Abstract
Aromatic cation activation is a useful strategy to promote deoxyfunctionalization; however, the deoxyfluorination of alcohols with cyclopropenium cation remains an unsolved problem due to the weak nucleophilicity of fluoride ion. Here we report the use of 3,3-difluoro-1,2-diarylcyclopropenes (CpFluors) as easily accessible and reactivity-tunable deoxyfluorination reagents. The electronic nature of CpFluors is critical for fluorination of monoalcohols via alkoxycyclopropenium cations, and CpFluors with electron-rich aryl substituents facilitate the transformation with high efficiency; however, selective monofluorination of 1,2- and 1,3-diols, which proceeds via cyclopropenone acetals, is less dependent on the electronic nature of CpFluors. Moreover, CpFluors are more sensitive to the electronic nature of alcohols than many other deoxyfluorination reagents, thus fluorination of longer diols can be achieved selectively at the relatively electron-rich position. This research not only unveils the first example of deoxyfluorination reagents that contain an all-carbon scaffold, but also sheds light on the divergent reactivity of cyclopropenium cation in deoxyfunctionalization of alcohols.
Fluorination of organic molecules can dramatically change the properties and is commonly used for the synthesis of bioactive molecules. Here, the authors report the deoxyfluorination of alcohols and the selective monofluorination of diols using bench stable, tunable difluorocyclopropene reagents.
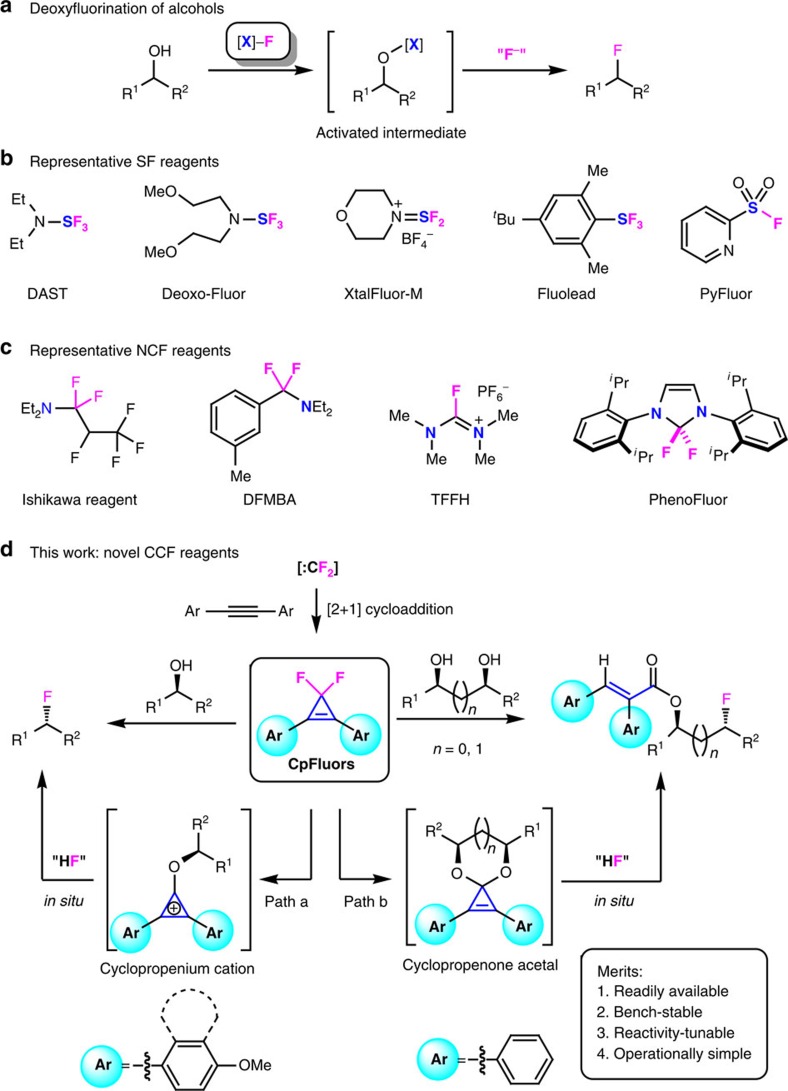
The introduction of fluorine atoms into organic molecules can induce marked changes in their chemical, physical and pharmacological properties, and fluorination has become a routine and powerful strategy in the design of new materials and drug candidates1,2,3. As a result, numerous efforts have been devoted to the development of fluorination methods4,5,6,7,8. Among them, direct deoxyfluorination of alcohols via nucleophilic fluorination of the in situ-generated activated intermediates, is a major approach to obtain alkyl fluorides, thanks to the abundance of natural and synthetic alcohols8,9,10,11,12 (Fig. 1a). Since the first report on the SF4-mediated deoxyfluorination reactions13, dozens of power-variable deoxyfluorination reagents have been developed8,9,10,11,12. Generally, these reagents can be divided into two categories: one is the sulfur fluorides and their derivatives (SF reagents)14,15,16,17,18 (Fig. 1b), and the other is the α-fluorinated alkylamines and their derivatives (NCF reagents)19,20,21,22 (Fig. 1c). The deoxyfluorination with these heteroatom-based reagents, however, suffers from disadvantages such as high cost, thermal instability, harsh reaction conditions or the requirement of external fluoride sources or bases. Moreover, these fluorination reagents rely on heteroatom-based motifs both as the fluoride carrier and as the C−O bond activator of alcohols. Carbon is the core of organic compounds and the use of an all-carbon moiety to activate a molecule is of great interest for both organic synthesis and mechanistic organic chemistry23,24. In this context, we have become interested in developing a new deoxyfluorination protocol using 3,3-difluorocyclopropenes as a class of novel fluorination reagents with an all-carbon scaffold (CCF reagents).
Figure 1. Methods for deoxyfluorination of alcohols.
(a) General route for deoxyfluorination of alcohols, proceeding through the activation of alcohol followed by displacement with a fluoride ion. (b) Representative SF reagents, including aminosulfur trifluorides (such as diethylaminosulfur trifluoride (DAST) and Deoxo-Fluor)14,15, aminodifluorosulfinium salts (such as XtalFluor-M)16, arylsulfur trifluorides (such as Fluolead)17 and sulfonyl fluorides (such as PyFluor)18. (c) Representative NCF reagents, including perfluoroolefin-secondary amine adducts (such as Ishikawa reagent)19, difluorobenzylamines (such as N,N-diethyl-a,a-difluoro-(m-methylbenzyl)amine (DFMBA))20, fluoroiminium salts (such as tetramethylfluoroformamidinium hexafluorophosphate (TFFH))21 and difluoromethanediamines (such as PhenoFluor)22. (d) Our method using difluorocarbene-derived CCF reagents, 3,3-difluoro-1,2-diarylcyclopropenes (CpFluors). CpFluors are reactivity-tunable and are capable of fluorinating both monoalcohols (path a) and diols (path b) through different activation mode.
3,3-Difluorocyclopropenes are readily prepared by difluoromethylenation of the corresponding alkynes with many difluorocarbene sources25,26 (Fig. 1d); however, their defluorinative transformations have been only focused on the use of fluorine as a leaving group, thus fluoride is eliminated as a waste27,28,29,30,31,32. Although the deoxychlorination of alcohols with their analogues 3,3-dichlorocyclopropenes via cyclopropenium cation activation has been disclosed since 2009 (refs 33, 34), the deoxyfluorination with 3,3-difluorocyclopropenes is still challenging due to the weak nucleophilicity of fluoride ion.
Here we report our success on deoxyfluorination of alcohols using crystalline, thermally stable solid reagents 3,3-difluoro-1,2-diarylcyclopropenes (CpFluors, 1) as reactivity-tunable fluorination reagents for the preparation of alkyl fluorides (Fig. 1d). The unique feature and potential application of this method are demonstrated in direct fluorination of monoalcohols through the cyclopropenium cation activation manner and acryloylative monofluorination of 1,2- and 1,3-diols through the cyclopropenone acetal activation manner. In the case of monoalcohols, tuning the electronic nature of the aryl substituents on CpFluors 1 is the key to suppress the competitive cyclopropenone acetal activation pathway, thus improving the efficiency of the desired deoxyfluorination. Furthermore, CpFluors are more sensitive to the electronic nature of alcohols than many other deoxyfluorination reagents, thus fluorination of longer diols can be achieved selectively at the relatively electron-rich position by using these new reagents.
Results
Reaction design
Previously, we were engaged in the development of novel difluorocarbene reagents for the difluoromethylation of heteroatom nucleophiles and difluoromethylenation of alkenes and alkynes26. During the course of our studies, we noticed that 3,3-difluorocyclopropenes can be hydrolyzed to cyclopropenones upon prolonged storage in a wet atmosphere and that their stability towards water is closely related to their molecular structures35,36,37. On the basis of these findings, we have initiated a program aiming to use the safe and readily available difluorocarbene reagents for deoxyfluorination by virtue of 3,3-difluorocyclopropenes. This research program was inspired by Lambert's deoxychlorination with 3,3-dichlorocyclopropenes, which utilizes aromatic cyclopropenium cations as the activator for the transformation of monoalcohols and carboxylic acids to alkyl chlorides and acyl chlorides, respectively33,34,38. We envisioned that under similar conditions, 3,3-difluorocyclopropenes may be used for deoxyfluorination of alcohols via nucleophilic attack of the alkoxycyclopropenium cation intermediate by the in situ-generated fluoride ion (Fig. 1d, path a).
Initial attempt
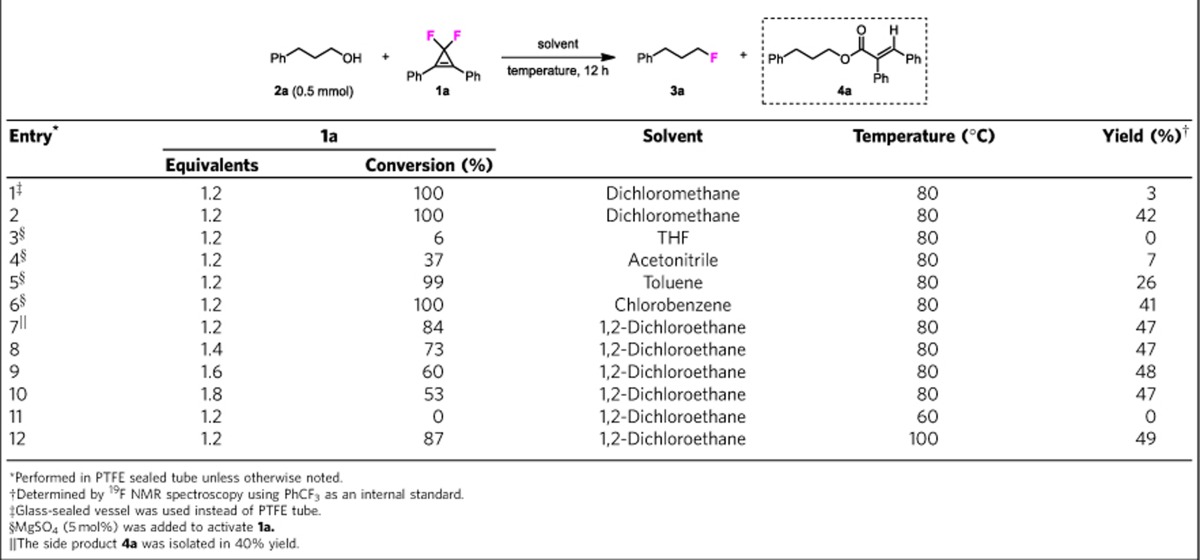
At the onset, we chose 3,3-difluoro-1,2-diphenylcyclopropene (CpFluor 1a) as the reagent to survey the suitable conditions for deoxyfluorination. After numerous trials and failures, we found that, unlike dichlorocyclopropenes33,34,38, CpFluor 1a was only effective for the dehydroxyfluorination of carboxylic acids and benzyl alcohols39, and its fluorination of non-activated alcohols such as 3-phenylpropan-1-ol (2a) was unexpectedly difficult. Table 1 shows the representative conditions that were screened with 2a as the model substrate. When the reaction was conducted in dichloromethane at room temperature, CpFluor 1a was found to be inert towards alcohol 2a, and at an elevated temperature (80 °C), 1a readily reacts with glass (mainly SiO2) rather than 2a due to the formation of HF (Table 1, entry 1). Therefore, a non-glass vessel should be used to avoid the undesired deoxyfluorination of glass. To our delight, when a polytetrafluoroethylene (PTFE) tube was used as the reaction vessel, the desired alkyl fluoride 3a was produced in 42% yield (Table 1, entry 2). In terms of reaction solvents, no product 3a was formed when THF was used, and acetonitrile provided only a very low yield (Table 1, entries 3 and 4). Other solvents such as toluene, chlorobenzene and 1,2-dichloroethane (DCE) were found to be much more efficient than THF and acetonitrile (Table 1, entries 5–7). These results indicate that Lewis basic solvents, which can serve as proton acceptors, are harmful to this reaction. To further improve the yield, we turned our attention to optimize the reactant ratio using DCE as the optimal solvent. However, to our great surprise, the highest yield of alkyl fluoride 3a was only moderate even a large excess of CpFluor 1a was used (entries 8–10). According to the conversion of 1a and the yield of 3a, we speculated that alcohol 2a also took part in a side reaction. Moreover, we found that raising the temperature to 100 °C had little influence on the reaction, whereas lowering the temperature to 60 °C resulted in no desired reaction (entries 11 and 12).
Table 1. Initial deoxyfluorination of alcohols with CpFluor 1a.
Modification of the reagent
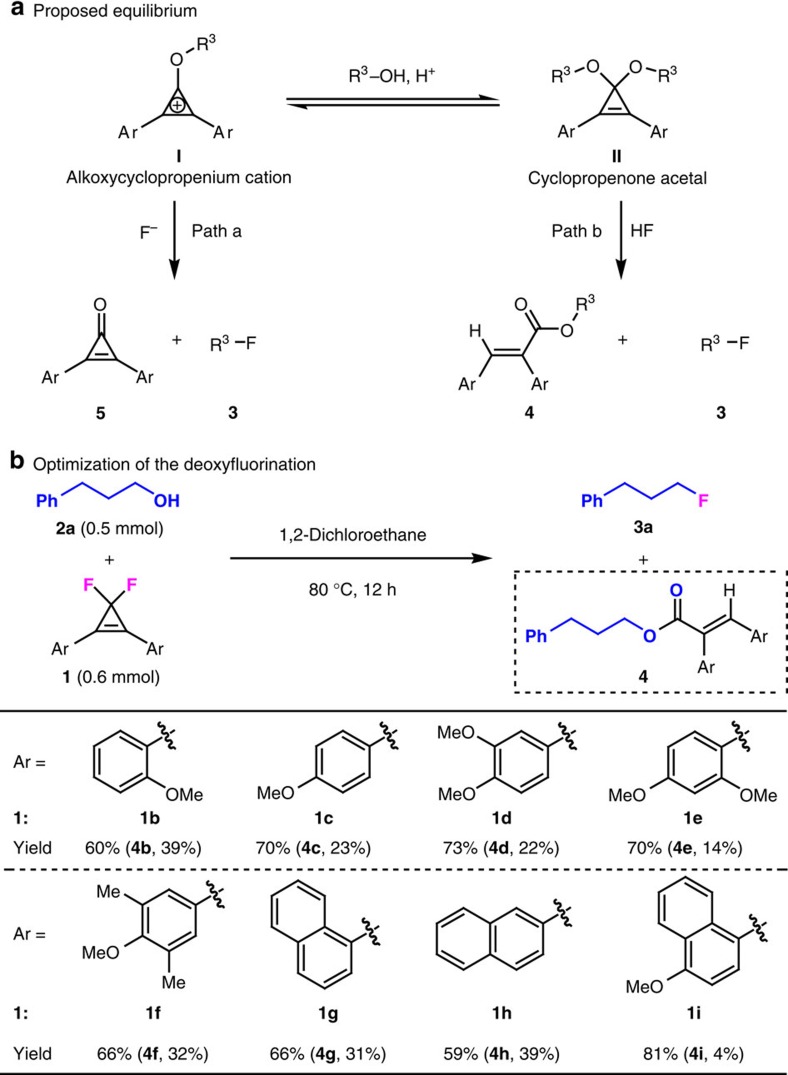
To further improve the yield of alkyl fluoride 3a, it is necessary to figure out and suppress the undesired competitive reaction pathway(s). An analysis of the crude reaction mixture with gas chromatography—mass spectrometry showed that 2,3-diphenylacrylate 4a (Table 1) was formed as the major side product, along with a substantial amount of 2,3-diphenylcyclopropenone (5a). The identity of 4a was confirmed by its separation (40% yield) and characterization. According to the above results, we proposed that the side product 4a may arise from hydrogen fluoride-promoted ring-opening of a cyclopropenone acetal intermediate II that is in equilibrium with an alkoxycyclopropenium cation intermediate I (Fig. 2a)40,41,42. The occurrence of the cyclopropenone acetal pathway (path b) is attributed to the weak nucleophilicity of fluoride ion. The slow consumption of aromatic cation I by fluoride ion (path a) results in its accumulation and thus promotes the formation of acetal II. On the basis of this rationalization, we envisioned that the desired fluorination of alcohols via cyclopropenium cation might be significantly improved by tuning the electronic nature of CpFluors 1. In other words, if we could further stabilize the cyclopropenium cation I (ref. 43), the formation of the cyclopropenone acetal intermediate II might be diminished, thus improving the desired fluorination reaction.
Figure 2. Substitution effect on the outcome of the deoxyfluorination.
(a) A proposed competitive reaction pathway that arises from an equilibrium between alkoxycyclopropenium cation I and cyclopropenone acetal II. (b) Optimization on the deoxyfluorination via screening CpFluors with different aryl substitutents. Yields of 3a were determined by 19F NMR spectroscopy using PhCF3 as an internal standard. Yields of 4 refer to the isolated yields.
With aforementioned conceptions in mind, we made a survey on the deoxyfluorination of alcohol 2a by using a series of structurally symmetrical 3,3-difluorocyclopropenes bearing various electron-rich aryl substituents under the same reaction conditions as those shown in Table 1, entry 7 (Fig. 2b). To our delight, when a methoxy group was introduced at the ortho-position of the phenyl group (1b), the yield of alkyl fluoride 3a was enhanced to 60%, and changing the substitution pattern from ortho- to para-position could further improve the yield of 3a to 70% (1c). However, compared with 1b and 1c, installing more than one electron-donating substituent on the phenyl ring has little influence on the chemical outcomes (1d–1f). The use of naphthyl groups instead of phenyl groups is also beneficial for the deoxyfluorination, and 1-naphthyl substituted 1g gives a better result than 2-naphthyl substituted 1h (1g and 1h). Finally, we established that 4-methoxy-1-naphthyl-substituted difluorocyclopropene 1i was the optimal reagent, giving the desired product 3a in up to 81% yield with the generation of side product 4i in only 4% yield (1i). Reducing the amount of 1i (1.1 equivalents) and shortening the reaction time (8 h) gives an identical result (Supplementary Table 1). Note that the increase of the yield of alkyl fluoride 3 was always accompanied by the decrease of the yield of ester 4, suggesting the existence of two tunable reaction pathways.
Fluorination of monoalcohols
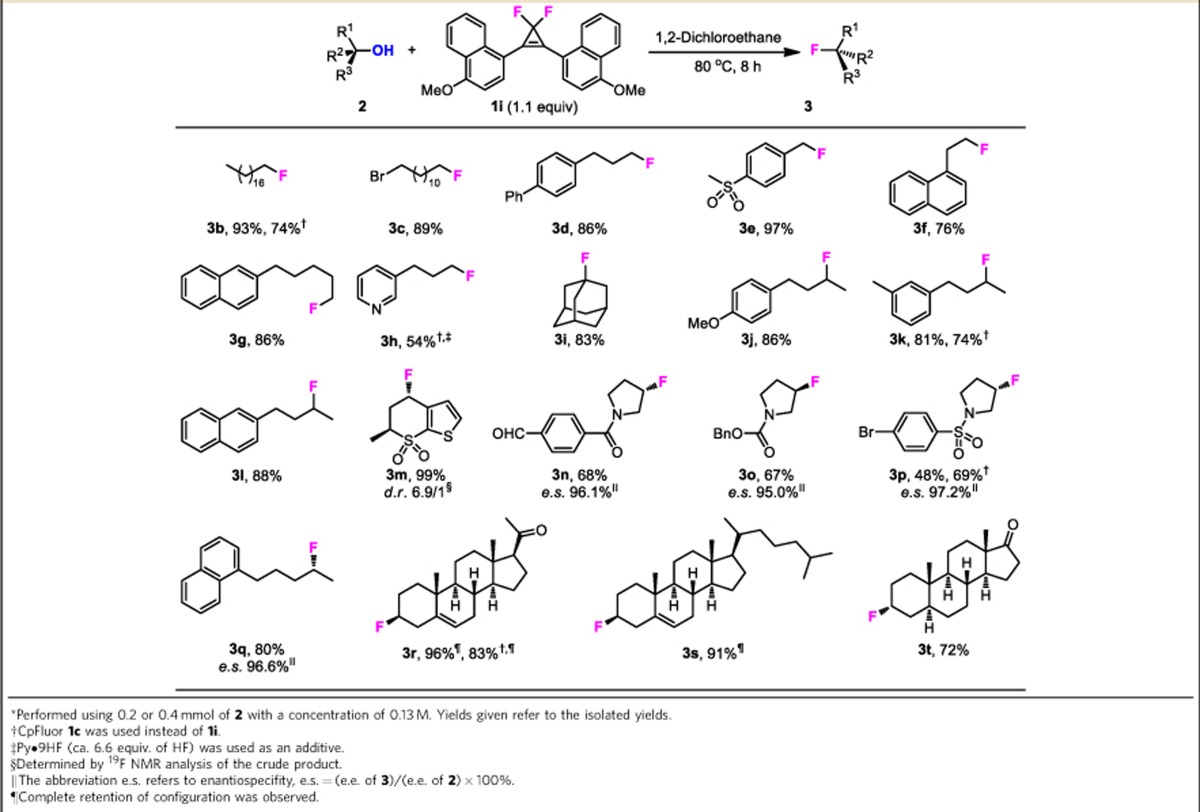
To evaluate the application scope of this deoxyfluorination protocol, we explored the transformation of structurally diverse monoalcohols by using CpFluor 1i as a useful reagent under the optimized conditions (Table 2; Supplementary Table 1). The reaction of a broad range of primary (3b–3g) and secondary (3j–3t) monoalcohols proceeded smoothly to afford the corresponding alkyl fluorides in moderate to excellent yields. Chiral secondary alcohols were deoxyfluorinated to furnish products with inversion of configuration (3n–3q). Tertiary alcohols often give rise to poor results due to the predominant elimination reaction; however, as an exception, 1-adamantanol could be effectively deoxyfluorinated (3i), which is in accordance with the Bredt's rule44. Although fluoride ion is involved in this fluorination reaction, alkyl bromide does not undergo competing halex reaction (3c). Aldehyde and ketone functionalities, which readily undergo gem-difluorination with DAST and its derivatives14,15, can keep intact under our reaction conditions (3n, 3r and 3t). Other functionalities such as amide (3n), carbamate (3o) and sulfonamide (3p) are also tolerated under the present conditions. Benzylic alcohols are the most reactive and can be fluorinated with very high efficiency, and the carbonium ion-induced self-polymerization or elimination reaction was not observed (3e and 3m)17. A homobenzylic alcohol also underwent smooth fluorination, albeit giving the alkyl fluoride in slightly lower yield due to the competitive elimination (3f). In addition to the good chemoselectivity and high stereospecificity, its ability for late-stage fluorination of complex molecules is another feature of this method. For examples, several steroids were selectively fluorinated at the hydroxyl group with high diastereoselectivity (3r–3t). Note that products 3r and 3s were obtained with retention of the configuration due to a homoallylic participation45. The para-methoxyphenyl-substituted CpFluor 1c is also capable of fluorinating many alcohols (3b, 3k, 3p and 3r), albeit usually in somewhat lower yields. However, although this method is compatible with several amide moieties, the substrates containing electron-rich amine groups, such as pyridine, cannot take part in reaction due to their inhibition effect on the initial activation of CpFluors, revealing a limitation of the additive-free deoxyfluorination method. We found that this limitation can be alleviated by the addition of an acid activator. For example, the reaction of alcohol 2h with CpFluor 1c in the presence of pyridine-hydrogen fluoride (Py·9HF) afforded the desired alkyl fluoride 3h in moderate yield (54%).
Table 2. Deoxyfluorination of monoalcohols with CpFluor 1i*.
Fluorination of 1,2- and 1,3-diols
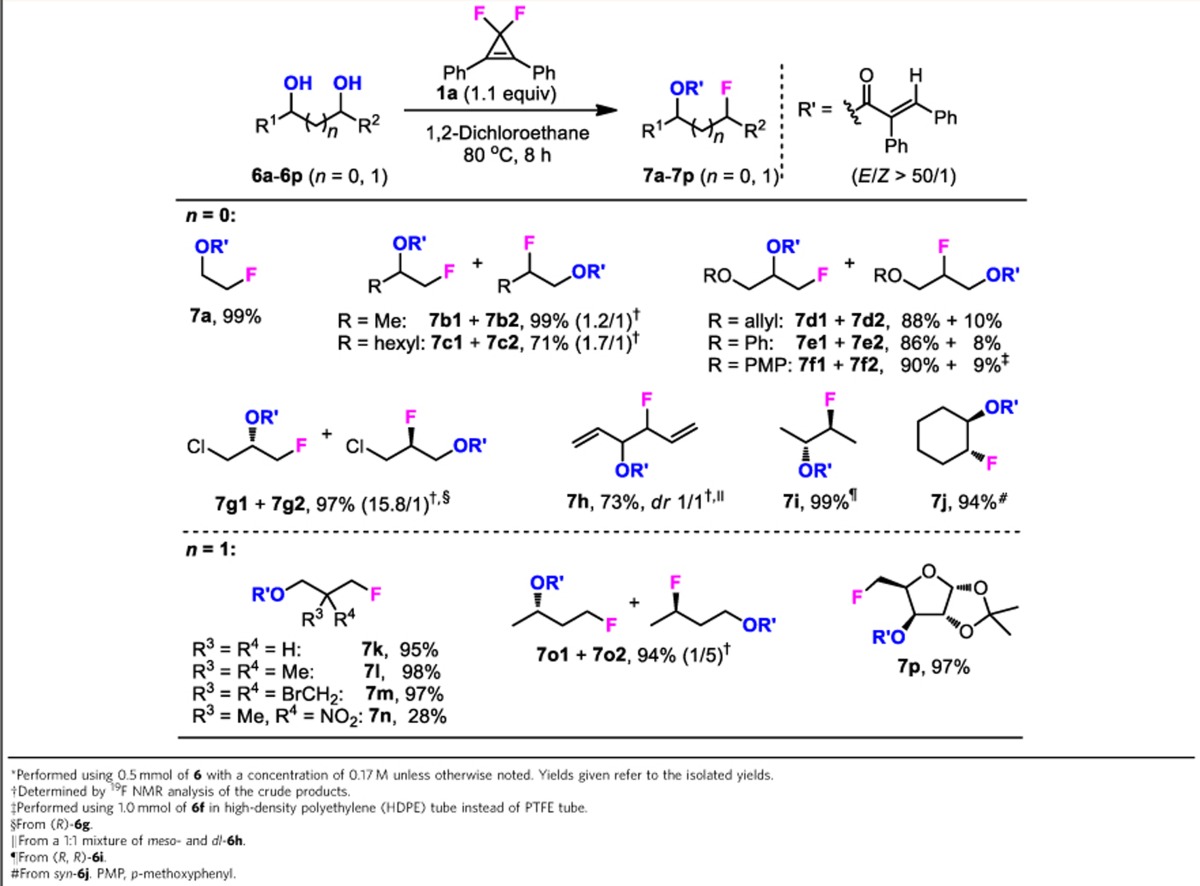
Selective fluorination of multiple alcohols, especially the symmetrical diols, using common deoxyfluorination reagents such as DAST, is a challenging synthetic task46,47. To date, available examples for selective deoxyfluorination of 1,2- and 1,3-diols mainly depend on the employment of sterically hindered reagents or reagents that can in situ generate stable hydroxyl protection groups17,18,22,48,49. On the basis of our finding that the competitive reaction of alcohol and fluoride ion towards alkoxycyclopropenium cation intermediate is tunable, we envisioned that the formation of a cyclic cyclopropenone acetal, by using 1,2- or 1,3-diols as substrates may lead to a productive monofluorination. Thus, the reactions of 2,2-dimethylpropane-1,3-diol (6l) with CpFluors 1a and 1i were examined under the optimized conditions for monoalcohols. To our delight, both of the reactions proceeded smoothly to afford the corresponding acryloylative monofluorination products selectively (see Supplementary Methods for deoxyfluorination of 1,2- and 1,3-diols). However, we found that CpFluor 1a was more efficient than CpFluor 1i, probably due to a relatively faster formation of the cyclic acetal intermediate from 1a. By using 1a as the reagent, we further investigated the scope of diols. As shown in Table 3, a series of symmetrical and unsymmetrical 1,2- and 1,3-diols were monofluorinated in high yields with good functional group tolerance. Functionalities such as alkyl halides (7g and 7m) and alkenes (7d and 7h) were intact during the reaction; however, a nitro substituent could retard the reaction significantly (7n). For unsymmetrical 1,2-diols, fluorination occurred preferentially at the less sterically hindered carbon, and the oxymethyl- and chloromethyl-substituted ones (7d–7g) were fluorinated more selectively than the simple alkyl-substituted ones (7b and 7c). The transformation of unsymmetrical 1,3-diols also proceeded regioselectively (7k–7p). When 3-alkyl substituted 1,3-diol underwent the reaction, 3-position fluorination was predominated (7o); however, when a sugar derivative with 1,3-diol motif was subjected to this reaction, the primary alkyl fluoride was obtained in excellent yield (7p). The observed unusual preference of the fluoride ion for the secondary position in the case of 7o can be attributed to the inductive and conformational effects present in the cyclic dioxanylium cation intermediate50. Moreover, fluorination at a chiral carbon proceeded with the inversion of the stereochemistry, as is indicated by single-crystal diffraction of product 7j derived from syn-cyclohexane-1,2-diol (6j).
Table 3. Deoxyfluorination of 1,2- and 1,3-diols with CpFluor 1a*.
Fluorination of longer diols
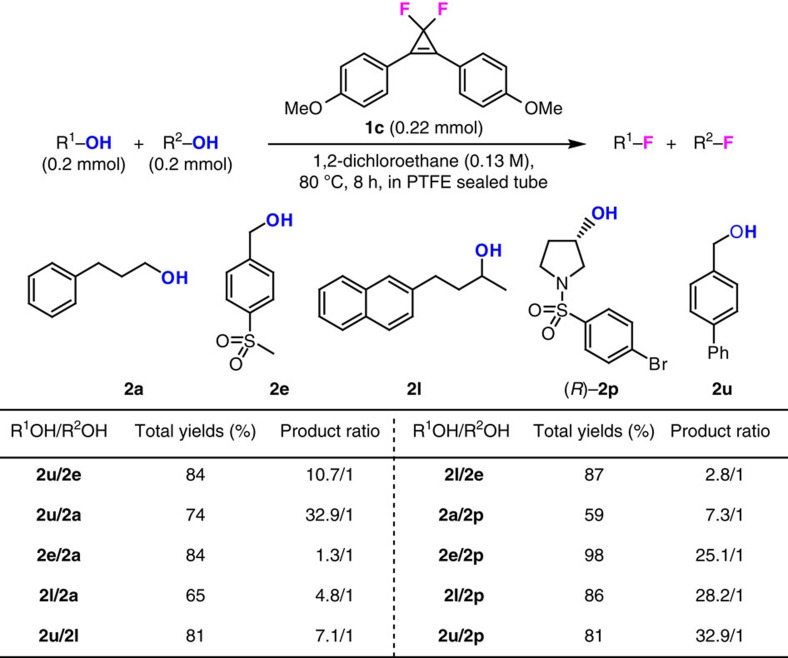
To expand the synthetic application of our developed reagents, we also investigated the selective deoxyfluorination of longer diols, where the two hydroxyl groups are separated by several carbon centers. In this case, the formation of a cyclic acetal intermediate is disfavoured; therefore, the challenge associated with this synthetic goal is to distinguish two separated hydroxyl groups, especially when they possess similar steric hindrance. To evaluate the selective deoxyfluorination capability of our method, we first conducted the competitive fluorination of different monoalcohols with the much more easily accessible CpFluor 1c, as the reagent under standard reaction conditions (Fig. 3). Among the primary alcohols tested, benzylic alcohols 2e and 2u are more active than the normal primary alcohol 2a, and the reactivity of benzylic alcohols are tunable, with the relatively electron-rich 2u being more reactive than the electron-deficient 2e. To our great surprise, when comparing primary alcohol 2a with secondary alcohol 2l, we found that 2a was less reactive, which is distinct from the expected reactivity order of typical SN2 reactions. Moreover, alcohol 2l was found to be even more reactive than electron-deficient benzylic alcohol 2e. These results indicate that it is the electronic nature of the alcohol rather than the steric hindrance of the alcohol that controls the selectivity of our deoxyfluorination reactions, at least for primary and secondary alcohols. This inference is further supported by the competitive reactions of electron-deficient 3-pyrrolidinol 2p and other alcohols, with 2p being the least reactive. Taking together, the reactivity of all the tested alcohols decreases in the following order: 2u>2l>2e>2a>2p. This interesting reactivity feature of alcohols may arise from the unique activation mode of our deoxyfluorination method, where not only the electronic nature of the CpFluor reagents, but also the electronic nature of the alcohol substrates can influence the formation of the reactive alkoxycyclopropenium cation intermediate. The electron-rich alcohol can promote the formation and stabilization of the alkoxycyclopropenium cation, thus favoring deoxyfluorination.
Figure 3. Competitive deoxyfluorination of monoalcohols.
Competitive deoxyfluorination of primary and secondary alcohols with CpFluor 1c. The yields and product ratios were determined by 19F NMR spectroscopy using PhCF3 as an internal standard.
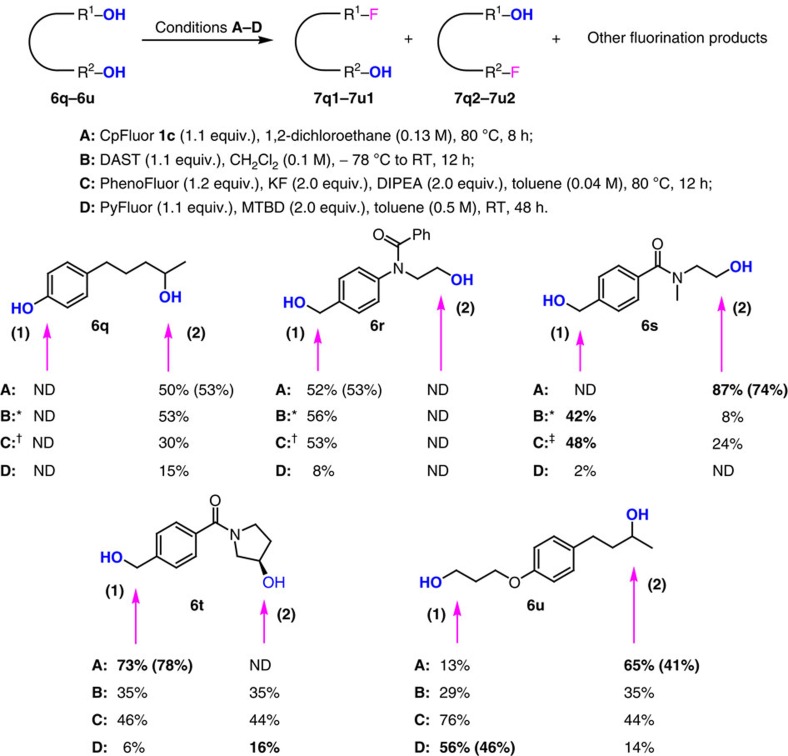
Inspired by the above finding, we investigated the deoxyfluorination of several diols (or functionalized alcohol) with CpFluor 1c and made a comparison with the results of other representative reagents DAST, PhenoFluor, as well as PyFluor (Fig. 4). By using CpFluor 1c as the reagent, alcohols 6q–6u were selectively fluorinated to give the monofluorinated compounds with retention of the other hydroxy group in moderate to good yields. Secondary alcohol 6q with a phenolic hydroxyl group smoothly underwent deoxyfluorination at the alcoholic position to give the monofluorinated phenol 7q2. Diols 6r and 6s with similar steric hindrances at both ends were selectively fluorinated at the relatively electron-rich position, affording the monofluorinated compounds 7r1 and 7s2 as the only products. In the case of diol 6t bearing an electron-deficient benzyl alcohol moiety and an electron-deficient 3-pyrrolidinol moiety, deoxyfluorination occurred selectively at the benzylic position. A comparison between the results of 6s and 6t showed that in addition to the electronic effect, the stereoelectronic effect also can influence the selectivity of deoxyfluorination with CpFluor reagents. Moreover, in the case of diol 6u containing both primary and acyclic secondary alcohol moieties, the relatively electron-rich secondary position preferred to be fluorinated, which is in accordance with the selectivity of intramolecular competitive reactions (2a/2l). Although the fluorination of 6q and 6r with DAST, PhenoFluor and PyFluor resulted in the same selectivity (not considering the yield) as CpFluor 1c did, the fluorination of 6s–6u with the three reagents afforded either opposite selectivity or poor selectivity. These preliminary results demonstrate that the chemical outcome of deoxyfluorination with various reagents is complicatedly influenced by both the steric hindrance and electronic nature of the alcohol substrates. Among the four kinds of reagents tested, CpFluor reagents are the most sensitive towards the electronic nature of the alcohols and are potentially useful for selective deoxyfluorination not otherwise achievable.
Figure 4. Selective deoxyfluorination of longer diols.
Selective deoxyfluorination of longer diols, where the two hydroxyl groups are separated by several carbon centers. The total fluorination yields at the given position were determined by 19F NMR spectroscopy using PhCF3 as an internal standard. The yields in parentheses refer to isolated yields of the monofluorination products with the retention of the other hydroxyl group. *1.5 equiv. of DAST was used. †1.5 equiv. of PhenoFluor was used. ‡The reaction was performed using 1.0 equiv. of PhenoFluor at RT for 48 h. DIPEA, N,N-diisopropylethylamine; MTBD, 7-methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene; ND, not detected.
Experimental investigation of reaction mechanism
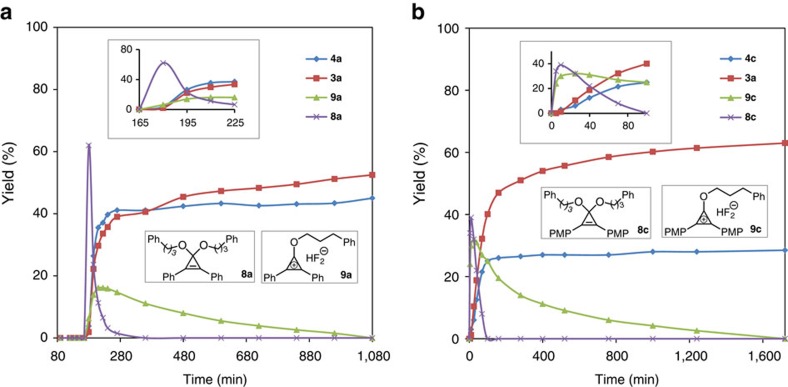
To better understand the reaction mechanism of CpFluors with different substitutions, we monitored the reaction of monoalcohol 2a with CpFluor 1a or 1c in DCE-d4 by 19F and 1H nuclear magnetic resonance (NMR) spectroscopy (Fig. 5). CpFluor 1c was chosen instead of 1i, because the progress of the reaction with 1c could be followed easily due to its good solubility.
Figure 5. Mechanistic study on the deoxyfluorination of alcohols with CpFluors.
CpFluors with electron-netural (1a) and electron-rich (1c) aryl substitutents were chosen to monitor the influence of electronic nature on the chemical outcome. Both reactions were perforformed in PTFE NMR tubes with DCE-d4 as the solvent. (a,b) Yield profiles of products and intermediates generated in the reactions of 3-phenylpropan-1-ol (2a, 1.0 equiv.) (a,b) with CpFluors 1a (1.0 equiv.) (a) and 1c (1.0 equiv.) (b) during the whole monitoring process. Insets on the top (a,b): the enlarged yield profiles at the early stage of the reactions. PMP, p-methoxyphenyl.
For the reaction between alcohol 2a and CpFluor 1a (Fig. 5a; Supplementary Table 2), there was a significant induction period. During this period (t=0–165 min), the consumption of a small amount of 1a and formation of HF were detected by 19F NMR analysis. The formation of trace HF is also supported by the 1H NMR chemical shift change of the alcoholic hydroxyl group (from 1.64 to 2.38 p.p.m.). After the induction period, a fast reaction between 1a and 2a gave intermediate 8a and HF as the major products; however, only a small amount of alkyl fluoride 3a was formed (t=165–180 min). According to the estimated molar ratio of consumed 1a and formed 8a, as well as the diagnostic 1H NMR spectrum of 8a (see Supplementary Methods for investigation of reaction mechanism), the structure of observed 8a is consistent with a cyclopropenone acetal. Subsequently (t=180–195 min), a fast consumption of 8a led to a fast formation of alkyl fluoride 3a and ester 4a; meanwhile, a new intermediate that is assigned to alkoxycyclopropenium 9a (ref. 33) was detected in substantial amount. As the reaction proceeded, a complete consumption of 8a (t=195–300 min) and 9a (t=210–1,080 min) furnished alkyl fluoride 3a in moderate yield. It should be noted that during the first 300 min, alkyl fluoride 3a and ester 4a were produced at the similar rate. From these results, we confirmed that the fluorination of 2a with the less electron-rich CpFluor 1a proceeds predominantly through the ring-opening of a cyclopropenone acetal intermediate, and the cyclopropenium cation activation pathway (mainly occurred at the late stage of the reaction) contributed less to this transformation.
When monitoring the reaction between alcohol 2a and CpFluor 1c (Fig. 5b; Supplementary Table 3), no significant induction period was observed. At the initial stage (t=0–10 min), a very fast reaction between 2a and 1c afforded HF and a nearly 1:1 mixture of intermediates 8c and 9c as the major products. The structures of 8c and 9c were assigned on the basis of their diagnostic 1H NMR spectra (see Supplementary Methods for investigation of reaction mechanism). In the subsequent course, cyclopropenone acetal 8c and alkoxycyclopropenium 9c were converted to alkyl fluorides at different rates. Judging from the rate for the formation of alkyl fluoride 3a and ester 4c, acetal 8c was consumed much faster than cyclopropenium 9c (t=10–100 min versus t=10–1,720 min). These results suggest that changing the aryl substituents' electronic nature from electron neutral to electron rich not only facilitates the activation of CpFluors, but also significantly inhibits the formation of cyclopropenone acetal from alkoxycyclopropenium cation, thus improving the fluorination efficiency.
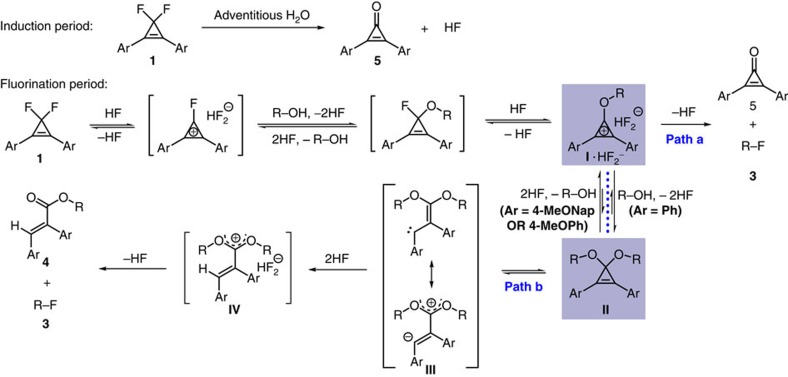
According to above results and discussion, the proposed mechanism for the fluorination of monoalcohols with CpFluors is shown in Fig. 6. Under the activation of the trace amount of hydrogen fluoride that is probably generated from the hydrolysis of CpFluor 1 by adventitious water, CpFluor 1 undergoes a fast reaction with monoalcohols to afford intermediate I. Because the reaction of I with fluoride is slow, the stability of I that is determined by the electronic nature of the aryl substituents can significantly influence the fate of I. In the case of CpFluor 1 with electron-rich aryl substituents, such as 4-methoxynaphthalen-1-yl group, the stabilization of intermediate I benefits the fluorination of monoalcohols via nucleophilic displacement of the cyclopropenium oxide by fluoride (path a). When CpFluor 1 with less electron-rich substituents, such as phenyl group undergoes the reaction (path b), the formed less stable intermediate I further reacts with monoalcohols to give cyclopropenone acetal II as the more stable intermediate. The so-formed intermediate II is in equilibrium with intermediate I under the action of hydrogen fluoride and readily undergoes thermal-induced ring-opening reaction to give vinylcarben intermediate III (refs 40, 41, 42). Protonation of the vinylcarbene III by hydrogen fluoride followed by a fluoride attack delivers a mixture of alkyl fluoride 3 and acrylate 4. The monofluorination of diols can be rationalized with the same depiction as shown in Fig. 6. In this case, the formation of the cyclic acetal of a diol is always favored regardless of the electronic nature of the CpFluors, and thus the fluorination proceeds through the second pathway (Fig. 6, path b).
Figure 6. Proposed mechanism for deoxyfluorination of monoalcohols with electronically different CpFluors.
CpFluor 1 undergoes a reaction with monoalcohols to afford intermediate I. The stability of I that is determined by the electronic nature of the aryl substituents can significantly influence its reaction pathway (path a or path b) to give the corresponding products.
Discussion
We have developed a fluorination protocol by adapting readily available and sufficiently stable CpFluors as a novel class of deoxyfluorination reagents that contain an all-carbon scaffold. We found that the electronic nature of the aryl substituents on the gem-difluorocyclopropene scaffold is critical for the transformation of monoalcohols. CpFluors with electron-rich aryl substituents furnish the deoxyfluorination with high efficiency through a stable alkoxycyclopropenium intermediate, and the spectroscopic investigations have disclosed the mechanistic pathways of this unprecedented deoxyfluorination reaction. On the other hand, the selective fluorination of 1,2- and 1,3-diols proceeds through a stable cyclopropenone acetal intermediate and is less dependent on the electronic nature of CpFluors. Furthermore, the monofluorination via alkoxycyclopropenium cation is sensitive to the electronic nature of the alcohols, thus deoxyfluorination of longer diols can be achieved selectively at the relatively electron-rich position rather than the less sterically hindered position. This research not only provides a practical method for the fluorination of various alcohols, using reagents that can be readily prepared from easily accessible alkynes and difluorocarbene reagents, but also sheds light on the divergent reactivity of cyclopropenium cations in the transformation of alcohols. We believe that in selective deoxyfluorination of multiple alcohols, CpFluors are complementary to other reagents, such as DAST, PhenoFluor and PyFluor, and should be as important as these reagents. Further research on the synthetic application of CpFluors is underway.
Methods
General
The general procedures for deoxyfluorination of monoalcohols 2 with CpFluor 1i are as follows. In a typical experiment, into a dried polytetrafluoroethylene tube equipped with a magnetic stir bar were sequentially added octadecan-1-ol (2b, 54.1 mg, 0.2 mmol), CpFluor 1i (85.4 mg, 0.22 mmol) and DCE (1.5 ml). The tube was sealed and immersed in an oil bath at 80 °C. After being stirred for 8 h, the reaction mixture was cooled to room temperature and precipitated with ether (6 ml). The precipitate (mainly 2,3-bis(4-methoxynaphthalen-1-yl)cycloprop-2-enone) was filtered and washed with ether. The filtrate was concentrated under reduced pressure and the residue was purified by flash chromatography on silica gel to give alkyl fluoride 3b (51.3 mg, 93%). The deoxyfluorination of 1,2- and 1,3-diols 6a-6p with CpFluor 1a, as well as longer diols 6q–6u with CpFluor 1c were carried out similarly and the procedures are presented in Supplementary Methods.
CpFluors 1a-1e were prepared from diarylalkynes and (chlorodifluoromethyl)trimethylsilane according to procedures presented in Supplementary Methods.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. For the experimental procedures, and spectroscopic and physical data of compounds, see Supplementary Methods. For NMR analysis of the compounds in this article, see Supplementary Figs 1–222 and Fig 227. The CCDC 1443649, CCDC 1443627, CCDC 1443644 and CCDC 1443654 contain the crystallographic data for compounds 3m, 3r, 3t and 7j, respectively (Supplementary Figs 223–226; Supplementary Tables 4–19). These data can be obtained free of charge from the Cambridge Crystallographic Data Center (www.ccdc.cam.ac.uk).
Additional information
How to cite this article: Li, L. et al. Deoxyfluorination of alcohols with 3,3-difluoro-1,2-diarylcyclopropenes. Nat. Commun. 7, 13320 doi: 10.1038/ncomms13320 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary Figures 1-227, Supplementary Tables 1-19, Supplementary Methods and Supplementary References.
Acknowledgments
Support of this work by the National Basic Research Program of China (2015CB931900 and 2012CB821600), the National Natural Science Foundation of China (21372246, 21421002, 2147222 and 21632009), the Chinese Academy of Sciences, Shanghai Academic Research Leader Program (15XD1504400), Shanghai Rising-Star Program (16QA1404600) and the Youth Innovation Promotion Association CAS (2014231) is gratefully acknowledged. L.L. thanks Yanqing Gong (SIOC) for assistance with the X-ray crystallographic analysis.
Footnotes
J.H. and F.W. have a patent on the synthetic application of CpFluors described in the manuscript: Method for preparing organic fluorides. Chinese Patent, CN 102285849A (2011).
Author contributions J.H. conceived the project. J.H., L.L. and C.N. designed the experiments, analysed the data and co-wrote the manuscript. L.L. and F.W. performed the synthetic and mechanistic experiments. All authors discussed the results and commented on the manuscript.
References
- Kirsch P. in Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications 2nd edn Wiley-VCH (2013). [Google Scholar]
- Ojima I. in Fluorine in Medicinal Chemistry and Chemical Biology Wiley-Blackwell (2009). [Google Scholar]
- Kirk K. L. Fluorination in medicinal chemistry: methods, strategies, and recent developments. Org. Process Res. Dev. 12, 305–321 (2008). [Google Scholar]
- Furuya T., Kamlet A. S. & Ritter T. Catalysis for fluorination and trifluoromethylation. Nature 473, 470–477 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. G. & Ritter T. Modern carbon–fluorine bond forming reactions for aryl fluoride synthesis. Chem. Rev. 115, 612–633 (2015). [DOI] [PubMed] [Google Scholar]
- Yang X., Wu T., Phipps R. J. & Toste F. D. Advances in catalytic enantioselective fluorination, mono-, di-, and trifluoromethylation, and trifluoromethylthiolation reactions. Chem. Rev. 115, 826–870 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatalova Sazepin C., Hemelaere R., Paquin J.-F. & Sammis G. Recent advances in radical fluorination. Synthesis 47, 2554–2569 (2015). [Google Scholar]
- Champagne P. A., Desroches J., Hamel J.-D., Vandamme M. & Paquin J.-F. Monofluorination of organic compounds: 10 years of innovation. Chem. Rev. 115, 9073–9174 (2015). [DOI] [PubMed] [Google Scholar]
- Singh R. P. & Shreeve J. M. Recent advances in nucleophilic fluorination reactions of organic compounds using Deoxofluor and DAST. Synthesis 34, 2561−2578 (2002). [Google Scholar]
- Al-Maharik N. & O'Hagan D. Organofluorine chemistry: deoxyfluorination reagents for C–F bond synthesis. Aldrichimica Acta 44, 65−75 (2011). [Google Scholar]
- Turcotte-Savard M.-O., Mahe O. & Paquin J.-F. Dialkylaminodifluorosulfinium tetrafluoroborate salts: synthesis and applications. Chim. Oggi 31, 14 (2013). [Google Scholar]
- Campbell M. G. & Ritter T. Late-stage fluorination: from fundamentals to application. Org. Process Res. Dev. 18, 474−480 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. C. et al. Fluorination reactions of sulfur tetrafluoride. J. Am. Chem. Soc. 81, 3165−3166 (1959). [Google Scholar]
- Middleton W. J. New fluorinating reagents. Dialkylaminosulfur fluorides. J. Org. Chem. 40, 574−578 (1975). [Google Scholar]
- Lal G. S., Pez G. P., Pesaresi R. J., Prozonic F. M. & Cheng H. Bis(2-methoxyethyl)aminosulfur trifluoride: a new broad-spectrum deoxofluorinating agent with enhanced thermal stability. J. Org. Chem. 64, 7048−7054 (1999). [Google Scholar]
- Beaulieu F. et al. Aminodifluorosulfinium tetrafluoroborate salts as stable and crystalline deoxofluorinating reagents. Org. Lett. 11, 5050−5053 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto T., Singh R. P., Xu Y. & Saito N. Discovery of 4-tert-butyl-2,6-dimethylphenylsulfur trifluoride as a deoxofluorinating agent with high thermal stability as well as unusual resistance to aqueous hydrolysis, and its diverse fluorination capabilities including deoxofluoro-arylsulfinylation with high stereoselectivity. J. Am. Chem. Soc. 132, 18199−18205 (2010). [DOI] [PubMed] [Google Scholar]
- Nielsen M. K., Ugaz C. R., Li W. & Doyle A. G. PyFluor: a low-cost, stable, and selective deoxyfluorination reagent. J. Am. Chem. Soc. 137, 9571–9574 (2015). [DOI] [PubMed] [Google Scholar]
- Ishikawa N., Iwakiri H. & Takaoka A. F-propene-dialkylamine reaction products as fluorinating agents. Bull. Chem. Soc. Jpn 52, 3377–3380 (1979). [Google Scholar]
- Kobayashi S., Yoneda A., Fukuhara T. & Hara S. Deoxyfluorination of alcohols using N,N-diethyl-α,α-difluoro-(m-methylbenzyl)-amine. Tetrahedron 60, 6923–6930 (2004). [Google Scholar]
- Bellavance G., Dubé P. & Nguyen B. Tetramethylfluoroformamidinium hexafluorophosphate (TFFH) as a mild deoxofluorination reagent. Synthesis 23, 569–572 (2012). [Google Scholar]
- Sladojevich F., Arlow S. I., Tang P. & Ritter T. Late-stage deoxyfluorination of alcohols with PhenoFluor. J. Am. Chem. Soc. 135, 2470–2473 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naredla R. R. & Klumpp D. A. Contemporary carbocation chemistry: applications in organic synthesis. Chem. Rev. 113, 6905–6948 (2013). [DOI] [PubMed] [Google Scholar]
- Sereda O., Tabassum S. & Wilhelm R. Lewis acid organocatalysts. Top. Curr. Chem. 291, 349–393 (2010). [DOI] [PubMed] [Google Scholar]
- Dolbier W. R. Jr & Battiste M. A. Structure, synthesis, and chemical reactions of fluorinated cyclopropanes and cyclopropenes. Chem. Rev. 103, 1071–1098 (2003). [DOI] [PubMed] [Google Scholar]
- Ni C. & Hu J. Recent advances in the synthetic application of difluorocarbene. Synthesis 46, 842–863 (2014). [Google Scholar]
- Crabbe P., Carpio H., Velarde E. & Fried J. H. Chemistry of difluorocyclopropenes. Application to the synthesis of steroidal allenes. J. Org. Chem. 38, 1478–1483 (1973). [Google Scholar]
- Bessard Y. & Schlosser M. gem-Difluorocyclopropenes by [1+2] cycloaddition reactions between difluorocarbene and acetylenes having terminal or internal triple bonds. Tetrahedron 47, 7323–7328 (1991). [Google Scholar]
- Xu W. & Chen Q.-Y. 3,3-Difluoro-1-iodocyclopropenes: a simple synthesis and their reactions. J. Org. Chem. 67, 9421–9427 (2002). [DOI] [PubMed] [Google Scholar]
- Cheng Z.-L. & Chen Q.-Y. Difluorocarbene chemistry: synthesis of gem-difluorocyclopropenylalkynes and 3,3,3′,3′-tetrafluorobicyclopropyl-1,1′-dienes. J. Fluorine Chem. 126, 39–43 (2005). [Google Scholar]
- Cheng Z.-L. & Chen Q.-Y. Chemistry of difluorocarbene adducts: stereospecific synthesis of conjugated fluoroolefins and 1,3-azabutadienes from gem-difluorocyclopropenyl ketones. J. Fluorine Chem. 127, 894–900 (2006). [Google Scholar]
- Ni F. et al. Reduction of fluorinated cyclopropene by nitrogenase. J. Am. Chem. Soc. 135, 10346–10352 (2013). [DOI] [PubMed] [Google Scholar]
- Kelly B. D. & Lambert T. H. Aromatic cation activation of alcohols: conversion to alkyl chlorides using dichlorodiphenylcyclopropene. J. Am. Chem. Soc. 131, 13930–13931 (2009). [DOI] [PubMed] [Google Scholar]
- Vanos C. M. & Lambert T. H. Development of a catalytic platform for nucleophilic substitution: cyclopropenone-catalyzed chlorodehydration of alcohols. Angew. Chem. Int. Ed. 50, 12222–12226 (2011). [DOI] [PubMed] [Google Scholar]
- Wang F. et al. Chloride ion-catalyzed generation of difluorocarbene for efficient preparation of gem-difluorinated cyclopropenes and cyclopropanes. Chem. Commun. 47, 2411−2413 (2011). [DOI] [PubMed] [Google Scholar]
- Wang F. et al. Synthesis of gem-difluorinated cyclopropanes and cyclopropenes:trifluoromethyltrimethylsilane as a difluorocarbene source. Angew. Chem. Int. Ed. 50, 7153−7157 (2011). [DOI] [PubMed] [Google Scholar]
- Li L., Wang F., Ni C. & Hu J. Synthesis of gem-difluorocyclopropa(e)nes and O-, S-, N-, and P-difluoromethylated compounds with TMSCF2Br. Angew. Chem. Int. Ed. 52, 12390−12394 (2013). [DOI] [PubMed] [Google Scholar]
- Hardee D. J., Kovalchuke L. & Lambert T. H. Nucleophilic acyl substitution via aromatic cation activation of carboxylic acids: rapid generation of acid chlorides under mild conditions. J. Am. Chem. Soc. 132, 5002–5003 (2010). [DOI] [PubMed] [Google Scholar]
- Hu J., Wang F., Hu M., Shen X. & Luo T. Method for preparing organic fluorides. Chinese Patent CN 102285849A (2011). [Google Scholar]
- Nakamura M., Isobe H. & Nakamura E. Cyclopropenone acetals: synthesis and reactions. Chem. Rev. 103, 1295–1326 (2003). [DOI] [PubMed] [Google Scholar]
- Boger D. L. & Brotherton C. E. Thermal reactions of cyclopropenone ketals. Key mechanistic features and scope of the cycloaddition reactions of delocalized singlet vinylcarbenes: three-carbon 1,1-/1,3-dipoles. J. Am. Chem. Soc. 108, 6695–6713 (1986). [Google Scholar]
- Tokuyama H., Isaka M. & Nakamura E. Thermal reactions of substituted cyclopropenone acetals. Regio- and stereochemistry of vinylcarbene formation and low-temperature [3+2] cycloaddition. J. Am. Chem. Soc. 114, 5523–5530 (1992). [Google Scholar]
- Kerber R. C. & Hsu C.-M. Substituent effects on cyclopropenium ions. J. Am. Chem. Soc. 95, 3239–32450 (1973). [Google Scholar]
- Bredt J. Über sterische Hindering in Brückenringen (Bredtsche regel) und über die meso-trans-Stellung in kondensierten Ringsystemen des Hexamethylens. Liebigs Ann. Chem. 437, 1–13 (1924). [Google Scholar]
- Petrov V. A., Swearingen S., Hong W. & Petersen W. C. 1,1,2,2-Tetrafluoroethyl-N,N-dimethylamine: a new selective fluorinating agent. J. Fluorine Chem. 109, 25–31 (2001). [Google Scholar]
- Shellhamer D. F. et al. Reaction of diethylaminosulfur trifluoride with diols. J. Chem. Soc. Perkin Trans. 2, 861−866 (1995). [Google Scholar]
- Ye C. & Shreeve J. M. Rearrangements accompanying fluorination of 2-amino alcohols and 1,2-diols with Deoxo-FluorTM. J. Fluorine Chem. 125, 1869−1872 (2004). [Google Scholar]
- Yoneda A., Fukuhara T. & Hara S. Selective monofluorination of diols using DFMBA. Chem. Commun. 41, 3589−3590 (2005). [DOI] [PubMed] [Google Scholar]
- Suwada M., Fukuhara T. & Hara S. Selective mono-fluorination of diols via a cyclic acetal of N,N-diethyl-4-methoxybenzamide. J. Fluorine Chem. 128, 1121−1125 (2007). [Google Scholar]
- Eras J., Méndez J. J., Balcells M. & Canela R. Chlorotrimethylsilane: a suitable reagent for the synthesis of chlorohydrin esters. J. Org. Chem. 67, 8631–8634 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-227, Supplementary Tables 1-19, Supplementary Methods and Supplementary References.
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. For the experimental procedures, and spectroscopic and physical data of compounds, see Supplementary Methods. For NMR analysis of the compounds in this article, see Supplementary Figs 1–222 and Fig 227. The CCDC 1443649, CCDC 1443627, CCDC 1443644 and CCDC 1443654 contain the crystallographic data for compounds 3m, 3r, 3t and 7j, respectively (Supplementary Figs 223–226; Supplementary Tables 4–19). These data can be obtained free of charge from the Cambridge Crystallographic Data Center (www.ccdc.cam.ac.uk).