Abstract
Objective
Since the introduction of diffusion tensor imaging, white matter abnormalities in epilepsy have been studied extensively. However, the affected areas reported, the extent of abnormalities and the association with relevant clinical parameters are highly variable. We aimed to obtain a more consistent estimate of white matter abnormalities and their association with clinical parameters in different epilepsy types.
Methods
We systematically searched for differences in white matter fractional anisotropy and mean diffusivity, at regional and voxel level, between people with epilepsy and healthy controls. Meta-analyses were used to quantify the directionality and extent of these differences. Correlations between white matter differences and age of epilepsy onset, duration of epilepsy and sex were assessed with meta-regressions.
Results
Forty-two studies, with 1027 people with epilepsy and 1122 controls, were included with regional data. Sixteen voxel-based studies were also included. People with temporal or frontal lobe epilepsy had significantly decreased fractional anisotropy (Δ –0.021, 95% confidence interval –0.026 to –0.016) and increased mean diffusivity (Δ0.026 × 10–3 mm2/s, 0.012 to 0.039) in the commissural, association and projection white matter fibers. White matter was much less affected in generalized epilepsy. White matter changes in people with focal epilepsy correlated with age at onset, epilepsy duration and sex.
Significance
This study provides a better estimation of white matter changes in different epilepsies. Effects are particularly found in people with focal epilepsy. Correlations with the duration of focal epilepsy support the hypothesis that these changes are, at least partly, a consequence of seizures and may warrant early surgery. Future studies need to guarantee adequate group sizes, as white matter differences in epilepsy are small.
Keywords: Focal epilepsy, Generalized epilepsy, White matter abnormalities, Meta-analysis, Meta-regression, Diffusion tensor imaging
Highlights
-
•
White matter FA and MD are more affected in focal than in generalized epilepsy.
-
•
Epilepsy subtypes show distinct patterns of affected white matter regions.
-
•
White matter integrity is altered both ipsi- and contralaterally in focal epilepsy.
-
•
White matter changes in focal epilepsy seem to be a consequence of seizures.
1. Introduction
Epilepsy has long been considered a grey-matter only disease. With the introduction of diffusion tensor imaging, allowing the non-invasive quantification of white matter integrity throughout the brain, region-specific as well as global white matter integrity changes were also found in people with epilepsy (Arfanakis et al., 2002, Concha et al., 2005, Concha et al., 2007, Gross et al., 2006). Decreased fractional anisotropy (FA) and increased mean diffusivity (MD) was shown for a wide variety of white matter fiber bundles in focal epilepsy (Concha et al., 2005, Meng et al., 2010a) but also in generalized epilepsy syndromes, particularly juvenile myoclonic epilepsy (Deppe et al., 2008, Keller et al., 2011, Liu et al., 2011).
Fractional anisotropy quantifies the preferred direction, and MD the average extent, of water diffusion in white matter. Both measures are proxies for underlying tissue integrity. The pathophysiologic mechanism of FA and MD changes in epilepsy remains unknown, but changes could be explained by i) the initial epileptogenic lesion that underlies and precedes the seizure onset, ii) the direct effect of axonal damage in ipsilateral white matter, iii) repetitive seizure spread and distal effects of frequent interictal spikes propagating through white matter parts of the epileptic network (Otte et al., 2012b), and iv) altered brain development and plasticity-related reorganization of local and global white matter structures. Despite these mechanistic uncertainties it is clinically relevant to know where, and to what extent, white matter is damaged in the brain across different epilepsy types. That information would increase our understanding of the potential detrimental effects of recurrent seizures and may serve as a quantitative biomarker in future treatment studies. Currently, no conclusive overview of data is available from individual studies that reliably quantifies the spatial characteristics and severity of white matter change.
Combining all diffusion data from single studies and correcting for between-study heterogeneity may enhance statistical power, reduce variability and allow more robust correlations with relevant clinical outcome parameters. In this systematic review with random and mixed-effects meta-analysis we combined evidence from all available individual studies with regional and voxel-based data and addressed the following questions: i) Is epilepsy associated with brain-wide white matter diffusivity change?, ii) Is this effect stronger in focal epilepsy in comparison to generalized epilepsy?, iii) Which white matter regions are affected in focal and generalized epilepsy and how do these regions correspond with regions found to be affected in voxel-based analyses?, iv) Is white matter diffusivity in focal epilepsy affected differently in the hemisphere ipsilateral to the epileptogenic zone in comparison to the contralateral hemisphere?, v) Are age at epilepsy onset, duration of epilepsy and sex related to the extent of white matter diffusivity changes?, and vi) How are individual studies statistically powered given the aggregated meta-analysis effect-sizes and an interest in global white matter differences?
We systematically reviewed existing literature on white matter FA and MD changes at a regional and voxel level in people with temporal lobe epilepsy (TLE), frontal lobe epilepsy (FLE), benign epilepsy with centro-temporal spikes (BECTS), and idiopathic-generalized epilepsy, in comparison to healthy controls. Sex, age at onset and duration of epilepsy were related to the extent of epilepsy-associated white matter changes, globally and region-specific. We expected i) changes in FA and MD to be more pronounced in focal epilepsy compared to generalized types of epilepsy, ii) focal and generalized epilepsies to show different patterns of affected white matter fibers, iii) the severity of changes to be associated with the distance to the epileptogenic zone (i.e. ipsilateral white matter affected more than contralateral white matter), and iv) the extent of white matter changes to be related to the disease duration and age of onset, but not to sex.
2. Methods
2.1. Information source and search
Studies were identified by searching the database PubMed (NCBI) with the following query: epilepsy AND (dwi OR dti OR dki OR “diffusion weighted imaging” OR “diffusion tensor imaging” OR “diffusion kurtosis imaging” OR “diffusion mri” OR “diffusion magnetic resonance imaging”) AND (“white matter lesions” OR “white matter abnormalities” OR “white matter anomalies” OR microstructural). Diffusion kurtosis imaging was added to the query as this technique extends tensor imaging but may provide similar FA and MD information. The search date was February 11th, 2016. Additional studies were identified by screening reference lists of downloaded full text articles.
2.2. Inclusion criteria
We included studies that reported regional or voxel-based data. Regional data – obtained from reported manual or automatic area delineations – were used for quantitative meta-analysis. Complementary spatial characteristics on white matter changes were extracted from voxel-based studies. Voxel-based studies are available in two flavors and may either compare all FA values from the central areas within all white matter bundles, called tract-based spatial statistics (Smith et al., 2006), or spatially normalize all FA voxels to an atlas, called voxel-based morphometry (Ashburner and Friston, 2000).
Studies meeting the following criteria were included:
-
-
Study design: cross-sectional or longitudinal design, including a control group, investigating the relation between any form of epilepsy and white matter properties by performing diffusion tensor imaging or diffusion kurtosis imaging (with acquisition always prior to possible epilepsy surgery).
-
-
Analysis method: white matter region-of-interest analysis, summary data on specific tracts, or voxel-based analysis (included for qualitative description).
-
-
Subjects: participants of any age and with any form of epilepsy.
-
-
Outcome measures: mean or median FA and MD or apparent diffusion coefficient with uncertainty (only for articles included for our meta-analysis) reported as standard deviation, interquartile range, standard error of the mean, standard error or 95% confidence interval (CI). If published studies provided outcome measures as charts, data were manually read and extracted from these charts. Trace apparent diffusion coefficient values were converted to MD values.
2.3. Study selection and data collection
One reviewer (GS) performed the literature search. All titles and abstracts, as well as the full-text versions of potentially relevant articles, were independently screened by two reviewers (GS and MRTS). Disagreements were discussed together with WMO to resolve them. Data extraction was carried out by one reviewer (GS) and checked at random data entries by two others (MRTS and WMO).
2.4. Data items
From all studies included in the meta-analysis, the following general data were extracted: first author, journal, year of publication, characteristics of people with epilepsy and controls (number of participants, type of epilepsy, sex, age at investigation, age at epilepsy onset and epilepsy duration), diffusion parameters (field strength, highest b-value, number of diffusion-weighed directions and slice thickness) and the mean/median with corresponding uncertainty of FA and MD values for the different white matter regions in the ipsilateral and contralateral hemisphere (if applicable and reported). These values, extracted from the papers' main texts, tables or figures, are typically reported for white matter structures as a whole and do not take into account within-structure heterogeneity. For the general analysis we labeled studies on BECTS, FLE and TLE as ‘focal epilepsy’ and studies on IGE and JME as ‘generalized epilepsy’.
Voxel-based studies commonly present changes in FA or MD as brain-wide p-value maps. We therefore extracted for each of these studies which white matter regions – defined similarly as in the regional meta-analysis – were significantly affected (i.e. p-value < 0.05). We summed for each white matter region how many voxel-based studies found significant white matter changes.
2.5. Methods of analysis
We performed meta-analyses to quantify regional white matter changes with multivariate random and mixed-effects meta-analysis (R package metaphor (Viechtbauer, 2010)). This analysis allows pooling of data obtained from studies with similar populations and provides a powerful mean to better estimate the extent of white matter alterations and their correlations with clinical parameters (Viechtbauer, 2010). First, effect sizes were estimated for FA and MD separately for the pooled data of all studies, irrespective of epilepsy type. This provides brain-wide estimates on FA and MD differences between people with epilepsy and controls. Next, the models were extended with distinct effect modifiers to obtain answers on our specific research questions. Effect modifiers included i) epilepsy type (i.e. BECTS, FLE, TLE, and ‘generalized epilepsy’), ii) binary epilepsy type (i.e. ‘focal epilepsy’ and ‘generalized epilepsy’), iii) side relative to epileptogenic zone (i.e. contralateral and ipsilateral hemisphere), iv) white matter categories (i.e. cerebellum/brainstem, commissural fibers, association fibers and projection fibers), v) white matter categories subdivided into smaller white matter regions (i.e. cerebellar regions: brainstem/cerebellum; commissural fibers: corpus callosum, anterior commissure and fornix; association fibers: arcuate fasciculus, parahippocampal fasciculus, superior longitudinal fasciculus, inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, uncinated fasciculus, cingulum, fronto-parietal tract, temporal lobe, occipital lobe, parietal lobe and frontal lobe; projection fibers: thalamo-cortical radiations, corona radiata, corticospinal tract, supplementary motor area tract, external capsule, internal capsule and subcortical white matter), vi) age at epilepsy onset, vii) duration of epilepsy, and viii) percentage males in epilepsy group. Effect modifier effects were considered statistically significant at a p-value of < 0.05.
2.6. Power analysis
Power analyses were performed with the R package pwr (https://cran.r-project.org/web/packages/pwr/index.html). Input was based on a meta-analysis with adjusted outcome. In this adjustment the summary estimate is expressed as ‘standardized mean difference’ rather than ‘mean difference’. This normalization step removes unwanted scaling differences between FA and MD. Based on the whole-brain standardized mean FA and MD difference – reflecting the best estimates of the (unknown) ground truth – the actual power of each individual study was determined with respect to detecting brain-wide white matter changes irrespective of specific a priori defined regional hypotheses. We assumed a two samples t-tests of means as the statistical model with two-sided testing at significance-level 0.05 taking into account the actual epilepsy and control group sizes. Similarly, we determined the minimal sample size – useful for future studies on white matter diffusion quantification – given a statistical power of 0.8 and 0.9 and assuming equal group sizes to detect changes in white matter in people with epilepsy.
3. Results
The study selection flow chart is shown in Fig. 1. We identified 214 potentially relevant articles. Full text was screened in 71 cases and reference lists were inspected to detect additional studies not identified with the initial search. Forty-two studies met the inclusion criteria for the meta-analysis (Ahmadi et al., 2009, Andrade et al., 2014, Arfanakis et al., 2002, Braakman et al., 2014, Campos et al., 2015, Concha et al., 2005, Concha et al., 2007, Concha et al., 2009, Deppe et al., 2008, Diao et al., 2015, Diehl et al., 2008, Duning et al., 2010, Gao et al., 2012, Govindan et al., 2008, Gross et al., 2006, Holt et al., 2011, Hutchinson et al., 2010, Keller et al., 2011, Keller et al., 2012, Kemmotsu et al., 2011, Kemmotsu et al., 2014, Kim et al., 2011, Knake et al., 2009, Kori et al., 2013, Labate et al., 2015, Lee et al., 2013, Lee et al., 2014, Li et al., 2010, Liacu et al., 2012, Lin et al., 2008, Liu et al., 2011, Mao et al., 2011, McDonald et al., 2008, Meng et al., 2010a, Nilsson et al., 2008, Powell et al., 2007, Pustina et al., 2015, Rodrigo et al., 2007, Scanlon et al., 2013, Vulliemoz et al., 2011, Wang et al., 2010, Xiao et al., 2014). The total number of people with epilepsy and controls was 1027 and 1122, respectively. Sixteen voxel-based studies were included, with 312 patients and 500 controls (Amarreh et al., 2014, Bonilha et al., 2015, Concha et al., 2012, Focke et al., 2014, Keller et al., 2013, Kim et al., 2012, Kim et al., 2014, Li et al., 2010, Liu et al., 2015, O'Muircheartaigh et al., 2011, Peng et al., 2014, Riley et al., 2010, Rugg-Gunn et al., 2001, Wang et al., 2011, Whelan et al., 2015, Yang et al., 2012). One of these studies was also included in the meta-analysis (Li et al., 2010). Demographic information and acquisition characteristics of all included regional and voxel-based studies are shown in Suppl. Tables 1 and 2, respectively.
Fig. 1.
Flow chart of the identification of studies for the meta-analysis and qualitative description. FA, fractional anisotropy; MD, mean diffusivity.
3.1. Meta-analysis
Mixed effects meta-analysis was first performed on the pooled FA and MD data from all studies included, independent of the type of epilepsy. Presented values are FA and MD differences with respect to control data. White matter FA was significantly reduced in people with epilepsy as compared to controls: –0.020 (95% CI –0.025 to –0.016; p < 0.001) and the MD was significantly increased: 0.025 (95% CI 0.012 to 0.037; p < 0.001).
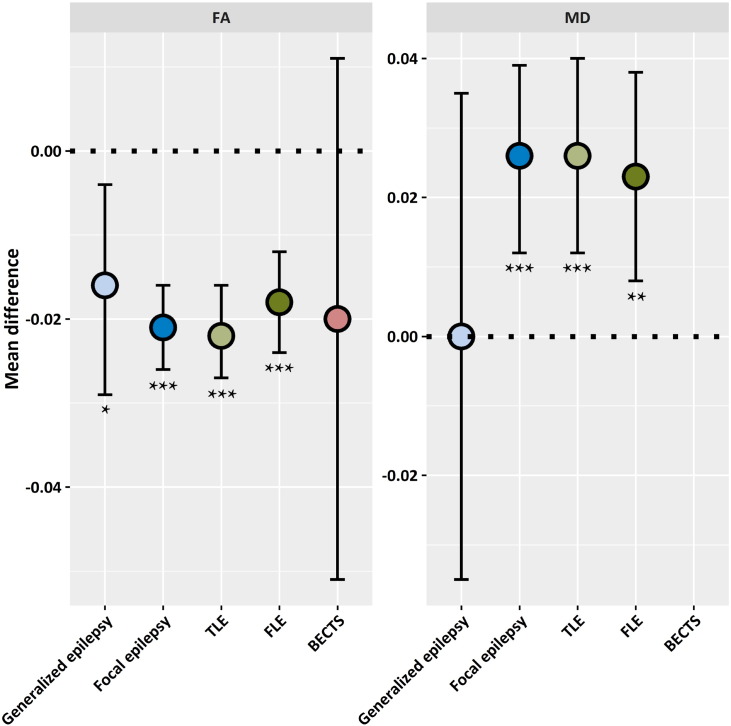
Separate analyses of ‘focal epilepsy’ and ‘generalized epilepsy’ studies revealed that whole brain white matter FA was more affected in focal epilepsy: –0.021 (95% CI –0.026 to –0.016; p < 0.001) than in generalized epilepsy: –0.016 (95% CI –0.029 to –0.004; p = 0.010). Moreover, MD values were significantly increased in focal epilepsy: 0.025 (95% CI 0.012 to 0.039; p < 0.001), but not in generalized epilepsy: 0.0002 (95% CI –0.035 to 0.035; p = 0.99). Although the diffusivity was significantly changed in focal epilepsies as a group, in one sub-type of focal epilepsy, benign epilepsy with centro-temporal spikes (BECTS), FA was not significantly altered (Fig. 2).
Fig. 2.
Mean fractional anisotropy (FA; left) and mean diffusivity (MD; right) differences between people with epilepsy and controls (reference-group; indicated with dotted line). Error bars represent the 95% confidence intervals. BECTS, benign epilepsy with centro-temporal spikes; FLE, frontal lobe epilepsy; TLE, temporal lobe epilepsy; * = p < 0.05; ** = p < 0.01; *** = p < 0.001. [No MD estimate available for BECTS (not reported).]
Mixed effects meta-analysis results for the effects of epilepsy types on four white matter categories are shown in Table 1. Diffusivity changes were predominantly seen in TLE and FLE for all reported white matter categories. In generalized epilepsy there was only a significant FA reduction in the projection fibers: –0.020 (95% CI –0.036 to –0.005; p = 0.010). Imaging data from patients with BECTS did, again, not show significant changes.
Table 1.
Mean fractional anisotropy (ΔFA; left) and mean diffusivity (ΔMD; right) differences between people with epilepsy and controls (reference-group) per white matter category (hemispheres pooled).
| Category | ΔFA | 95% CI | p-Value | ΔMD | 95% CI | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| Focal | |||||||||
| TLE | Commissural fibers | –0.020 | –0.026 | –0.014 | < 0.001 | 0.024 | 0.009 | 0.039 | 0.002 |
| Association fibers | –0.021 | –0.027 | –0.016 | < 0.001 | 0.028 | 0.014 | 0.042 | < 0.001 | |
| Projection fibers | –0.025 | –0.031 | –0.019 | < 0.001 | 0.017 | 0.002 | 0.031 | 0.03 | |
| Cerebellum/brainstem | –0.033 | –0.051 | –0.015 | < 0.001 | 0.066 | 0.012 | 0.120 | 0.02 | |
| FLE | Commissural fibers | –0.014 | –0.022 | –0.006 | < 0.001 | 0.046 | 0.013 | 0.079 | 0.006 |
| Association fibers | –0.019 | –0.026 | –0.012 | < 0.001 | 0.025 | 0.010 | 0.039 | 0.001 | |
| Projection fibers | –0.021 | –0.029 | –0.013 | < 0.001 | 0.031 | 0.015 | 0.046 | < 0.001 | |
| Cerebellum/brainstem | – | – | – | – | – | – | – | – | |
| BECTS | Commissural fibers | –0.020 | –0.052 | 0.012 | 0.22 | – | – | – | – |
| Association fibers | – | – | – | – | – | – | – | – | |
| Projection fibers | – | – | – | – | – | – | – | – | |
| Cerebellum and brainstem | – | – | – | – | – | – | – | – | |
| Generalized | Commissural fibers | –0.017 | –0.044 | 0.010 | 0.21 | – | – | – | – |
| Association fibers | –0.009 | –0.029 | 0.011 | 0.39 | – | – | – | – | |
| Projection fibers | –0.020 | –0.036 | –0.005 | 0.010 | 0.003 | –0.032 | 0.038 | 0.86 | |
| Cerebellum/brainstem | –0.010 | –0.028 | 0.008 | 0.30 | – | – | – | – | |
95% CI, 95% confidence interval; BECTS, benign epilepsy with centro-temporal spikes; FLE, frontal lobe epilepsy; Focal, focal epilepsy; Generalized, generalized epilepsy; TLE, temporal lobe epilepsy. ‘–’ indicates that no data is available. Significant p-values are typeset in bold.
White matter categories were further analyzed at the level of individual white matter regions for all different types of epilepsy. Results for ‘focal epilepsy’ are provided in Supp. Table 3. Results for ‘generalized epilepsy’ and provided in Suppl. Table 4. In TLE, FA but not MD, was significantly changed in all white matter regions. People with FLE showed significantly lower FA values in the fornix: –0.019 (95% CI –0.027 to –0.011; p < 0.001), cingulum: –0.027 (95% CI –0.034 to –0.019; p < 0.001), inferior fronto-occipital fasciculus: –0.026 (95% CI –0.035 to –0.017; p < 0.001) and uncinate fasciculus: –0.015 (95% CI –0.024 to –0.006; p = 0.002). MD was significantly increased in the fornix: 0.053 (95% CI 0.018 to 0.087; p = 0.003), cingulum: 0.036 (95% CI 0.017 to 0.055; p < 0.001) and inferior fronto-occipital fasciculus: 0.034 (95% CI 0.013 to 0.054; p = 0.001). Patients with BECTS or generalized epilepsy did not have significant white matter changes in this analysis.
In the ‘focal epilepsy’ studies, white matter regions can be either in the ipsilateral or the contralateral hemisphere with respect to the epileptogenic zone (Suppl. Table 5). Mixed effects meta-analysis on the overall effect in ‘focal epilepsy’ on total ipsilateral and contralateral white matter showed significantly reduced FA values both ipsilaterally: –0.023 (95% CI –0.023 to –0.029; p < 0.001) and contralaterally: –0.019 (95% CI –0.024 to –0.013; p < 0.001). MD was also significantly increased for the ipsilateral: 0.045 (95% CI 0.027 to 0.060; p < 0.001) as well as for the contralateral white matter: 0.030 (95% CI 0.013 to 0.046; p < 0.001).
3.2. Voxel-based studies
The scoring of voxel-based studies included ten studies on focal epilepsy, five on generalized epilepsy and one on both focal and generalized epilepsy (Table 2). The TLE studies most consistently reported a decrease in FA in the corpus callosum, the cerebellum, the uncinate fasciculus, and temporal lobe. An increase in MD was most often reported for the corpus callosum and temporal lobe. Studies on generalized epilepsy most frequently reported a reduction in FA in the corpus callosum, cingulum, corona radiata, and white matter of the parietal lobe and an increase in MD in both the corpus callosum and parietal lobe white matter.
Table 2.
White matter regions reported as changed in fractional anisotropy (FA) or mean diffusivity (MD) in 16 voxel-based studies.
| Epilepsy | N | Regions with decrease in FA | Regions with increase in MD |
|---|---|---|---|
| BECTS | 1 | – | Internal capsule, superior longitudinal fasciculus, thalamo-cortical radiations (100%) |
| FLE | 1 | External capsule (100%) | Frontal lobe (100%) |
| TLE | 7 | Arcuate fasciculus, thalamo-cortical radiations (14%); cortico-spinal tracts, external capsule, fornix, internal capsule, inferior longitudinal fasciculus, motor projections, superior longitudinal fasciculus (29%); cerebellum, temporal lobe, uncinate fasciculus (43%); corpus callosum (57%) | Arcuate fasciculus, brainstem, cortico-spinal tract, fornix, external capsule, internal capsule, inferior fronto-occipital fasciculus, superior longitudinal fasciculus (14%); inferior longitudinal fasciculus, uncinate fasciculus (29%); temporal lobe (43%); corpus callosum (57%) |
| Partial | 1 | Fornix, thalamo-cortical radiations (100%) | Fornix (100%) |
| Generalized | 5 | Brainstem, cerebellum, cortico-spinal tract, frontal lobe, internal capsule, inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, parahippocampal fasciculus, superior longitudinal fasciculus, thalamo-cortical radiations (20%); cingulum, corona radiata, parietal lobe (40%); corpus callosum (60%) | Cerebellum, cingulum, corona radiata, cortico-spinal tract, frontal lobe, internal capsule, inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, superior longitudinal fasciculus, thalamo-cortical radiations (20%); corpus callosum, parietal lobe (40%) |
| Various | 1 | Cingulum, fornix (100%) | Corpus callosum, thalamo-cortical radiations (100%) |
BECTS, benign epilepsy with centro-temporal spikes; FLE, frontal lobe epilepsy; Generalized, generalized epilepsy; N, number; Partial, different types of partial epilepsy; TLE, temporal lobe epilepsy; Various, generalized and partial epilepsy together. Data given as part (%) of total number of included voxel-based studies on the same type of epilepsy (N).
3.3. Meta-regression
Meta-regression results are presented in Table 3. Age and onset of epilepsy were negatively correlated in people with TLE with an overall FA difference and positively correlated with an overall MD difference. In FLE a significantly positive correlation was found between age of disease onset and MD difference. Furthermore, this analysis revealed that disease duration, in both TLE and FLE, was positively correlated with an overall difference in both FA and MD. There was also a significantly positive correlation between the proportion of males and the extent of white matter diffusivity changes (FA and MD) in people with TLE, FLE or generalized epilepsy.
Table 3.
Regression coefficient (β) of mean fractional anisotropy (ΔFA; left) and mean diffusivity (ΔMD; right) differences and age at epilepsy onset, epilepsy duration and male sex.
| β ΔFA | 95% CI | p-Value | β ΔMD | 95% CI | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| Age at epilepsy onset | ||||||||
| TLE | –0.0003 | –0.0005 | –0.0001 | 0.008 | 0.0009 | 0.0004 | 0.0013 | < 0.001 |
| FLE | –0.0002 | –0.0004 | 0.0000 | 0.11 | 0.0009 | 0.0004 | 0.0014 | < 0.001 |
| BECTS | –0.0025 | –0.0065 | 0.0015 | 0.23 | – | – | – | – |
| Generalized | –0.0003 | –0.0007 | 0.0002 | 0.22 | 0.0000 | –0.0009 | 0.0010 | 0.93 |
| Epilepsy duration | ||||||||
| TLE | 0.0022 | 0.0019 | 0.0024 | < 0.001 | 0.0023 | 0.0015 | 0.0031 | < 0.001 |
| FLE | 0.0022 | 0.0020 | 0.0025 | < 0.001 | 0.0021 | 0.0013 | 0.0029 | < 0.001 |
| BECTS | – | – | – | – | – | – | – | – |
| Generalized | –0.0020 | –0.0060 | 0.0021 | 0.34 | – | – | – | – |
| Sex (male) | ||||||||
| TLE | 0.0011 | 0.0010 | 0.0012 | < 0.001 | –0.0008 | –0.0012 | –0.0004 | < 0.001 |
| FLE | 0.0010 | 0.0008 | 0.0011 | < 0.001 | –0.0007 | –0.0011 | –0.0003 | < 0.001 |
| BECTS | 0.0004 | –0.0013 | 0.0020 | 0.67 | – | – | – | – |
| Generalized | 0.0008 | 0.0003 | 0.0013 | 0.001 | –0.0013 | –0.0024 | –0.0003 | 0.01 |
95% CI, 95% confidence interval; BECTS, benign epilepsy with centro-temporal spikes; FLE, frontal lobe epilepsy; Generalized, generalized epilepsy; TLE, temporal lobe epilepsy. Significant p-values are typeset in bold.
3.4. Power analysis
Hypothesis-free analysis of brain-wide white matter changes between groups (i.e., without a priori focus on one or two regions of interest) would render, based on the global aggregated meta-analysis estimates, approximately 31% of studies reporting ΔFA and 9% of studies reporting ΔMD statistically sufficiently powered. The actual power of all included studies had a mean ± standard deviation of 0.68 ± 0.19 for studies on FA differences and 0.49 ± 0.18 for studies on MD differences. The minimal sample size per group to achieve a power of at least 0.80 was 21 (with outcome overall FA difference) and 32 (with outcome overall MD difference) for diffusion tensor assessments of white matter in people with epilepsy. Twenty-eight (FA) and 42 (MD) subjects per single group are required if the power is set to 0.9.
4. Discussion
In this systematic review with quantitative meta-analysis based on data from 2149 subjects we have assessed the possible association of several epilepsy subtypes with changes in local and global white matter integrity as measured with diffusion tensor imaging. The overall white matter FA is significantly reduced in epilepsy, whereas the overall MD is significantly increased. In focal epilepsy white matter changes are more pronounced than in generalized epilepsy. We also demonstrated distinct patterns of white matter abnormalities across different epilepsy subtypes. White matter changes in focal epilepsy were significantly associated with age at epilepsy onset, epilepsy duration and male sex.
A previous meta-analysis, based on fewer studies, specifically assessed white matter changes in temporal lobe epilepsy only (Otte et al., 2012a). The majority of white matter regions found to be significantly affected in the current study is in line with this previous meta-analysis. We now pooled more data and were able to identify additional affected regions, including the arcuate fasciculus, corticospinal tract, and cingulum. No previous white matter meta-analyses had previously been performed on epilepsy subtypes other than TLE.
The consistent widespread white matter alterations in people with focal epilepsy are relevant in the search of biological substrates underlying cognitive changes. Previous papers have linked results from cognitive tests across distinct domains with white matter modifications (Diao et al., 2015, McDonald et al., 2008, Riley et al., 2010). For instance, diffusivity changes in the uncinate fasciculus and inferior fronto-occipital fasciculus correlated with commonly reported cognitive comorbidities such as impaired memory and altered executive functioning (McDonald et al., 2008, Riley et al., 2010). Our study provides more accurate estimates of the degree to which these regions are affected, and will allow cognitive studies in people with epilepsy to tailor their assessments on white matter substrates with respect to effect sizes and spatial locations.
In focal epilepsy, white matter diffusivity changes were not restricted to the ipsilateral hemisphere, although the effect sizes were smaller in areas remote from the epileptogenic zone. The exact mechanism underlying these bilateral and widespread white matter changes is unknown. However, since the extent of the changes differs between different focal epilepsy types and the severity of the changes differs between the ipsi- and contralateral hemisphere in TLE, it seems likely that the underlying mechanisms are linked with intrinsic processes, including frequent seizure propagation, rather than external factors, such as treatment with antiepileptic drugs. Our regression analysis with age at onset indicates that diagnosis of focal epilepsy at young age and longer duration of epilepsy results in larger white matter FA and MD changes. This effect was not found in people with generalized epilepsy. These findings provide new support for the hypothesis that local and remote white matter changes in focal epilepsy are – at least to some extent – a consequence of recurrent seizures, as previously suggested based on preclinical epilepsy rodent models (Otte et al., 2012b). These relationships remained inconsistent in individual human studies with smaller sample sizes (Andrade et al., 2014, Gross et al., 2006, Liu et al., 2011, Meng et al., 2010b). Our results implicate that early seizure control, pharmacologically or surgically, is important to inhibit progression of white matter alterations.
The relative low statistical powers, given an interest in detecting brain-wide white matter changes between groups, is in line with data that showed the average statistical power of studies in neurosciences to be very low (Button et al., 2013). Low statistical power reduces the chance of detecting a true effect and results in overestimating effect sizes and consequently low reproducibility of results (Button et al., 2013, Ioannidis, 2005). With pooling of evidence from individual studies into aggregated effect estimates, we overcame this low power. However, correcting for potential confounders at subject level or correlating individual demographic factors with white matter values is not possible with a standard meta-analysis. Even when an underpowered study discovers a true effect, it is likely that this effect will be overestimated (Button et al., 2013). This could be an explanation for the inconsistencies discussed here between individual studies' results (including the results of the voxel-based studies) and the meta-analysis results. We should note that powers were determined with effect sizes based on brain-wide white matter changes. Not all studies focused primarily on brain-wide changes. Effect sizes calculated from ipsilateral white matter structures are larger. Nonetheless, pooling of data in a meta-analysis is highly desirable to boost the statistical power. Individual patient data (IPD) meta-analysis (Simmonds et al., 2005) would potentially resolve this issue, but requires collecting individuals' scans from the original datasets, which is hard to do in practice. Sharing of data in open-access repositories is strongly suggested to allow future pooling and analysis with sufficient statistical power.
Our study has limitations. Although we corrected for statistical heterogeneity, there was a considerable variability in age, age at onset, and epilepsy duration between studies. Acquisition parameters also varied between studies. Slice thickness and the number of diffusion-weighed directions may have influenced the accuracy of FA and MD values. Variability is also expected in the size and exact 3D location of white matter regions. Within-structure variation remains thus unmeasured. Voxel-based meta-methods would take this local variation into account, but require access to the raw FA and MD voxel data. We were unable to fully differentiate epilepsy subtypes due to missing detailed data on epilepsy characteristics in some studies and not all authors separated ipsilateral and contralateral white matter, resulting in less data for this particular meta-analysis.
This study has implications for further research. The systematic overview reveals that the majority of white matter assessments in the last decades has been limited to temporal lobe epilepsy. More data, obtained with sufficiently powered studies, from other focal and general epilepsy types would greatly help to increase the accuracy of the white matter characterization throughout the brain. These studies should also systematically assess white matter regions both in close vicinity of the epileptogenic zone as well as remote regions. More detailed and standardized epilepsy characterization (e.g. mesial versus lateral temporal lobe epilepsy) would also help to further elucidate the complex relationship between seizures and white matter alterations.
A better understanding of white matter changes and their causes across epilepsy subtypes could lead to a better understanding of symptoms and the development of comorbidities. It may potentially facilitate in more personalized diagnosis and could serve as biomarker to evaluate treatments with respect to (preventing) brain damage.
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Acknowledgements
This work was supported by the Dutch National Epilepsy Fund [NEF 08-10, NEF 12-05]; the Netherlands Organisation for Scientific Research [VENI 016.168.038]; and the Dutch Brain Foundation [F2014(1)-06].
Footnotes
Supplementary data to this article can be found online at doi:10.1016/j.nicl.2016.10.025.
Appendix A. Supplementary data
Supplementary tables
References
- Ahmadi M.E., Hagler D.J., Jr., McDonald C.R., Tecoma E.S., Iragui V.J., Dale A.M., Halgren E. Side matters: diffusion tensor imaging tractography in left and right temporal lobe epilepsy. AJNR Am. J. Neuroradiol. 2009;30:1740–1747. doi: 10.3174/ajnr.A1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarreh I., Meyerand M.E., Stafstrom C., Hermann B.P., Birn R.M. Individual classification of children with epilepsy using support vector machine with multiple indices of diffusion tensor imaging. Neuroimage Clin. 2014;4:757–764. doi: 10.1016/j.nicl.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade C.S., Leite C.C., Otaduy M.C., Lyra K.P., Valente K.D., Yasuda C.L., Beltramini G.C., Beaulieu C., Gross D.W. Diffusion abnormalities of the corpus callosum in patients with malformations of cortical development and epilepsy. Epilepsy Res. 2014;108:1533–1542. doi: 10.1016/j.eplepsyres.2014.08.023. [DOI] [PubMed] [Google Scholar]
- Arfanakis K., Hermann B.P., Rogers B.P., Carew J.D., Seidenberg M., Meyerand M.E. Diffusion tensor MRI in temporal lobe epilepsy. Magn. Reson. Imaging. 2002;20:511–519. doi: 10.1016/s0730-725x(02)00509-x. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry – the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bonilha L., Lee C.Y., Jensen J.H., Tabesh A., Spampinato M.V., Edwards J.C., Breedlove J., Helpern J.A. Altered microstructure in temporal lobe epilepsy: a diffusional kurtosis imaging study. AJNR Am. J. Neuroradiol. 2015;36:719–724. doi: 10.3174/ajnr.A4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman H.M., Vaessen M.J., Jansen J.F., Debeij-van Hall M.H., de Louw A., Hofman P.A., Vles J.S., Aldenkamp A.P., Backes W.H. Pediatric frontal lobe epilepsy: white matter abnormalities and cognitive impairment. Acta Neurol. Scand. 2014;129:252–262. doi: 10.1111/ane.12183. [DOI] [PubMed] [Google Scholar]
- Button K.S., Ioannidis J.P., Mokrysz C., Nosek B.A., Flint J., Robinson E.S., Munafo M.R. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Campos B.M., Coan A.C., Beltramini G.C., Liu M., Yassuda C.L., Ghizoni E., Beaulieu C., Gross D.W., Cendes F. White matter abnormalities associate with type and localization of focal epileptogenic lesions. Epilepsia. 2015;56:125–132. doi: 10.1111/epi.12871. [DOI] [PubMed] [Google Scholar]
- Concha L., Beaulieu C., Gross D.W. Bilateral limbic diffusion abnormalities in unilateral temporal lobe epilepsy. Ann. Neurol. 2005;57:188–196. doi: 10.1002/ana.20334. [DOI] [PubMed] [Google Scholar]
- Concha L., Beaulieu C., Wheatley B.M., Gross D.W. Bilateral white matter diffusion changes persist after epilepsy surgery. Epilepsia. 2007;48:931–940. doi: 10.1111/j.1528-1167.2007.01006.x. [DOI] [PubMed] [Google Scholar]
- Concha L., Beaulieu C., Collins D.L., Gross D.W. White-matter diffusion abnormalities in temporal-lobe epilepsy with and without mesial temporal sclerosis. J. Neurol. Neurosurg. Psychiatry. 2009;80:312–319. doi: 10.1136/jnnp.2007.139287. [DOI] [PubMed] [Google Scholar]
- Concha L., Kim H., Bernasconi A., Bernhardt B.C., Bernasconi N. Spatial patterns of water diffusion along white matter tracts in temporal lobe epilepsy. Neurology. 2012;79:455–462. doi: 10.1212/WNL.0b013e31826170b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppe M., Kellinghaus C., Duning T., Moddel G., Mohammadi S., Deppe K., Schiffbauer H., Kugel H., Keller S.S., Ringelstein E.B., Knecht S. Nerve fiber impairment of anterior thalamocortical circuitry in juvenile myoclonic epilepsy. Neurology. 2008;71:1981–1985. doi: 10.1212/01.wnl.0000336969.98241.17. [DOI] [PubMed] [Google Scholar]
- Diao L., Yu H., Zheng J., Chen Z., Huang D., Yu L. Abnormalities of the uncinate fasciculus correlate with executive dysfunction in patients with left temporal lobe epilepsy. Magn. Reson. Imaging. 2015;33:544–550. doi: 10.1016/j.mri.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Diehl B., Busch R.M., Duncan J.S., Piao Z., Tkach J., Luders H.O. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia. 2008;49:1409–1418. doi: 10.1111/j.1528-1167.2008.01596.x. [DOI] [PubMed] [Google Scholar]
- Duning T., Kellinghaus C., Mohammadi S., Schiffbauer H., Keller S., Ringelstein E.B., Knecht S., Deppe M. Individual white matter fractional anisotropy analysis on patients with MRI negative partial epilepsy. J. Neurol. Neurosurg. Psychiatry. 2010;81:136–139. doi: 10.1136/jnnp.2008.160820. [DOI] [PubMed] [Google Scholar]
- Focke N.K., Diederich C., Helms G., Nitsche M.A., Lerche H., Paulus W. Idiopathic-generalized epilepsy shows profound white matter diffusion-tensor imaging alterations. Hum. Brain Mapp. 2014;35:3332–3342. doi: 10.1002/hbm.22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Zhang Y., Wong C.S., Wu P.M., Zhang Z., Gao J., Qiu D., Huang B. Diffusion abnormalities in temporal lobes of children with temporal lobe epilepsy: a preliminary diffusional kurtosis imaging study and comparison with diffusion tensor imaging. NMR Biomed. 2012;25:1369–1377. doi: 10.1002/nbm.2809. [DOI] [PubMed] [Google Scholar]
- Govindan R.M., Makki M.I., Sundaram S.K., Juhasz C., Chugani H.T. Diffusion tensor analysis of temporal and extra-temporal lobe tracts in temporal lobe epilepsy. Epilepsy Res. 2008;80:30–41. doi: 10.1016/j.eplepsyres.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D.W., Concha L., Beaulieu C. Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia. 2006;47:1360–1363. doi: 10.1111/j.1528-1167.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- Holt R.L., Provenzale J.M., Veerapandiyan A., Moon W.J., De Bellis M.D., Leonard S., Gallentine W.B., Grant G.A., Egger H., Song A.W., Mikati M.A. Structural connectivity of the frontal lobe in children with drug-resistant partial epilepsy. Epilepsy Behav. 2011;21:65–70. doi: 10.1016/j.yebeh.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson E., Pulsipher D., Dabbs K., Myers y Gutierrez A., Sheth R., Jones J., Seidenberg M., Meyerand E., Hermann B. Children with new-onset epilepsy exhibit diffusion abnormalities in cerebral white matter in the absence of volumetric differences. Epilepsy Res. 2010;88:208–214. doi: 10.1016/j.eplepsyres.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis J.P. Why most published research findings are false. PLoS Med. 2005;2 doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S.S., Ahrens T., Mohammadi S., Moddel G., Kugel H., Ringelstein E.B., Deppe M. Microstructural and volumetric abnormalities of the putamen in juvenile myoclonic epilepsy. Epilepsia. 2011;52:1715–1724. doi: 10.1111/j.1528-1167.2011.03117.x. [DOI] [PubMed] [Google Scholar]
- Keller S.S., Schoene-Bake J.C., Gerdes J.S., Weber B., Deppe M. Concomitant fractional anisotropy and volumetric abnormalities in temporal lobe epilepsy: cross-sectional evidence for progressive neurologic injury. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S.S., Ahrens T., Mohammadi S., Gerdes J.S., Moddel G., Kellinghaus C., Kugel H., Weber B., Ringelstein E.B., Deppe M. Voxel-based statistical analysis of fractional anisotropy and mean diffusivity in patients with unilateral temporal lobe epilepsy of unknown cause. J. Neuroimaging. 2013;23:352–359. doi: 10.1111/j.1552-6569.2011.00673.x. [DOI] [PubMed] [Google Scholar]
- Kemmotsu N., Girard H.M., Bernhardt B.C., Bonilha L., Lin J.J., Tecoma E.S., Iragui V.J., Hagler D.J., Jr., Halgren E., McDonald C.R. MRI analysis in temporal lobe epilepsy: cortical thinning and white matter disruptions are related to side of seizure onset. Epilepsia. 2011;52:2257–2266. doi: 10.1111/j.1528-1167.2011.03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmotsu N., Kucukboyaci N.E., Leyden K.M., Cheng C.E., Girard H.M., Iragui V.J., Tecoma E.S., McDonald C.R. Frontolimbic brain networks predict depressive symptoms in temporal lobe epilepsy. Epilepsy Res. 2014;108:1554–1563. doi: 10.1016/j.eplepsyres.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.H., Chung C.K., Koo B.B., Lee J.M., Kim J.S., Lee S.K. Changes in language pathways in patients with temporal lobe epilepsy: diffusion tensor imaging analysis of the uncinate and arcuate fasciculi. World Neurosurg. 2011;75:509–516. doi: 10.1016/j.wneu.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Suh S.I., Park S.Y., Seo W.K., Koh I., Koh S.B., Seol H.Y. Microstructural white matter abnormality and frontal cognitive dysfunctions in juvenile myoclonic epilepsy. Epilepsia. 2012;53:1371–1378. doi: 10.1111/j.1528-1167.2012.03544.x. [DOI] [PubMed] [Google Scholar]
- Kim S.E., Lee J.H., Chung H.K., Lim S.M., Lee H.W. Alterations in white matter microstructures and cognitive dysfunctions in benign childhood epilepsy with centrotemporal spikes. Eur. J. Neurol. 2014;21:708–717. doi: 10.1111/ene.12301. [DOI] [PubMed] [Google Scholar]
- Knake S., Salat D.H., Halgren E., Halko M.A., Greve D.N., Grant P.E. Changes in white matter microstructure in patients with TLE and hippocampal sclerosis. Epileptic Disord. 2009;11:244–250. doi: 10.1684/epd.2009.0272. [DOI] [PubMed] [Google Scholar]
- Kori P., Garg R.K., Malhotra H.S., Gupta R.K., Verma R., Singh M.K., Rathore R.K., Gupta P.K. Evaluation of cerebral white-matter micro-structural alterations in patients with medically refractory epilepsy using diffusion tensor tractography. Epilepsy Res. 2013;107:82–90. doi: 10.1016/j.eplepsyres.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Labate A., Cherubini A., Tripepi G., Mumoli L., Ferlazzo E., Aguglia U., Quattrone A., Gambardella A. White matter abnormalities differentiate severe from benign temporal lobe epilepsy. Epilepsia. 2015;56:1109–1116. doi: 10.1111/epi.13027. [DOI] [PubMed] [Google Scholar]
- Lee C.Y., Tabesh A., Benitez A., Helpern J.A., Jensen J.H., Bonilha L. Microstructural integrity of early- versus late-myelinating white matter tracts in medial temporal lobe epilepsy. Epilepsia. 2013;54:1801–1809. doi: 10.1111/epi.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.Y., Tabesh A., Spampinato M.V., Helpern J.A., Jensen J.H., Bonilha L. Diffusional kurtosis imaging reveals a distinctive pattern of microstructural alternations in idiopathic generalized epilepsy. Acta Neurol. Scand. 2014;130:148–155. doi: 10.1111/ane.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Du H., Xie B., Wu N., Wang J., Wu G., Feng H., Jiang T. Cerebellum abnormalities in idiopathic generalized epilepsy with generalized tonic-clonic seizures revealed by diffusion tensor imaging. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liacu D., Idy-Peretti I., Ducreux D., Bouilleret V., de Marco G. Diffusion tensor imaging tractography parameters of limbic system bundles in temporal lobe epilepsy patients. J. Magn. Reson. Imaging. 2012;36:561–568. doi: 10.1002/jmri.23678. [DOI] [PubMed] [Google Scholar]
- Lin J.J., Riley J.D., Juranek J., Cramer S.C. Vulnerability of the frontal-temporal connections in temporal lobe epilepsy. Epilepsy Res. 2008;82:162–170. doi: 10.1016/j.eplepsyres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Liu M., Concha L., Beaulieu C., Gross D.W. Distinct white matter abnormalities in different idiopathic generalized epilepsy syndromes. Epilepsia. 2011;52:2267–2275. doi: 10.1111/j.1528-1167.2011.03313.x. [DOI] [PubMed] [Google Scholar]
- Liu Z., Xu Y., An J., Wang J., Yin X., Huang R., Lv X., Chen L., Wang W., Qiu S. Altered brain white matter integrity in temporal lobe epilepsy: a TBSS study. J. Neuroimaging. 2015;25:460–464. doi: 10.1111/jon.12154. [DOI] [PubMed] [Google Scholar]
- Mao L.Y., Ding J., Peng W.F., Ma Y., Zhang Y.H., Chen C.Z., Cheng W.Z., Wang H., Fan W., Wang X. Disease duration and arcuate fasciculus abnormalities correlate with psychoticism in patients with epilepsy. Seizure. 2011;20:741–747. doi: 10.1016/j.seizure.2011.07.002. [DOI] [PubMed] [Google Scholar]
- McDonald C.R., Ahmadi M.E., Hagler D.J., Tecoma E.S., Iragui V.J., Gharapetian L., Dale A.M., Halgren E. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71:1869–1876. doi: 10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L., Xiang J., Kotecha R., Rose D., Zhao H., Zhao D., Yang J., Degrauw T. White matter abnormalities in children and adolescents with temporal lobe epilepsy. Magn. Reson. Imaging. 2010;28:1290–1298. doi: 10.1016/j.mri.2010.03.046. [DOI] [PubMed] [Google Scholar]
- Meng L., Xiang J., Kotecha R., Rose D., Zhao H., Zhao D., Yang J., Degrauw T. White matter abnormalities in children and adolescents with temporal lobe epilepsy. Magn. Reson. Imaging. 2010;28:1290–1298. doi: 10.1016/j.mri.2010.03.046. [DOI] [PubMed] [Google Scholar]
- Nilsson D., Go C., Rutka J.T., Rydenhag B., Mabbott D.J., Snead O.C., III, Raybaud C.R., Widjaja E. Bilateral diffusion tensor abnormalities of temporal lobe and cingulate gyrus white matter in children with temporal lobe epilepsy. Epilepsy Res. 2008;81:128–135. doi: 10.1016/j.eplepsyres.2008.05.002. [DOI] [PubMed] [Google Scholar]
- O'Muircheartaigh J., Vollmar C., Barker G.J., Kumari V., Symms M.R., Thompson P., Duncan J.S., Koepp M.J., Richardson M.P. Focal structural changes and cognitive dysfunction in juvenile myoclonic epilepsy. Neurology. 2011;76:34–40. doi: 10.1212/WNL.0b013e318203e93d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte W.M., van Eijsden P., Sander J.W., Duncan J.S., Dijkhuizen R.M., Braun K.P. A meta-analysis of white matter changes in temporal lobe epilepsy as studied with diffusion tensor imaging. Epilepsia. 2012;53:659–667. doi: 10.1111/j.1528-1167.2012.03426.x. [DOI] [PubMed] [Google Scholar]
- Otte W.M., Dijkhuizen R.M., van Meer M.P., van der Hel W.S., Verlinde S.A., van Nieuwenhuizen O., Viergever M.A., Stam C.J., Braun K.P. Characterization of functional and structural integrity in experimental focal epilepsy: reduced network efficiency coincides with white matter changes. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S.J., Harnod T., Tsai J.Z., Huang C.C., Ker M.D., Chiou J.C., Chiueh H., Wu C.Y., Hsin Y.L. Through diffusion tensor magnetic resonance imaging to evaluate the original properties of neural pathways of patients with partial seizures and secondary generalization by individual anatomic reference atlas. Biomed. Res. Int. 2014;2014:419376. doi: 10.1155/2014/419376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell H.W., Parker G.J., Alexander D.C., Symms M.R., Boulby P.A., Wheeler-Kingshott C.A., Barker G.J., Koepp M.J., Duncan J.S. Abnormalities of language networks in temporal lobe epilepsy. NeuroImage. 2007;36:209–221. doi: 10.1016/j.neuroimage.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Pustina D., Doucet G., Sperling M., Sharan A., Tracy J. Increased microstructural white matter correlations in left, but not right, temporal lobe epilepsy. Hum. Brain Mapp. 2015;36:85–98. doi: 10.1002/hbm.22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley J.D., Franklin D.L., Choi V., Kim R.C., Binder D.K., Cramer S.C., Lin J.J. Altered white matter integrity in temporal lobe epilepsy: association with cognitive and clinical profiles. Epilepsia. 2010;51:536–545. doi: 10.1111/j.1528-1167.2009.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo S., Oppenheim C., Chassoux F., Golestani N., Cointepas Y., Poupon C., Semah F., Mangin J.F., Le Bihan D., Meder J.F. Uncinate fasciculus fiber tracking in mesial temporal lobe epilepsy. Initial findings. Eur. Radiol. 2007;17:1663–1668. doi: 10.1007/s00330-006-0558-x. [DOI] [PubMed] [Google Scholar]
- Rugg-Gunn F.J., Eriksson S.H., Symms M.R., Barker G.J., Duncan J.S. Diffusion tensor imaging of cryptogenic and acquired partial epilepsies. Brain. 2001;124:627–636. doi: 10.1093/brain/124.3.627. [DOI] [PubMed] [Google Scholar]
- Scanlon C., Mueller S.G., Cheong I., Hartig M., Weiner M.W., Laxer K.D. Grey and white matter abnormalities in temporal lobe epilepsy with and without mesial temporal sclerosis. J. Neurol. 2013;260:2320–2329. doi: 10.1007/s00415-013-6974-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds M.C., Higgins J.P., Stewart L.A., Tierney J.F., Clarke M.J., Thompson S.G. Meta-analysis of individual patient data from randomized trials: a review of methods used in practice. Clin. Trials. 2005;2:209–217. doi: 10.1191/1740774505cn087oa. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010;36:1–48. [Google Scholar]
- Vulliemoz S., Vollmar C., Koepp M.J., Yogarajah M., O'Muircheartaigh J., Carmichael D.W., Stretton J., Richardson M.P., Symms M.R., Duncan J.S. Connectivity of the supplementary motor area in juvenile myoclonic epilepsy and frontal lobe epilepsy. Epilepsia. 2011;52:507–514. doi: 10.1111/j.1528-1167.2010.02770.x. [DOI] [PubMed] [Google Scholar]
- Wang X.Q., Lang S.Y., Hong L.U., Lin M.A., Yan-ling M.A., Yang F. Changes in extratemporal integrity and cognition in temporal lobe epilepsy: a diffusion tensor imaging study. Neurol. India. 2010;58:891–899. doi: 10.4103/0028-3886.73739. [DOI] [PubMed] [Google Scholar]
- Wang X.Q., Lang S.Y., Hong L.U., Lin M.A., Yan-Ling M.A., Yang F. Changes in extrafrontal integrity and cognition in frontal lobe epilepsy: a diffusion tensor imaging study. Epilepsy Behav. 2011;20:471–477. doi: 10.1016/j.yebeh.2010.12.039. [DOI] [PubMed] [Google Scholar]
- Whelan C.D., Alhusaini S., O'Hanlon E., Cheung M., Iyer P.M., Meaney J.F., Fagan A.J., Boyle G., Delanty N., Doherty C.P., Cavalleri G.L. White matter alterations in patients with MRI-negative temporal lobe epilepsy and their asymptomatic siblings. Epilepsia. 2015;56:1551–1561. doi: 10.1111/epi.13103. [DOI] [PubMed] [Google Scholar]
- Xiao F., Chen Q., Yu X., Tang Y., Luo C., Fang J., Liu L., Huang X., Gong Q., Zhou D. Hemispheric lateralization of microstructural white matter abnormalities in children with active benign childhood epilepsy with centrotemporal spikes (BECTS): a preliminary DTI study. J. Neurol. Sci. 2014;336:171–179. doi: 10.1016/j.jns.2013.10.033. [DOI] [PubMed] [Google Scholar]
- Yang T., Guo Z., Luo C., Li Q., Yan B., Liu L., Gong Q., Yao D., Zhou D. White matter impairment in the basal ganglia-thalamocortical circuit of drug-naive childhood absence epilepsy. Epilepsy Res. 2012;99:267–273. doi: 10.1016/j.eplepsyres.2011.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables