Abstract
In this issue of Blood, Marcos-Contreras and colleagues present convincing evidence that mechanistically links hyperfibrinolysis, typically seen as a bleeding risk, with increased brain endothelial permeability through plasmin-mediated cleavage of high-molecular-weight kininogen (HMWK) to bradykinin (BK). This study establishes plasmin as a key effector of what might be termed “hemovascular dysfunction”: a pathological state of blood enzymatic activity resulting in vascular structural and functional disruption. Their findings point the way toward improved treatment of patients with pharmacologically (stroke and myocardial infarction) or pathologically activated fibrinolysis (trauma and surgery) through selective blockade of bradykinin activity. Combined blockade of bradykinin and plasmin activation may provide additional therapeutic benefits in hemorrhagic shock by reducing tissue edema in resuscitation while enhancing hemostasis.1
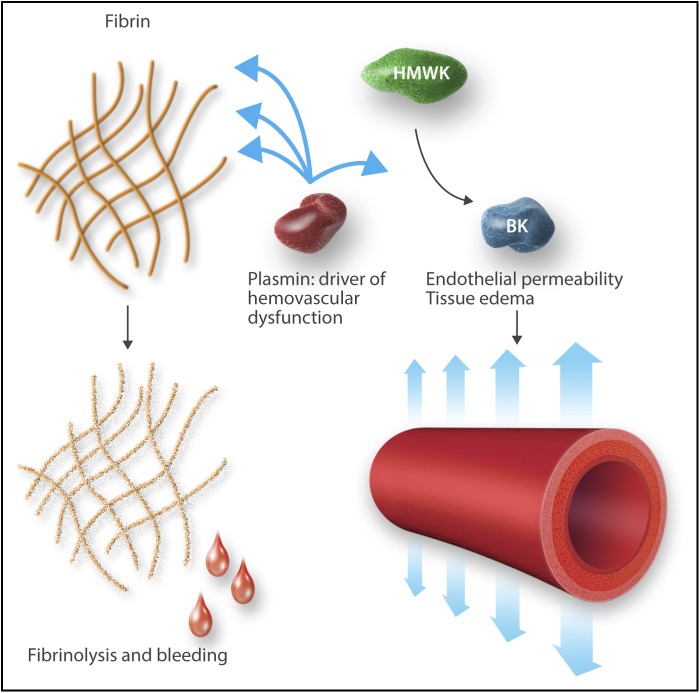
Plasmin as a driver of hemovascular dysfunction. Professional illustration by Somersault18:24.
Activation of plasmin for therapeutic thrombolysis is well established in the management of myocardial infarction and stroke. However, the systemic and intracranial bleeding risks of this therapy are well known, often limiting its use. Many investigators have suggested that an increase in blood–brain barrier permeability observed in association with tissue plasminogen activator (tPA) administration may underlie the bleeding risk, brain edema, and resultant neurological deterioration, through a number of different mechanisms. Curiously, oral and lingual angioedema has been observed in 8% of patients receiving tPA with the greatest frequency in those taking angiotensin-converting enzyme (ACE) inhibitors.2
HMWK has been suggested as the linker molecule between the coagulation and immune systems, and its cleavage product, bradykinin, the primary effector (see figure). Bradykinin, highly conserved in human evolution, produces vasodilation and increased vascular permeability, fever, and hypotension through stimulation of endothelial nitric oxide and prostaglandin formation, in addition to effects on regulation of neutrophil trafficking and nociception.3,4 Bradykinin drives the “calor, rubor, dolor, tumor” and ultimately “functio laesa” of inflammation. Bradykinin production has been thought to be primarily linked to coagulation through the activity of factor XII (FXII). FXIIa activates prekallikrein to kallikrein, which, in turn, cleaves HMWK to release bradykinin. The role of plasmin in bradykinin formation has remained unclear, and its role as an activator of inflammation has been called into question.5 However, plasmin, in addition to degrading fibrin, is known to cleave, transiently activate, and ultimately inactivate FV and FVIII, while also activating FXII.6,7 Thus, it has been proposed that plasmin could generate bradykinin by activating FXII.8 The current study shows that plasmin activity, independent of FXII activity, can drive bradykinin formation and thus directly activate inflammation and the innate immune system.
Through a combination of elegant in vitro mouse model and clinical studies, Marcos-Contreras and colleagues bring clarity to this confusing picture by demonstrating that plasmin activation by tPA directly generates bradykinin, which increases blood–brain barrier permeability. Blockade of bradykinin B2 receptors with icatibant protects brain vascular integrity and neurologic function in mice. The investigators systematically eliminated contributions from other proposed mediators, including N-methyl-d-aspartate receptor, low-density lipoprotein related protein, protease-activated receptor-1, matrix-metalloproteinases, neuronal cell death, or other inflammatory pathways. The central role of plasmin-generated bradykinin in blood–brain barrier disruption also illuminates the association of tPA with angioedema and the increased risk faced by patients on ACE inhibitors: bradykinin drives loss of endothelial integrity in hereditary angioedema and ACE is a primary clearance pathway for bradykinin. Tranexamic acid, a lysine analog inhibitor of plasmin activation best known for its role in treating and preventing hemorrhage, has been used to treat angioedema since the 1970s. We have thus closed an important gap in our understanding of how the fibrinolytic system, through bradykinin, drives changes in vascular structure and end-organ function.
The current study leaves unresolved several questions needing further exploration. Probably due to limitations of their animal model, the investigators did not observe increased vascular permeability outside of the brain, not even in the spinal cord. It is likely that multiple effectors and tissue-specific factors can drive changes in vascular permeability. A better understanding of associated phenomena such as shedding of the endothelial glycocalyx in shock and inflammatory states will improve our appreciation of the plasmin-bradykinin axis in hemovascular dysfunction. A recent clinical report links cerebrospinal fluid bradykinin levels to increased brain edema and intracranial pressure in patients with traumatic brain injury, corroborating the results in the current paper.9
In addition to further basic mechanistic studies, the work of Marcos-Contreras and coworkers suggests application of plasmin or bradykinin blockade, or the combination, in treatment of clinical scenarios for which current management is inadequate. Trauma care, for example, has been advanced by the CRASH-2 trial in which tranexamic acid reduced the all-cause relative risk of mortality by 9%—an unprecedented result for a pharmaceutical intervention in this patient population.10 Nevertheless, skepticism regarding tranexamic acid’s utility remains because the trial did not demonstrate significant differences in blood transfusion between study arms. Although blood transfusion may not be the optimal measure of bleeding intensity, this observation has prompted calls to study potential alternative beneficial mechanisms of tranexamic acid in trauma. The present study suggests a promising line of inquiry and an opportunity for studying tranexamic acid and icatibant together in trauma, particularly traumatic brain injury, and other indications in which plasmin activation is associated with pathology.
The opinions or assertions expressed herein are the private views of the author and are not to be construed as official or as reflecting the views of the US Department of the Army or the US Department of Defense.
Footnotes
Conflict-of-interest disclosure: The author declares no competing financial interests.
REFERENCES
- 1.Marcos-Contreras OA, Martinez de Lizarrondo S, Bardou I, et al. Hyperfibrinolysis increases blood–brain barrier permeability by a plasmin- and bradykinin-dependent mechanism. Blood. 2016;128(20):2423-2434. [DOI] [PubMed] [Google Scholar]
- 2.Hurford R, Rezvani S, Kreimei M, et al. Incidence, predictors and clinical characteristics of orolingual angio-oedema complicating thrombolysis with tissue plasminogen activator for ischaemic stroke. J Neurol Neurosurg Psychiatry. 2015;86(5):520-523. [DOI] [PubMed] [Google Scholar]
- 3.Cagliani R, Forni D, Riva S, et al. Evolutionary analysis of the contact system indicates that kininogen evolved adaptively in mammals and in human populations. Mol Biol Evol. 2013;30(6):1397-1408. [DOI] [PubMed] [Google Scholar]
- 4.Hofman Z, de Maat S, Hack CE, Maas C. Bradykinin: inflammatory product of the coagulation system. Clin Rev Allergy Immunol. 2016;51(2):152-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renckens R, Weijer S, de Vos AF, et al. Inhibition of plasmin activity by tranexamic acid does not influence inflammatory pathways during human endotoxemia. Arterioscler Thromb Vasc Biol. 2004;24(3):483-488. [DOI] [PubMed] [Google Scholar]
- 6.Tracy RP, Rubin DZ, Mann KG, et al. Thrombolytic therapy and proteolysis of factor V. J Am Coll Cardiol. 1997;30(3):716-724. [DOI] [PubMed] [Google Scholar]
- 7.Nogami K, Shima M, Matsumoto T, Nishiya K, Tanaka I, Yoshioka A. Mechanisms of plasmin-catalyzed inactivation of factor VIII: a crucial role for proteolytic cleavage at Arg336 responsible for plasmin-catalyzed factor VIII inactivation. J Biol Chem. 2007;282(8):5287-5295. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan AP, Ghebrehiwet B. The plasma bradykinin-forming pathways and its interrelationships with complement. Mol Immunol. 2010;47(13):2161-2169. [DOI] [PubMed] [Google Scholar]
- 9.Kunz M, Nussberger J, Holtmannspötter M, Bitterling H, Plesnila N, Zausinger S. Bradykinin in blood and cerebrospinal fluid after acute cerebral lesions: correlations with cerebral edema and intracranial pressure. J Neurotrauma. 2013;30(19):1638-1644. [DOI] [PubMed] [Google Scholar]
- 10.Collaborators, C.-t., et al., Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23-32. [DOI] [PubMed]