. doi: 10.1089/vim.2006.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Srivastava R, Aggarwal R, Sachdeva S, Alam MI, Jameel S, Naik S. Adaptive immune responses during acute uncomplicated and fulminant hepatitis E. J Gastroenterol Hepatol. 2011;26:306–311. doi: 10.1111/j.1440-1746.2010.06356.x. [DOI] [PubMed] [Google Scholar]
- 60.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 61.Wang X, Zhang L, Jiang Z. T-helper cell-mediated factors in drug-induced liver injury. J Appl Toxicol. 2015;35:695–700. doi: 10.1002/jat.3115. [DOI] [PubMed] [Google Scholar]
- 62.Masubuchi Y, Sugiyama S, Horie T. Th1/Th2 cytokine balance as a determinant of acetaminophen-induced liver injury. Chem Biol Interact. 2009;179:273–279. doi: 10.1016/j.cbi.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 63.Wang X, Jiang Z, Cao W, Yuan Z, Sun L, Zhang L. Th17/Treg imbalance in triptolide-induced liver injury. Fitoterapia. 2014;93:245–251. doi: 10.1016/j.fitote.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 64.Dong X, Gong Y, Zeng H, Hao Y, Wang X, Hou J, Wang J, Li J, Zhu Y, Liu H, et al. Imbalance between circulating CD4+ regulatory T and conventional T lymphocytes in patients with HBV-related acute-on-chronic liver failure. Liver Int. 2013;33:1517–1526. doi: 10.1111/liv.12248. [DOI] [PubMed] [Google Scholar]
- 65.Kar-Roy A, Korkaya H, Oberoi R, Lal SK, Jameel S. The hepatitis E virus open reading frame 3 protein activates ERK through binding and inhibition of the MAPK phosphatase. J Biol Chem. 2004;279:28345–28357. doi: 10.1074/jbc.M400457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ding L, Chen T, Wang XJ, Zhou L, Shi AC, Ning Q. CD69+NK cells contribute to the murine hepatitis virus strain 3-induced murine hepatitis. J Huazhong Univ Sci Technolog Med Sci. 2013;33:505–510. doi: 10.1007/s11596-013-1150-7. [DOI] [PubMed] [Google Scholar]
- 67.Bonorino P, Ramzan M, Camous X, Dufeu-Duchesne T, Thélu MA, Sturm N, Dariz A, Guillermet C, Pernollet M, Zarski JP, et al. Fine characterization of intrahepatic NK cells expressing natural killer receptors in chronic hepatitis B and C. J Hepatol. 2009;51:458–467. doi: 10.1016/j.jhep.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 68.Golden-Mason L, Castelblanco N, O’Farrelly C, Rosen HR. Phenotypic and functional changes of cytotoxic CD56pos natural T cells determine outcome of acute hepatitis C virus infection. J Virol. 2007;81:9292–9298. doi: 10.1128/JVI.00834-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meier UC, Owen RE, Taylor E, Worth A, Naoumov N, Willberg C, Tang K, Newton P, Pellegrino P, Williams I, et al. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol. 2005;79:12365–12374. doi: 10.1128/JVI.79.19.12365-12374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou Y, Callendret B, Xu D, Brasky KM, Feng Z, Hensley LL, Guedj J, Perelson AS, Lemon SM, Lanford RE, et al. Dominance of the CD4(+) T helper cell response during acute resolving hepatitis A virus infection. J Exp Med. 2012;209:1481–1492. doi: 10.1084/jem.20111906. [DOI] [PMC free article] [PubMed] [Google Scholar]


