Abstract
To determine what alternative pathways may act as mechanisms of bypass resistance to type 1 insulin-like growth factor receptor (IGF-1R) blockade in rhabdomyosarcoma (RMS), we compared expression of receptor tyrosine kinase activity in a number of IGF-1R antibody-resistant and -sensitive RMS cell lines. We found that platelet-derived growth factor receptor β (PDGFR-β) activity was upregulated in three xenograft-derived IGF-1R antibody-resistant cell lines that arose from a highly sensitive fusion-positive RMS cell line (Rh41). Furthermore, we identified four additional fusion-negative RMS cell lines that similarly upregulated PDGFR-β activity when selected for IGF-1R antibody resistance in vitro. In the seven cell lines described, we observed enhanced growth inhibition when cells were treated with dual IGF-1R and PDGFR-β inhibition in vitro. In vivo studies have confirmed the enhanced effect of targeting IGF-1R and PDGFR-β in several mouse xenograft models of fusion-negative RMS. These findings suggest that PDGFR-β acts as a bypass resistance pathway to IGF-1R inhibition in a subset of RMS. Therapy co-targeting these receptors may be a promising new strategy in RMS care.
Introduction
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma of childhood. The annual incidence in the United States is four to seven cases per million children under 15 years, which represent 250 new cases per year [1]. Two major histologic subtypes exist: embryonal or fusion negative and alveolar or fusion positive, the latter of which carries a particularly poor prognosis. Patients with metastatic and recurrent disease are essentially incurable with a 5-year overall survival of less than 20%, and outcomes have only minimally improved over the past several decades [2]. Thus, new therapies for RMS are critically needed.
The insulin-like growth factor (IGF) system plays an important role in the biology of many cancers. Overexpression of both the type 1 IGF receptor (IGF-1R) and its ligands has been observed in multiple malignancies, including pediatric sarcomas, and abnormal activation of this pathway contributes to sarcoma development and progression [3], [4], [5]. Downstream signaling cascades of IGF-1R further regulate tumor cell proliferation, survival, and metastasis through the MAPK/ERK and PI3K/mTOR pathways [6]. In samples from human RMS tumors, IGF-1R has been found to be highly expressed in about 60% of tumors [7]. Thus, inhibition of IGF-1R is a potentially important therapeutic target in RMS.
Monoclonal antibodies targeting IGF-1R interfere with ligand binding and decrease the expression of the receptor on cell surfaces by internalization and degradation of the receptor [8], [9], [10], [11]. A number of these antibodies have been tested in the clinical setting. Results from early-phase clinical trials using monotherapy with R1507, a monoclonal antibody against IGF-1R, indicated clinically meaningful responses in about 10% to 15% of patients with RMS. However, the vast majority of these responses were short-lived with a rapid onset of resistance [12].
We previously reported data from mouse xenograft models of RMS, which revealed a phenomenon similar to that seen in the adult clinical trials. Mice treated with h7C10, another monoclonal antibody against IGF-1R, showed a progression-free period of about 9 weeks compared with 3 weeks in control animals. Evaluation of the tumor samples from treated mice after regrowth showed persistent downregulation of IGF-1R but a rebound in AKT phosphorylation. This suggests that the resistance was not due to loss of activity of the antibody against IGF-1R but rather the result of a bypass resistance pathway [7].
By investigating the potential molecular bypass mechanisms that enable this type of acquired resistance, our goal is to shift the focus from targeting single pathways to targeting larger networks in an effort to overcome treatment failures. We recently published preclinical work showing that in both embryonal and alveolar RMS models, blockade of IGF-1R results in activation of YES, an Src family kinase member, and that YES activation is associated with resistance to IGF-1R blockade. In addition, combination treatment blocking both IGF-1R and YES resulted in enhanced growth inhibition of both embryonal and alveolar RMS in vitro and in vivo [13]. However, given the heterogeneous nature of these tumors, it is likely that multiple mechanisms of resistance to IGF-1R blockade exist. Hence, we sought to identify other specific molecular mechanisms mediating the bypass of IGF-1R blockade.
Material and Methods
Generation of IGF-1R Antibody-Resistant Cell Lines
Cell Lines
Alveolar (fusion-positive) RMS cell lines Rh30 and Rh41 and embryonal (fusion-negative) cell line RD have been previously described [14]. Alveolar (fusion-positive) cell lines Rh5, JR, and Rh28 and embryonal (fusion-negative) cell lines TTC-442 and TTC-516 were obtained from Dr. Javed Khan (NCI, Bethesda, MD). Embryonal (fusion-negative) cell line Rh18C was obtained from Dr. Frederick Barr (NCI, Bethesda, MD). Embryonal (fusion-negative) cell line Rh36 was obtained from Dr. Maria Tsokos (Beth Israel Medical Center, Boston, MA).
Compounds
R1507 antibody was obtained from Hoffman-La Roche Inc. (Nutley, NJ).
In Vitro Resistance
Cells were maintained in RPMI growth medium (Life Technologies, Grand Island, NY) with 10% FBS, heat-inactivated (Sigma-Aldrich, St. Louis, MO), 100 U/ml of penicillin and 100 μg/ml of streptomycin (Life Technologies), and 2 mM L-glutamine (Life Technologies) at 37°C in an atmosphere of 5% CO2. For each cell line, two flasks were maintained: one with media alone (termed parental) and one with media plus R1507 at 100 nM (termed resistant). Resistant cells were maintained in R1507 media with the drug replenished with each media change for at least 6 weeks before experiments commenced.
In Vivo Resistance
Animal studies were performed in accordance with the guidelines of the National Institutes of Health Animal Care and Use Committee. Four- to 6-week-old female Fox Chase severe combined immunodeficiency (SCID)-Beige mice were purchased from Charles River Laboratories (Wilmington, MA). Two million Rh41 cells were suspended in a solution of Hank’s balanced salt solution and Geltrex LDEV-free reduced growth factor basement membrane matrix (Matrigel) (Life Technologies) mixed at a 1:1 ratio and injected orthotopically into the gastrocnemius muscle in the left hind leg of each mouse. When tumors were palpable, mice began treatment with intraperitoneal (IP) R1507 at 6 mg/kg weekly. Tumors regressed and eventually regrew while mice continuously received R1507 treatments. When tumors reached 1500 to 2000 mm3, they were harvested under sterile conditions and aspirated or cut into small sections. Tumor aspirates and sections were incubated in cell culture flasks in complete RPMI until cell lines were established. Cell lines from individual mice were kept and named separately. Resistance to R1507 was confirmed in vitro with exposure to R1507 at 100 nM and in vivo with reinjection into mice and subsequent treatment with R1507 at 6 mg/kg weekly.
Identification of PDGFR-β Activation
PDGFR-β activation was identified and tested using the following methods: receptor tyrosine kinase (RTK) array, Western blotting, and MesoScale analysis.
RTK Array
Cell lines were prepared, lysed, and compared according to the protocol from the Proteome Profiler Human Phospho-RTK Array Kit (Bio-Techne, Minneapolis, MN).
Lysate Preparation and Ligand Stimulation for Western Blot and MesoScale Analysis
Cells were plated in RPMI growth medium for 24 hours. After 24 hours, medium was removed, and each plate was treated with ligand stimulation with 25 ng/ml of PDGF-BB (Bio-Techne) or vehicle in RPMI with glutamine only. Plates were incubated for 10 minutes at 37°C and then immediately placed on ice for harvest. Cells were washed twice with ice-cold PBS (Life Technologies) and then lysed with LDS lysis buffer (Sigma Aldrich) with phosphatase and protease inhibitors (Life Technologies).
Western Blot Analysis
Protein lysates (30-60 μg/lane), as determined by BCA protein assay (Life Technologies), were separated in 4% to 12% SDS-PAGE (Life Technologies) and transferred to nitrocellulose membranes (Amersham Pharmacia Biotech, Piscataway, NJ). Membranes were blocked with 5% nonfat dried milk in TBS (KPL, Gaithersburg, MD)-Tween 20 (Sigma Aldrich) (20 mm Tris-HCI, pH 7.5; 8 g/l of sodium chloride; 0.1% Tween 20). Blots were incubated with antibodies against total PDGFR-β and phospho-PDGFR-β (tyr751) (Cell Signaling Technology, Beverly, MA) at a 1:1000 dilution. Antiactin antibody from Abcam Inc. (Cambridge, MA) was used as a loading control. Bands were visualized on camera using West Femto ECL detection reagent (Life Technologies).
MesoScale Analysis
Protein lysates were quantified by BCA protein assay (Life Technologies) and added to MesoScale Discovery Multi-spot 4 Spot 96-well plates (Rockville, MD). Samples were analyzed in triplicate using the MesoScale Discovery Sector Imager 2400 plate reader.
In Vitro Proliferation Studies
Cell proliferation kinetics were monitored and recorded with the IncuCyte System (Essen BioScience, Ann Arbor, MI). Imatinib, pazopanib, and crenolanib were all obtained from Selleck (Houston, TX). For all in vitro experimentations, all three compounds were dosed at 1 μM. R1507 was dosed at 100 nM.
In Vivo Studies
SCID-Beige mice as described above were injected with two million cells of Rh36 or Rh18C (details of injection are specified in the in vivo resistance section above). Mice were randomized after tumors developed but prior to the start of treatment. Treatment with agents began when tumors were palpable, which was on day 21 for Rh36 and day 86 for Rh18C. R1507 was given by IP injection at 6 mg/kg weekly. Crenolanib was given by IP injection at 15 mg/kg daily 5 days per week. Treatments were continuous.
All mice were maintained in a pathogen-free environment and monitored weekly for tumor growth. Tumors were measured twice per week with calipers, and mice were weighed weekly to determine drug tolerability. Tumor volume was calculated by the following formula: V (mm3) = (D × d2)/6 × 3.14, where D (mm) is the longest tumor axis and d (mm) is the shortest tumor axis. Tumors were harvested at midpoints and at study end point for biology studies.
Statistical Analysis
Activity of PDGFR-β in cell lines was compared between groups using a nonparametric t test. Statistical significance was defined as P < .05.
Tumor volumes were compared between groups using a nonparametric t test at time points selected to be appropriate according to the data being presented in each plot. Measurements for mice that had already reached end point were carried forward until all mice in the group had reached end point or the experiment was terminated. Mice that had not developed tumor by the time of drug treatments were discarded from the analysis. Statistical significance was defined as P < .05.
Results
RMS cell lines with acquired resistance to IGF-1R blockade were analyzed to determine what other pathways might be contributing to bypass resistance. Upregulation of PDGFR-β activity was identified in three IGF-1R antibody-resistant cell lines that were generated in vivo from a previously highly sensitive parental line (Rh41) and in four of nine additional IGF-1R antibody-resistant cell lines that were generated in vitro from parental lines with varying IGF-1R antibody sensitivity. In vitro experiments using dual blockade of IGF-1R and PDGFR-β resulted in enhanced growth inhibition in cell lines that displayed upregulated PDGFR-β activity, whereas cell lines that did not upregulate PDGFR-β activity did not respond to the combination. In mouse xenograft models of cell lines that showed in vitro sensitivity to dual blockade of IGF-1R and PDGFR-β, combination therapy also produced superior inhibition of tumor growth.
PDGFR-β Activation in Xenograft-Derived Cell Lines Resistant to IGF-1R Blockade
In vitro testing of a parental cell line (Rh41) that was highly sensitive to IGF-1R blockade and its daughter cell lines (Rh41.R1 (R1), Rh41.R2 (R2), Rh41.R3 (R3), and Rh41.R4 (R4)) that were derived from xenografts of Rh41 treated with prolonged IGF-1R inhibition revealed that R2, R3, and R4 retained resistance to R1507 in culture, whereas R1 did not (Figure 1, A and B). Comparison of the sensitive (Rh41 and R1) and resistant lines (R2) by receptor phosphotyrosine kinase (RTK) array demonstrated that R2 resistant cells expressed increased phosphorylation of PDGFR-β, whereas the parental Rh41 cell line and the xenograft-generated nonresistant cell line R1 did not express any PDGFR-β. In addition, both sensitive (Rh41 and R1) and resistant (R2) cell lines expressed high levels of phosphorylated IGF-1R, HGFR, and FGFR-4, a finding that is consistent with published data on alveolar RMS cell lines [15], [16]. In the resistant cells, subsequent exposure to IGF-1R blockade in vitro further increased phosphorylation of PDGFR-β, reduced phosphorylation of IGF-1R, and did not significantly alter phosphorylation of HGFR or FGFR-4 (Figure 1C).
Figure 1.
(A and B) Xenograft-derived R1507 resistant cell lines R2, R3, and R4 maintained resistance to R1507 in vitro, whereas parental Rh41 and R1 remained sensitive. (A) Cells were plated in 96-well plates at a density of 3000 cells per well and placed in the IncuCyte. Cells were treated with 100 nM R1507 starting at time 0 and redosed every 3 days. Each data point represents an average of 32 wells. (B) Cells were plated in 96-well plates at a density of 2000 cells per well and placed in the IncuCyte. Cells were treated with one dose of R1507 (100 nM) at 24 hours. Each data point represents an average of 16 wells. (C) Phospho-PDGFR-β was increased in resistant cell line R2 (3), but not in R1 (2), when compared with parental Rh41 (1) by RTK array. When R2 was treated with R1507, phospho-IGF-1R decreased, as expected, and phospho-PDGFR-β increased further (4). HGF-R, FGFR-4, and IGF-1R were the most highly phosphorylated receptors across all tested cell lines. (D) With PDGF-BB ligand stimulation, phospho-PDGFR-β was detected by Western blot at high levels in R1507 resistant R2 but not in parental Rh41 or nonresistant R1, confirming the RTK array data. Cells were grown in 10-cm tissue culture plates in complete RPMI for 24 hours. After 24 hours, medium was removed, and each plate was treated with PDGF-BB ligand stimulation with 25 ng/ml of PDGF-BB or vehicle in RPMI with glutamine only. Plates were incubated for 10 minutes at 37°C and then immediately placed on ice for harvest.Fifty micrograms of protein was loaded per lane. A172 glioblastoma cells stimulated with ligand were used as positive controls. Blot was probed with anti-phospho-PDGFR-β. Beta-actin was used as loading control. (E) With PDGF-BB ligand stimulation, phospho-PDGFR-β was detected at significantly higher levels in R1507 resistant R2 compared with parental Rh41 (P = .001) by MesoScale. Cells were grown and treated with PDGF-BB ligand or vehicle as described above. Whole cell lysates were added to MSD Multi-spot 4 Spot 96-well plates after protein standardization (25 μg/well). Phosphorylated proteins were detected with MSD Sulfo-Tag detection antibodies. Experiments were done in triplicate. (F) With PDGF-BB ligand stimulation, phospho-PDGFR-β was detected at significantly higher levels in R1507 resistant R3 and R4 compared with parental Rh41 (P = .022 and P = .035, respectively). Methods were as described in (E).
Western blot and MesoScale analyses using PDGF-BB for ligand stimulation confirmed the findings of the RTK array. By Western blot, resistant cell line R2 displayed an increase in phosphorylated PDGFR-β with ligand stimulation. In contrast, in nonresistant cell lines Rh41 and R1, phosphorylated PDGFR-β was not detected with or without ligand stimulation (Figure 1D). By MesoScale analysis, R2, R3, and R4 expressed higher levels of phosphorylated PDGFR-β compared with Rh41 when stimulated with ligand. For all three cell lines, this difference reached statistical significance (P < .05) (Figure 1, E and F).
Upregulation of PDGFR-β Activation in Additional Cell Lines Under Prolonged IGF-1R Inhibition
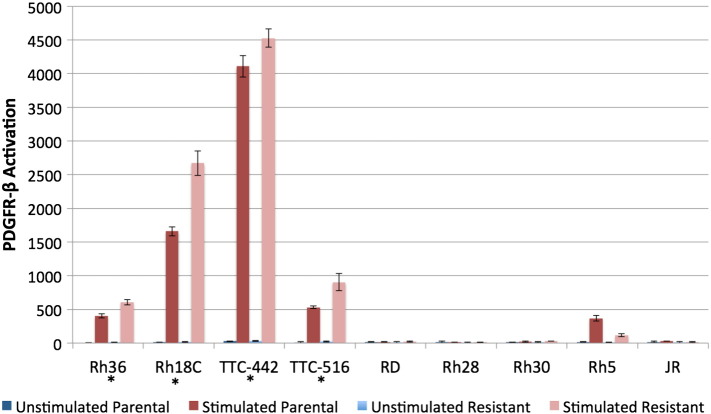
To determine whether the upregulation of phospho-PDGFR-β noted in R2, R3, and R4 was an isolated observation in Rh41-derived cell lines or if other RMS cell lines would similarly activate PDGFR-β when they developed resistance to IGF-1R blockade, nine additional RMS cell lines (four alveolar, five embryonal) were investigated. In vitro IGF-1R antibody resistance was generated through prolonged R1507 exposure, and the resulting resistant daughter cell lines were screened against their corresponding parental cell lines to identify expression differences in PDGFR-β phosphorylation. Four of the nine cell lines (Rh18C, Rh36, TTC-442, and TTC-516) displayed a statistically significant increase (P = .005, P = .002, P = .02, and P = .03, respectively) in phosphorylated PDGFR-β after 8 weeks of exposure to R1507, suggesting that PDGFR-β activation in this context was not unique to Rh41 cells. Interestingly, all four of the cell lines identified are of the embryonal subtype (Figure 2).
Figure 2.
Rh36 (P = .005), Rh18C (P = .002), TTC-442 (P = .02), and TTC-516 (P = .03) displayed significantly increased activation of PDGFR-β with acquired in vitro resistance to IGF-1R blockade. RD, Rh28, Rh30, Rh5, and JR did not show this effect. Cells were grown and selected for in vitro resistance to IGF-1R inhibition using continuous IGF-1R antibody (R1507) exposure for 8 weeks to create parental and resistant pairs. Ligand stimulation with PDGF-BB was performed as described in Figure 1D. MesoScale methods were as described in Figure 1E. Protein standardization by BCA was performed within each pair and quantified as follows: Rh36-58 ug, Rh18C-27 ug, TTC-442-65 ug, TTC-516-24 ug, RD-64 ug, Rh28-17 ug, Rh30-64 ug, Rh5-15 ug, JR-5 ug. Experiments were done in triplicate.
Co-Inhibition of IGF-1R and PDGFR-β In Vitro
Target inhibition of PDGFR-β in R2 using both imatinib and pazopanib demonstrated that both drugs downregulate PDGFR-β activity. Imatinib reduced levels of activation by approximately 7-fold (P = .0015), and pazopanib did so by about 25-fold (P = .0012) (Figure 3A). Of note, a pure PDGFR-β inhibitor does not exist, but several tyrosine kinase inhibitors that target PDGFR-β are commercially available. Imatinib, pazopanib, and crenolanib were used in these in vitro experiments, with crenolanib being the most specific and targeting only PDGFR-β, PDGFR-α, and FLT-3. Based on data from the RTK array, the resistant cell lines did not highly express PDGFR-α or FLT-3; thus, inhibitory activity on these kinases was not expected to significantly affect the cells.
Figure 3.
Upper panel: (A) Imatinib (P = .0015) and pazopanib (P = .0012) inhibited phospho-PDGFR-β activity in R2 cells. Phospho-PDGFR-β was inhibited most completely by pazopanib (P = .0019). Cells were plated in a 96-well plate at a density of 3000 cells per well. At 24 hours of growth, drug treatments of DMSO, imatinib (1 μM), or pazopanib (1 μM) were applied, and cells were incubated for an additional 24 hours before lysing. Ligand stimulation with PDGF-BB was performed as described in Figure 1(D). MesoScale methods were as described in Figure 1E. Experiments were done in triplicate with protein loaded at 10 μg/well.
(B) IncuCyte plot of R2 cells treated with DMSO, R1507 (100 nM), imatinib (1 μM), pazopanib (1 μM), imatinib plus R1507, and pazopanib plus R1507. Growth inhibition was most profound in cells receiving a combination of R1507 and pazopanib. Cells were plated in a 96-well plate at a density of 3000 cells per well. Treatments were applied at 24 hours. Retreatment was done every 3 days. Each data point represents an average of 16 wells.
Center panel: Dual inhibition of IGF-1R and PDGFR-β with R1507 and crenolanib resulted in enhanced growth inhibition in R3 (C) and R4 (D). Cells were plated in a 96-well plate at a density of 2000 cells per well. At 24 hours, cells were treated with DMSO, R1507 (100 nM), crenolanib (1 μM), or R1507 plus crenolanib. Each data point represents an average of 12 wells.
Lower panel: Dual inhibition of IGF-1R and PDGFR-β with R1507 (100 nM) and pazopanib (1 μM) (E) or with R1507 (100 nM) and crenolanib (1 μM) (F) resulted in enhanced growth inhibition in Rh18C, Rh36, TTC-442, and TTC-516. Cells were plated in a 96-well plate at various densities to optimize growth as follows: Rh18C and TTC-516 at 4000 cells/well, Rh36 and TTC-442 at 2000 cells/well. At 24 hours, treatment was administered. Each data point represents an average of 24 wells.
Combination treatment of R2, R3, and R4 with R1507 and the aforementioned tyrosine kinase inhibitors revealed enhanced growth inhibition in all three of the cell lines when compared with any single agent alone. In R2, R1507 plus pazopanib more profoundly inhibited cell growth than R1507 plus imatinib did (Figure 3B). Interestingly, this corresponded to the greater degree of PDGFR-β inhibition observed with pazopanib in this cell line (Figure 3A). In R3 and R4, combination treatment with R1507 and crenolanib similarly resulted in enhanced cell growth inhibition when compared with either single agent alone (Figure 3, C and D).
Additionally, in all four of the embryonal cell lines identified as upregulating PDGFR-β during development of IGF-1R antibody resistance (Rh18C, Rh36, TTC-442, and TTC-516), combination therapy with R1507 and pazopanib resulted in superior growth inhibition (Figure 3E). Repeating this experiment with crenolanib recapitulated these results (Figure 3F). In contrast, cell lines that did not show increased phosphorylation of PDGFR-β with development of resistance to IGF-1R blockade, such as RD and Rh30, were not sensitive to this combination (data not shown). These findings suggest that activation of PDGFR-β is responsible for bypass resistance to IGF-1R blockade in a selected subset of RMS.
Dual Blockade of IGF-1R and PDGFR-β in RMS Xenografts
Based on pilot studies to determine growth kinetics in vivo, Rh36 and Rh18C were selected from cell lines that displayed upregulation of PDGFR-β in vitro for use in xenograft studies due to their reliable tumor take and consistent growth pattern. Although consistent, the growth rate for Rh18C was slow, with a latency of about 80 days and a time to end point of about 120 days in untreated mice. Because the schedule for crenolanib administration was daily IP injections 5 days per week, Rh18C mice receiving crenolanib were treated with as many as 45 IP injections over a 9-week period, which resulted in substantial morbidity in some animals. Specifically, repetitive dosing of the agent resulted in IP adhesions and subsequent bowel obstruction, necessitating euthanasia in a number of mice receiving the combination before the tumors had reached end point. This also occurred in Rh36 mice receiving the combination but to a lesser degree, as these mice were treated for a shorter duration and the effect was more pronounced with longer exposure. Measurements from mice requiring euthanasia prior to reaching end point were only included in the analysis until the day they were euthanized and then were removed to avoid artificially depressing average tumor size in the combination groups.
In both models, combination therapy with R1507 and crenolanib resulted in greater suppression of tumor growth compared with all other treatment groups. This was reflected by a slower growth rate of the tumors in mice treated with the combination (Figure 4, A and B). In Rh36, the differences in tumor sizes between the group treated with R1507 and the group treated with the combination reached statistical significance between day 63 (P = .0499) and day 81 (P = .0241), which was the end of the experiment (Figure 4, C and D). In mice bearing Rh18C tumors, statistical significance between tumors sizes was not reached despite the apparently slower growth rate in the combination group likely because of the aforementioned toxicity reducing the number of animals remaining in the combination group. In both Rh36 and Rh18C, mice receiving crenolanib alone experienced tumor growth at the same rate as the vehicle group, and in Rh18C mice, R1507 had no effect as a single agent, suggesting that the advantage of the combination therapy was synergistic in these models.
Figure 4.
Dual inhibition of IGF-1R and PDGFR-β in Rh36 and Rh18C xenografts resulted in slower tumor growth (A and B). In Rh36, differences in tumor sizes between the R1507 group and the combination group reached statistical significance between day 63 (P = .0499) (C) and day 81 (P = .0241) (D). In Rh18C, statistical significance was not reached likely because of insufficient number of animals. Two million cells were injected orthotopically into the gastrocnemius muscle of SCID-Beige mice. Mice were allowed to develop palpable tumors before randomization into four groups [vehicle, R1507, crenolanib, and combination (R1507 plus crenolanib)]. Weight was measured weekly to determine tolerability. Tumors were measured twice weekly. Mice were sacrificed at interim time points for tissue collection and at end point (2 cm).
Discussion
Early-phase clinical trials in RMS have shown encouraging results using antibodies against IGF-1R. However, the potential of these agents has been limited because most patients respond for a short time before the onset of resistance. Our current work focused on identifying and targeting PDGFR-β in its capacity as an alternative bypass mechanism of resistance in RMS. Data from our preclinical RMS models suggest that signaling through PDGFR-β results in bypass of IGF-1R blockade and contributes to IGF-1R resistance in three cell lines (R2, R3, and R4) derived in xenografts from a previously sensitive alveolar RMS cell line (Rh41) as well as in four additional embryonal RMS cell lines (Rh18C, Rh36, TTC-441, and TTC-516). In these cell lines, an increase in PDGFR-β activation is observed with prolonged exposure to IGF-1R blockade and the development of IGF-1R antibody resistance. Both in vitro and in vivo data from multiple RMS models suggest that combination therapy against IGF-1R and PDGFR-β results in enhanced growth inhibition in the cell lines that upregulate PDGFR-β when exposed to IGF-1R blockade compared with single-agent therapy alone. These findings collectively suggest that PDGFR-β may be acting as a bypass resistance pathway in a subset of RMS.
Multiple studies have described mechanisms that aid in the development of resistance to IGF-1R blockade. We recently reported the identification of YES/Src family kinase signaling as a bypass resistance mechanism in preclinical models of RMS [13]. Additional preclinical studies of RMS have reported that Her2 heterodimers and PDGFRA-α expression contribute to IGF-1R inhibitor resistance [17], [18]. In other cancer types, overexpression of IGF binding proteins and heat shock 90 protein has been found to promote resistance to these agents [19], [20]. This is the first study to report on upregulation of PDGFR-β expression acting as a potential bypass pathway in IGF-1R antibody-resistant cell lines.
As we previously reported, an increase in AKT phosphorylation despite persistent downregulation of IGF-1R in RMS xenograft tumors treated with an IGF-1R antibody suggested that resistance was the result of a bypass resistance pathway signaling through AKT [7]. We again observed this effect in our xenograft samples treated with R1507. Interestingly, PDGFR-β and IGF-1R both signal through the same two major pathways, PI3K and MAPK/ERK, and as AKT is part of the PI3K pathway, PDGFR-β activation may explain the reactivation of AKT in resistant cells. Moreover, when resistant cells are exposed to continuous IGF-1R inhibition, PDGFR-β activation is further increased, suggesting that once PDGFR-β becomes activated as a bypass pathway, cells depend more heavily on signaling through it for growth.
Other studies have reported that PDGFR-β activity mediates resistance through the AKT pathway in various cancer types. Juliachs et al. described that acquired resistance to cisplatin in testicular cancer develops as a result of overexpression of PDGFR-β; Akhavan et al. found that derepression of PDGFR-β transcription contributes to resistance to EGFR inhibitors in glioblastoma [21], [22]. Clinically, an increase in PDGFR-β activation may act as a biomarker for predicting resistance to IGF-1R inhibition in patient tumors. Published data support this idea, as analysis of human RMS tumors has shown heterogeneous expression of PDGFR-β in patient samples, with high-expressing tumors portending worse prognoses (Oncogenomic Database, http://home.ccr.cancer.gov/oncology/oncogenomics/).
An enhanced inhibitory effect of rational combination therapy targeted against IGF-1R and other identified bypass pathways has been reported in the literature [13], [20]. Furthermore, targeted therapy against PDGFR-β in cell types where it has been identified as a resistance mechanism has restored sensitivity of those cells to the original agent [21], [22]. In our study, both in vitro and in vivo findings demonstrated an enhanced inhibitory effect of combination therapy against IGF-1R and PDGFR-β. The ability of these combinations to overcome acquired resistance to IGF-1R blockade in a number of RMS cell lines and xenograft tumors suggests that rational combination therapy has the potential to outpace tumor bypass and may be a promising combination for patients with RMS.
One limitation of our study is that the xenograft-derived resistant cell lines all originated from the same alveolar cell line (Rh41) and thus may not reflect RMS overall. Hence, the cell line screen was performed to test whether PDGFR-β activation was an isolated event in Rh41 or more broadly observed. The four additional embryonal cell lines identified in the screen further support the fact that PDGFR-β activation occurs not only in a single cell line but also in a larger subset of IGF-1R blockade-resistant RMS. Specifically, this mechanism appears to predominate in embryonal RMS.
An additional limitation affecting the in vivo experiments was the toxicity of long-term daily IP crenolanib administration, as described in the results section. Although other PDGFR inhibitors with less toxic dosing schedules were considered for these experiments, many have a large number of targets in addition to PDGFR. To minimize off-target effects, crenolanib was selected because of its specificity for inhibiting only PDGFR and FLT-3, which is not expressed in these cells. However, because of the unexpected toxicity associated with long-term dosing of crenolanib, a number of animals receiving this agent were lost, and the statistical power of the Rh18C experiment in particular was reduced. The toxicity appeared to be related specifically to the IP route of administration, as mice receiving crenolanib maintained body weight and normal activity throughout the experiment.
In summary, we have identified PDGFR-β activation as a potential bypass pathway contributing to IGF-1R blockade resistance in RMS and demonstrated that there is enhanced antitumor activity when both pathways are targeted in this subset of RMS. Although PDGFR-β activation appears to be an important bypass mechanism in the subset of RMS cell lines we have tested, we recognize that the development of resistance is complex and PDGFR-β activation will not be the only contributing factor. In fact, PDGFR-β activation is certainly one of many potential mechanisms at play. Hence, the clinical relevance of these findings may lie in the identification of patients with PDGFR-β activation in their tumors, who may experience an enhanced benefit from dual therapy against IGF-1R and PDGFR-β.
Funding Sources
This work was supported by grants from the Intramural Research Program of National Institutes of Health, the National Cancer Institute, and the Center for Cancer Research and by a Conquer Cancer Foundation of ASCO Young Investigator Award supported by the Quad W Foundation. Any opinions, findings, and conclusions expressed in this material are those of the authors and do not necessarily reflect those of the American Society of Clinical Oncology or the Conquer Cancer Foundation.
Acknowledgements
The authors would like to acknowledge Liang Cao, Ph.D., and Yunkai Yu for their assistance with this study.
Footnotes
This work was supported by grants from the Intramural Research Program of National Institutes of Health, the National Cancer Institute, and the Center for Cancer Research and by a Conquer Cancer Foundation of ASCO Young Investigator Award supported by the Quad W Foundation.
References
- 1.Dagher R., Helman L. Rhabdomyosarcoma: an overview. Oncologist. 1999;4(1):34–44. [PubMed] [Google Scholar]
- 2.Davicioni E., Anderson M.J., Finckenstein F.G., Lynch J.C., Qualman S.J., Shimada H., Schofield D.E., Buckley J.D., Meyer W.H., Sorensen P.H. Molecular classification of rhabdomyosarcoma—genotypic and phenotypic determinants of diagnosis: a report from the Children's Oncology Group. Am J Pathol. 2009;174(2):550–564. doi: 10.2353/ajpath.2009.080631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Badry O.M., Minniti C., Kohn E.C., Houghton P.J., Daughaday W.H., Helman L.J. Insulin-like growth factor II acts as an autocrine growth and motility factor in human rhabdomyosarcoma tumors. Cell Growth Differ. 1990;1(7):325–331. [PubMed] [Google Scholar]
- 4.Kalebic T., Blakesley V., Slade C., Plasschaert S., Leroith D., Helman L.J. Expression of a kinase-deficient IGF-I-R suppresses tumorigenicity of rhabdomyosarcoma cells constitutively expressing a wild type IGF-I-R. Int J Cancer. 1998;76(2):223–227. doi: 10.1002/(sici)1097-0215(19980413)76:2<223::aid-ijc9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Wang W., Kumar P., Wang W., Epstein J., Helman L., Moore J.V., Kumar S. Insulin-like growth factor II and PAX3-FKHR cooperate in the oncogenesis of rhabdomyosarcoma. Cancer Res. 1998;58(19):4426–4433. [PubMed] [Google Scholar]
- 6.Grimberg A., Cohen P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J Cell Physiol. 2000;183(1):1–9. doi: 10.1002/(SICI)1097-4652(200004)183:1<1::AID-JCP1>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao L., Yu Y., Darko I., Currier D., Mayeenuddin L.H., Wan X., Khanna C., Hellman L.J. Addiction to elevated insulin-like growth factor I receptor and initial modulation of the AKT pathway define the responsiveness of rhabdomyosarcoma to the targeting antibody. Cancer Res. 2008;68(19):8039–8048. doi: 10.1158/0008-5472.CAN-08-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller B.S., Yee D. Type I insulin-like growth factor receptor as a therapeutic target in cancer. Cancer Res. 2005;65(22):10123–10127. doi: 10.1158/0008-5472.CAN-05-2752. [DOI] [PubMed] [Google Scholar]
- 9.Tao Y., Pinzi V., Bourhis J., Deutsch E. Mechanisms of disease: signaling of the insulin-like growth factor 1 receptor pathway—therapeutic perspectives in cancer. Nat Clin Pract Oncol. 2007;4(10):591–602. doi: 10.1038/ncponc0934. [DOI] [PubMed] [Google Scholar]
- 10.Burtrum D., Zhu Z., Lu D., Anderson D.M., Prewett M., Pereira D.S., Bassi R., Abdullah R., Hooper A.T., Koo H. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res. 2003;63(24):8912–8921. [PubMed] [Google Scholar]
- 11.Pappo A.S., Patel S.R., Crowley J., Reinke D.K., Kuenkele K.P., Chawla S.P., Toner G.C., Maki R.G., Meyers P.A., Chugh R. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research Through Collaboration Study. J Clin Oncol. 2011;29(34):4541–4547. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pappo A.S., Vassal G., Crowley J.J., Bolejack V., Hogendoorn P.C., Chugh R., Ladanyi M., Grippo J.F., Dall G., Staddon A.P. A phase 2 trial of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF-1R), in patients with recurrent or refractory rhabdomyosarcoma, osteosarcoma, synovial sarcoma, and other soft tissue sarcomas: results of a Sarcoma Alliance for Research Through Collaboration study. Cancer. 2014;120(16):2448–2456. doi: 10.1002/cncr.28728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan X., Yeung C., Heske C., Mendoza A., Helman L.J. IGF-1R inhibition activates a YES/SFK bypass resistance pathway: rational basis for co-targeting IGF-1R and Yes/SFK kinase in rhabdomyosarcoma. Neoplasia. 2015;17(4):358–366. doi: 10.1016/j.neo.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan X., Yeung C., Kim S.Y., Dolan J.G., Ngo V.N., Burkett S., Khan J., Staudt L.M., Helman L.J. Identification of FoxM1/Bub1b signaling pathway as a required component for growth and survival of rhabdomyosarcoma. Cancer Res. 2012;72(22):5889–5899. doi: 10.1158/0008-5472.CAN-12-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crose L.E., Etheridge K.T., Chen C., Belyea B., Talbot L.J., Bentley R.C., Linardic C.M. FGFR4 blockade exerts distinct antitumorigenic effects in human embryonal versus alveolar rhabdomyosarcoma. Clin Cancer Res. 2012;18(14):3780–3790. doi: 10.1158/1078-0432.CCR-10-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou H.Y., Li Q., Lee J.H., Arango M.E., McDonell S.R., Yamazaki S., Koudriakova T.B., Alton G., Cui J.J., Kung P.P. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67(9):4408–4417. doi: 10.1158/0008-5472.CAN-06-4443. [DOI] [PubMed] [Google Scholar]
- 17.Abraham J., Prajapati S.I., Nishijo K., Schaffer B.S., Taniguchi E., Kilcoyne A., McCleish A.T., Nelon L.D., Giles F.G., Efstratiadis A. Evasion mechanisms to Igf1r inhibition in rhabdomyosarcoma. Mol Cancer Ther. 2011;10(4):697–707. doi: 10.1158/1535-7163.MCT-10-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang F., Hurlburt W., Greer A., Reeves K.A., Hillerman S., Chang H., Fargnoli J., Graf Finckenstein F., Gottardis M.M., Carboni J.M. Differential mechanisms of acquired resistance to insulin-like growth factor-i receptor antibody therapy or to a small-molecule inhibitor, BMS-754807, in a human rhabdomyosarcoma model. Cancer Res. 2010;70(18):7221–7231. doi: 10.1158/0008-5472.CAN-10-0391. [DOI] [PubMed] [Google Scholar]
- 19.Huang F., Greer A., Hurlburt W., Han X., Hafezi R., Wittenberg G.M., Reeves K., Chen J., Robinson D., Li A. The mechanisms of differential sensitivity to an insulin-like growth factor-1 receptor inhibitor (BMS-536924) and rationale for combining with EGFR/HER2 inhibitors. Cancer Res. 2009;69(1):161–170. doi: 10.1158/0008-5472.CAN-08-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martins A.S., Ordoñez J.L., Garcia-Sanchez A., Herrero D., Sevillano V., Osuna D., Mackintosh C., Caballero G., Otero A.P., Poremba C. A pivotal role for heat shock protein 90 in Ewing sarcoma resistance to anti-insulin-like growth factor 1 receptor treatment: in vitro and in vivo study. Cancer Res. 2008;68(15):6260–6270. doi: 10.1158/0008-5472.CAN-07-3074. [DOI] [PubMed] [Google Scholar]
- 21.Juliachs M., Muñoz C., Moutinho C.A., Vidal A., Condom E., Esteller M., Graupera M., Casanovas O., Germà J.R., Villanueva A. The PDGFRbeta-AKT pathway contributes to CDDP-acquired resistance in testicular germ cell tumors. Clin Cancer Res. 2014;20(3):658–667. doi: 10.1158/1078-0432.CCR-13-1131. [DOI] [PubMed] [Google Scholar]
- 22.Akhavan D., Pourzia A.L., Nourian A.A., Williams K.J., Nathanson D., Babic I., Villa G.R., Tanaka K., Nael A., Yang H. De-repression of PDGFRbeta transcription promotes acquired resistance to EGFR tyrosine kinase inhibitors in glioblastoma patients. Cancer Discov. 2013;3(5):534–547. doi: 10.1158/2159-8290.CD-12-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]