Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
CXCR4 and α4β1/7 inhibition by AMD3100 and firategrast mobilizes fetal liver HSCs with α4β1/7 inhibition having a stronger effect.
Fetal HSC mobilization followed by IUHCT results in increased donor HSC homing to the FL and enhanced long-term allogeneic engraftment.
Abstract
In utero hematopoietic cell transplantation (IUHCT) is a novel nonmyeloablative approach that results in donor-specific tolerance and mixed allogeneic chimerism. Clinical application is limited by low levels of donor cell engraftment. Competition from endogenous hematopoietic stem cells (HSCs) for limited “space” in fetal hematopoietic organs remains a significant barrier to successful IUHCT. AMD3100, a CXCR4 inhibitor, and firategrast, an α4β1 and α4β7 integrin inhibitor (α4β1/7), have been shown to disrupt HSC retention in the postnatal hematopoietic niche. We hypothesized that maternal administration of AMD3100 and/or firategrast prior to IUHCT would mobilize endogenous HSCs from the fetal liver (FL) and result in preferential FL homing of donor HSCs and enhanced long-term engraftment following IUHCT in an allogeneic mouse model. We demonstrate that (1) both agents cross the placenta with rapidly detectable fetal serum concentrations following maternal administration; (2) firategrast treatment alone or with AMD3100 mobilizes endogenous HSCs from the FL and results in increased FL homing of donor HSCs following IUHCT; and (3) enhanced donor HSC homing following firategrast treatment translates into increased long-term multilineage donor cell engraftment. This approach highlights the potential of mobilization strategies to overcome barriers to successful engraftment and increase the clinical promise of IUHCT.
Introduction
In utero hematopoietic cell transplantation (IUHCT) is a nonmyeloablative nonimmunosuppressive transplant approach that results in donor cell engraftment across immune barriers and donor-specific tolerance secondary to fetal immunologic immaturity.1,2 Despite the potential to treat many congenital hematologic diseases, engraftment has been subtherapeutic for most diseases or below levels necessary to reliably induce tolerance.3 A barrier to enhanced engraftment is competition with endogenous hematopoietic stem cells (HSCs) for limited fetal hematopoietic niches (HNs) as supported by increased engraftment following IUHCT when a competitive advantage exists for donor cells.2-5 The lack of a fetal immune barrier allows for nontoxic novel conditioning regimens to provide a donor cell competitive advantage and increase alloengraftment.
The CXCR4|SDF-1α and α4β1|VCAM-1 pathways are critical to HSC engraftment in postnatal bone marrow (BM) HNs. Studies also implicate these pathways in endogenous fetal HSC engraftment of the fetal liver (FL), the hematopoietic organ at the time of IUHCT.6-11 Postnatal blockade of these pathways mobilizes HSCs from BM in mice, nonhuman primates, and humans.12-16 Evidence also suggests that the α4β7|MAdCAM-1 pathway contributes to HSC recruitment into the BM after postnatal transplantation.17 In the current study, we present data on mobilization of endogenous FL HSCs by inhibiting CXCR4 and α4β1/7 independently, or in combination, with small molecule inhibitors AMD3100 and firategrast. Furthermore, we evaluate FL HSC mobilization before IUHCT as a novel strategy to create “space” in the FL and enhance allo-engraftment.
Study design
Mice
Balb/c (H2d) mice were purchased from Jackson Laboratories. C57Bl/6TgN(act-EGFP)OsbY01 (H2b, green fluorescent protein [GFP]+) (called B6GFP) mice were provided by Dr Okabe (Osaka University, Osaka, Japan). The Institutional Animal Care and Use Committee at The Children’s Hospital of Philadelphia approved the experimental protocols.
Mobilizing agent pharmacokinetics
AMD3100 (Abcam; 5 mg/kg, subcutaneous) or firategrast (synthesized in the DiPersio laboratory [published structure: C27H27F2NO6; patent #US2014051655]; 100 mg/kg, intravenous) were dissolved in Ca+2/Mg+2-free phosphate-buffered saline (AMD3100) plus 1% ethanol (firategrast) and maternally administered to gestational day (E)17 Balb/c dams. Maternal and fetal sera were analyzed for mobilizing agent concentrations by mass spectrometry using the Agilent system (6410 triple quad mass spectrometer and 1260 liquid chromatography).
Endogenous fetal HSC mobilization
AMD3100 and/or firategrast were administered to E14 Balb/c dams. FLs were harvested 60 minutes after administration, and low-density mononuclear cells (LDMCs) isolated over a Ficoll gradient were analyzed by flow cytometry (FACSAria; antibodies used are shown in supplemental Table 1, available on the Blood Web site) for absolute number of Lineage−Sca-1+c-Kit+ cells.
IUHCT
BM LDMCs (10 × 106) from 6- to 8-week-old B6GFP mice were injected into E14 Balb/c fetuses (2.9 × 1010 cells/kg) via vitelline vein as previously described.2 Sixty minutes before IUHCT, dams were administered AMD3100 (subcutaneous), firategrast (intravenous), AMD3100+firategrast, or vehicle.
Donor cell homing and postnatal analysis
FL and peripheral blood LDMCs were analyzed by flow cytometry for donor HSC homing, total donor chimerism, and multilineage engraftment. Mice were weighed and evaluated for graft-versus-host-disease (GVHD) monthly.
Statistics
Data are presented as means ± standard error of the mean. Statistical comparisons were performed with the Student t test for 2 samples assuming unequal variances or analysis of variance with Bonferroni multiple comparisons test using GraphPad Prism 6.0. P ≤ .05 was considered significant.
Results and discussion
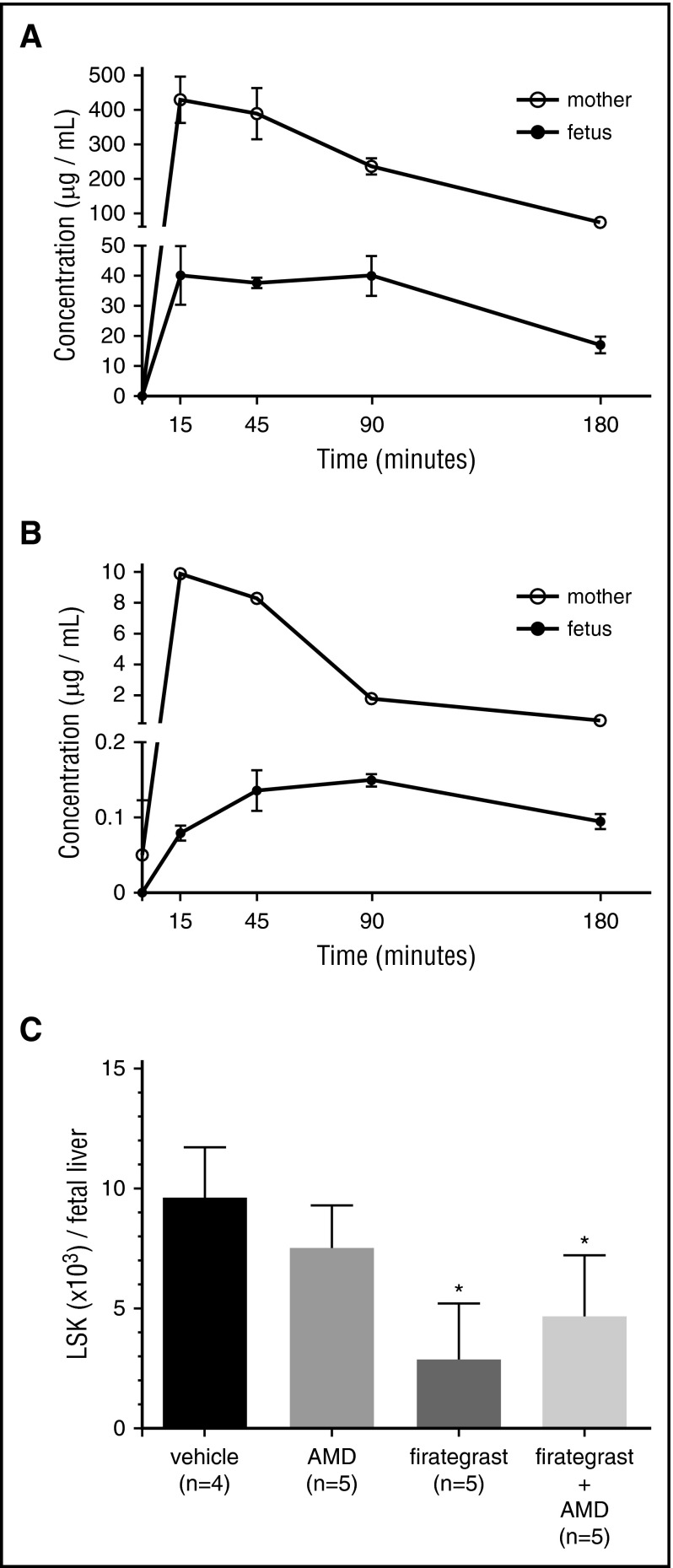
Small-molecule antagonists of CXCR4 and α4β1 efficiently mobilize HSCs from adult BM HNs.15,16 As an initial step to evaluate whether small molecule inhibitors could mobilize fetal HSCs before IUHCT, we confirmed expression of CXCR4, α4β1/7, and their ligands on FL HSCs and FL (supplemental Figures 1 and 2) and assessed transplacental passage of AMD3100, a CXCR4 antagonist, and firategrast,18 an α4β1/7 integrin antagonist, after maternal administration. Applying a dosing protocol that mobilizes adult BM HSCs16 and results in transient mobilization of maternal lymphocytes and myeloid cells (supplemental Figures 3 and 4), we demonstrate that both agents reach peak maternal serum concentrations at 15 minutes and rapidly cross the placenta with peak fetal serum concentrations at 15 to 90 (firategrast) and 45 to 90 (AMD3100) minutes after administration (Figure 1A-B). The higher and more rapid peak fetal concentration of firategrast is likely related to the higher dose, intravenous administration, and more efficient transplancental passage (fold decrease fetal vs adult peak concentration: AMD3100 = 66-fold; firategrast = 11-fold).
Figure 1.
Pharmacokinetics of transplacental passage of AMD3100 and firategrast and fetal HSC mobilization. (A) Firategrast (100 mg/kg, intravenous) or (B) AMD3100 (5 mg/kg, subcutaneous) was maternally administered to E17 Balb/c dams, and the concentration of the mobilizing agent in ∼100 μL maternal (n = 3 per time point) and fetal (n = 4-5 per time point) serum was determined at 15, 45, 90, and 180 minutes after treatment by mass spectrometry by spiking the serum with either deuterated-AMD3100 (AMD3100 analysis) or reserpine (firategrast analysis). To obtain an adequate volume of fetal serum for analysis, pharmacokinetics was performed at E17, and each fetal sample consisted of blood pooled from 4 to 5 fetuses obtained by fetal decapitation. (C) Mobilization of endogenous HSCs from the FL was determined after administering AMD3100 and/or firategrast to E14 Balb/c dams. FLs were harvested 60 minutes after treatment, and the absolute number of lineage− (CD3−B220−Gr1−Ter119−) Sca-1+c-Kit+ cells per FL was determined based on the average total number of LDMCs per FL of 3.5 × 105. *P < .05 vs vehicle.
We next evaluated whether maternal administration of AMD3100 and/or firategrast mobilized FL HSCs (Figure 1C). FLs from mice treated with firategrast and firategrast+AMD3100 demonstrated a significant reduction in HSCs (3.4-fold and 2.1-fold, respectively, reduction vs controls) supporting efficient HSC mobilization. There was a trend toward increased mobilization following AMD3100 alone. In contrast to postnatal studies, which demonstrate increased mobilization following combined treatment,13,16 we found no significant additive effect of combined therapy over firategrast alone. These findings may suggest a more important role of α4β1 in HSC retention in fetal HNs as supported by the high percentage of fetal HSCs expressing α4β1 (supplemental Figure 1). Inhibition of α4β1 may maximize HSC mobilization, and no significant increase can be attained by the addition of AMD3100. Alternatively, enhanced mobilization with firategrast may be related to its higher fetal serum concentrations. We elected not to increase the AMD3100 dose due to increased lethality with higher doses.16
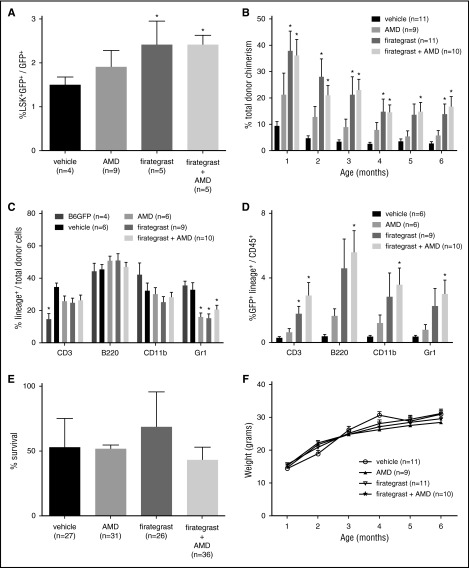
We have shown that augmenting the CXCR4|SDF-1α pathway on donor cells before IUHCT enhances engraftment.2 Alternatively, treating donor cells with anti-α4β1 antibodies before IUHCT inhibited engraftment.19 These studies suggest that manipulation of these pathways can affect donor cell engraftment following IUHCT. In the current study, we evaluated whether mobilization of endogenous FL HSCs with pathway inhibitors could create space before IUHCT and increase donor cell homing and long-term engraftment. AMD3100, firategrast, and firategrast+AMD3100 treatment increased the percentage of donor HSCs in FL 24 hours after IUHCT (Figure 2A). Similar to mobilization studies, this increase was significant in recipients of firategrast and firategrast+AMD3100, suggesting that mobilization allowed for enhanced short-term HSC engraftment. Enhanced short-term engraftment translated into significantly increased long-term engraftment in recipients of firategrast without an additive effect of firategrast+AMD3100 over firategrast alone (Figure 2B). Donor engraftment was stable and multilineage at 6 months following all treatments supporting engraftment at the HSC level (Figure 2B-D).
Figure 2.
Enhanced donor cell homing and long-term engraftment following fetal HSC mobilization and IUHCT without detrimental effects on fetal recipients. B6GFP BM cells (10 × 106) were injected into E14 Balb/c fetuses via the vitelline vein 60 minutes after maternal administration of vehicle, AMD3100, firategrast, or firategrast+AMD3100. (A) The percentage of donor lineage- (CD3−B220−Gr1−CD11b−) Sca-1+c-Kit+ cells out of total donor CD45+ cells in the FL 24 hours after IUHCT was assessed by flow cytometry. (B) The peripheral blood (PB) of recipients of IUHCT ± mobilizing agent was assessed monthly from 1 to 6 months of life for total donor chimerism defined as the %CD45+ cells that were GFP+. (C) At 6 to 9 months of age, the PB was assessed for multilineage engraftment by determining the percentage of donor cells that express CD3 (T cells), B220 (B cells), CD11b (macrophages/monocytes), and Gr1 (granulocytes). Of note, surface markers, including CD11b and Gr1, are expressed by other cell lineages in addition to macrophages and granulocytes, thus explaining the total percentage of lineage cells >100%. (D) Analysis of the percentage of the CD45+ cells that are GFP+Lineage+ suggests that increased engraftment after treatment with firategrast or firategrast+AMD3100 results from an increase in donor cells of all lineages. (★P < .05 vs vehicle for A-E; see supplemental Figure 5 for lineage flow cytometry plots). (E) Survival to birth was assessed in recipients of IUHCT ± mobilizing agent, and no significant difference was noted between groups (P = .6; values represent the mean survival of 4-5 injected litters per group, n represents total number of fetuses injected per group). (F) Recipients of IUHCT ± mobilizing agent were weighed monthly from 1 to 6 months of life, and no significant difference was noted between treatment groups and the control group (P = .52). At the time of weighing, recipients were also evaluated and demonstrated no evidence of GVHD including ruffled fur, mucositis, fur loss, and hunched posture (data not shown).
Although human FL hematopoiesis transitions to fetal BM more quickly, we believe the current strategy remains an option to enhance engraftment following clinical IUHCT. The FL is the primary hematopoietic organ at the time of clinical IUHCT. Increased FL engraftment would allow for a fractional increase in donor HSCs that can migrate to the BM as supported by enhanced 6-month engraftment in our study. Furthermore, HSC mobilization from postnatal BM with CXCR4 and α4β1 blockade and their expression on fetal HSCs suggests our strategy may mobilize fetal BM HSCs if hematopoiesis has transitioned to BM at the time of IUHCT.16,20-22
Mobilizing agents did not affect survival and mice demonstrated appropriate weight gain (Figure 2E-F) without GVHD. Although encouraging, potential adverse effects of mobilizing agents on fetal development must be considered. AMD3100 has reported teratogenicity.23 SDF-1α and CXCR4 expression have been localized to neuronal, cardiac, vascular, hematopoietic, and craniofacial organogenesis.24 Studies in VCAM-1– or α4-deficient mice support their role in placental and cardiac development.25,26 Thus, large animal studies are required to investigate side effects of CXCR4 and α4β1/7 inhibitors in fetuses.
The current study supports an important function of the CXCR4|SDF-1α and α4β1|VCAM-1 pathways in homing and retention of endogenous fetal HSCs in the FL as indicated by mobilization of FL HSCs by inhibiting these pathways. Furthermore, it provides proof-in-principle that this strategy of creating space through HSC mobilization is feasible and enhances allogeneic engraftment by increasing donor HSC homing to the FL following IUHCT.
Acknowledgments
The authors thank the Metabolomics Core at Children’s Hospital of Philadelphia for guidance and assistance in performing mass spectrometry.
This work was supported by grants from The American College of Surgeons (W.H.P.), generous family gifts to the Center for Fetal Research at the Children’s Hospital of Philadelphia, and National Institutes of Health, National Cancer Institute grants R01 CA152329 and U54 CA199092-01 (principal investigator for both: J. F. DiPersio).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.G.K. designed the research, performed the research, analyzed the data, and wrote the paper; J.D.V., M.M.B., L.E., M.P.R., J.S.R., M.S.H., M.A.C., S.P.L., and H.L. performed the research, analyzed the data, and provided vital technical skills; J.F.D. and A.W.F. designed the research, contributed vital analytical skills, and wrote the paper; W.H.P. designed and performed the research, provided vital analytical skills, and wrote the paper; and all authors discussed the results and commented on the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William H. Peranteau, Department of Surgery, Children’s Hospital of Philadelphia, Abramson Research Building, Room 1116E, 3615 Civic Center Blvd, Philadelphia, PA 19104-4318; e-mail: peranteauw@email.chop.edu.
References
- 1.Nijagal A, Derderian C, Le T, et al. Direct and indirect antigen presentation lead to deletion of donor-specific T cells after in utero hematopoietic cell transplantation in mice. Blood. 2013;121(22):4595-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peranteau WH, Endo M, Adibe OO, Merchant A, Zoltick PW, Flake AW. CD26 inhibition enhances allogeneic donor-cell homing and engraftment after in utero hematopoietic-cell transplantation. Blood. 2006;108(13):4268-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flake AW, Zanjani ED. In utero hematopoietic stem cell transplantation: ontogenic opportunities and biologic barriers. Blood. 1999;94(7):2179-2191. [PubMed] [Google Scholar]
- 4.Blazar BR, Taylor PA, McElmurry R, et al. Engraftment of severe combined immune deficient mice receiving allogeneic bone marrow via In utero or postnatal transfer. Blood. 1998;92(10):3949-3959. [PubMed] [Google Scholar]
- 5.Flake AW, Roncarolo MG, Puck JM, et al. Treatment of X-linked severe combined immunodeficiency by in utero transplantation of paternal bone marrow. N Engl J Med. 1996;335(24):1806-1810. [DOI] [PubMed] [Google Scholar]
- 6.Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382(6592):635-638. [DOI] [PubMed] [Google Scholar]
- 7.Nagasawa T, Tachibana K, Kishimoto T. A novel CXC chemokine PBSF/SDF-1 and its receptor CXCR4: their functions in development, hematopoiesis and HIV infection. Semin Immunol. 1998;10(3):179-185. [DOI] [PubMed] [Google Scholar]
- 8.Ara T, Tokoyoda K, Sugiyama T, Egawa T, Kawabata K, Nagasawa T. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity. 2003;19(2):257-267. [DOI] [PubMed] [Google Scholar]
- 9.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393(6685):595-599. [DOI] [PubMed] [Google Scholar]
- 10.Hamamura K, Matsuda H, Takeuchi Y, Habu S, Yagita H, Okumura K. A critical role of VLA-4 in erythropoiesis in vivo. Blood. 1996;87(6):2513-2517. [PubMed] [Google Scholar]
- 11.Mazo IB, Gutierrez-Ramos JC, Frenette PS, Hynes RO, Wagner DD, von Andrian UH. Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J Exp Med. 1998;188(3):465-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craddock CF, Nakamoto B, Andrews RG, Priestley GV, Papayannopoulou T. Antibodies to VLA4 integrin mobilize long-term repopulating cells and augment cytokine-induced mobilization in primates and mice. Blood. 1997;90(12):4779-4788. [PubMed] [Google Scholar]
- 13.Bonig H, Watts KL, Chang KH, Kiem HP, Papayannopoulou T. Concurrent blockade of alpha4-integrin and CXCR4 in hematopoietic stem/progenitor cell mobilization. Stem Cells. 2009;27(4):836-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rettig MP, Ansstas G, DiPersio JF. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia. 2012;26(1):34-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201(8):1307-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramirez P, Rettig MP, Uy GL, et al. BIO5192, a small molecule inhibitor of VLA-4, mobilizes hematopoietic stem and progenitor cells. Blood. 2009;114(7):1340-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katayama Y, Hidalgo A, Peired A, Frenette PS. Integrin alpha4beta7 and its counterreceptor MAdCAM-1 contribute to hematopoietic progenitor recruitment into bone marrow following transplantation. Blood. 2004;104(7):2020-2026. [DOI] [PubMed] [Google Scholar]
- 18.Miller DH, Weber T, Grove R, et al. Firategrast for relapsing remitting multiple sclerosis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2012;11(2):131-139. [DOI] [PubMed] [Google Scholar]
- 19.Zanjani ED, Flake AW, Almeida-Porada G, Tran N, Papayannopoulou T. Homing of human cells in the fetal sheep model: modulation by antibodies activating or inhibiting very late activation antigen-4-dependent function. Blood. 1999;94(7):2515-2522. [PubMed] [Google Scholar]
- 20.Aiuti A, Tavian M, Cipponi A, et al. Expression of CXCR4, the receptor for stromal cell-derived factor-1 on fetal and adult human lympho-hematopoietic progenitors. Eur J Immunol. 1999;29(6):1823-1831. [DOI] [PubMed] [Google Scholar]
- 21.Cao B, Zhang Z, Grassinger J, et al. Therapeutic targeting and rapid mobilization of endosteal HSC using a small molecule integrin antagonist. Nat Commun. 2016;7:11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy V, Verfaillie CM. Expression and function of cell adhesion molecules on fetal liver, cord blood and bone marrow hematopoietic progenitors: implications for anatomical localization and developmental stage specific regulation of hematopoiesis. Exp Hematol. 1999;27(2):302-312. [DOI] [PubMed] [Google Scholar]
- 23.Mozobil (plerixafor) [package insert]. Cambridge, MA: Genzyme Corporation; 2015.
- 24.McGrath KE, Koniski AD, Maltby KM, McGann JK, Palis J. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol. 1999;213(2):442-456. [DOI] [PubMed] [Google Scholar]
- 25.Kwee L, Baldwin HS, Shen HM, et al. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development. 1995;121(2):489-503. [DOI] [PubMed] [Google Scholar]
- 26.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121(2):549-560. [DOI] [PubMed] [Google Scholar]