Abstract
Purpose
Tobacco smoking and occupational exposures are the leading risk factors for developing urothelial bladder carcinoma (UBC), yet little is known about the contribution of these two factors to risk of UBC recurrence. We evaluated whether smoking status and usual adult occupation are associated with time to UBC recurrence for 406 patients with muscle-invasive bladder cancer submitted to The Cancer Genome Atlas (TCGA) project.
Methods
Kaplan-Meier and Cox proportional hazard methods were used to assess the association between smoking status, employment in a high-risk occupation for bladder cancer, occupational diesel exhaust exposure, and 2010 Standard Occupational Classification (SOC) group and time to UBC recurrence.
Results
Data on time to recurrence was available for 358 patients over a median follow-up time of 15 months. Of these, 133 (37.2%) experienced a recurrence. Current smokers who smoked for more than 40 pack-years had an increased risk of recurrence compared to never smokers (HR 2.1, 95% CI 1.1, 4.1). Additionally, employment in a high-risk occupation was associated with a shorter time to recurrence (log-rank P=0.005). We found an increased risk of recurrence for those employed in occupations with probable diesel exhaust exposure (HR: 1.8, 95% CI 1.1, 3.0) and for those employed in production occupations (HR: 2.0, 95% CI 1.1, 3.6).
Conclusions
These findings suggest smoking status impacts risk of UBC recurrence, although several previous studies provided equivocal evidence regarding this association. In addition to the known causal relationship between occupational exposure and bladder cancer risk, our study suggests that occupation may also be related to increased risk of recurrence.
Keywords: bladder cancer, recurrence, occupation, smoking status, diesel exhaust
INTRODUCTION
Tobacco smoking and occupational exposures are the leading risk factors for urothelial bladder carcinoma (UBC) incidence. Smoking accounts for about 50% of bladder cancer cases in both men and women in the U.S. [1]. Following smoking, occupational exposures are the second most important risk factor for UBC, with up to 25% of all cases being attributed to these exposures [2]. Occupational groups that have been linked to increased incidence of UBC include dyestuffs workers, aromatic amine manufacturing workers, painters, aluminum workers, and drivers of trucks and other motor vehicles [3].
Little is known about the contribution of smoking and occupational exposures to the risk of UBC recurrence. A recent meta-analysis of the evidence for smoking found that both smoking status and pack-years appear to have an effect on disease prognosis (in terms of recurrence and progression) in patients with non-muscle invasive bladder carcinoma (NMIBC) [4]. In contrast, the studies that include patients with muscle-invasive bladder carcinoma (MIBC) had equivocal results about the association of smoking with disease recurrence [4]. We are not aware of any studies that have evaluated the impact of occupation or specific occupational exposures on risk of recurrence.
UBC is highly recurrent and costly to treat, making research into possible risk factors for recurrence an important public health priority. The objective of our study is to evaluate whether smoking status and usual adult occupation are associated with time to UBC recurrence for patients with muscle-invasive bladder cancer in The Cancer Genome Atlas (TCGA) project.
MATERIALS AND METHODS
Study Population
There were 406 muscle-invasive bladder cancer cases, diagnosed between 2010–2014, included in The Cancer Genome Atlas project (TCGA) (https://tcga-data.nci.nih.gov/, accessed 06/05/2015). Patients included in TCGA were those who underwent surgical resection with either transurethral resection of the bladder (TURB) or radical cystectomy (RC). No patients received prior chemotherapy or radiotherapy treatment for their disease, although prior intravesical Bacille Calmette Guerin (BCG) was allowed. Of 406 unique records, 358 cases had data on recurrence and time to recurrence. Recurrence was defined as first new tumor event: locoregional recurrence or distant metastasis. Subjects were censored at date of first recurrence after follow-up. Those who did not experience recurrence were censored at last known follow-up date or date of death. A total of 133 patients had a recurrence, while 225 patients did not have a recurrence.
Exposure Assessment
Smoking status was assessed by patients’ self-reported smoking history as either: “lifelong non-smoker (<100 cigarettes smoked in lifetime)”, “current smoker (includes daily and non-daily smokers)”, “current reformed smoker for >15 years”, “current reformed smoker for ≤15 years”, “current reformed smoker (duration not specified)”, or “smoking history not documented”. For current and former smokers, additional questions were asked to determine age of onset of tobacco smoking and year of quitting tobacco smoking. This information was combined with the number of cigarettes smoked per day to calculate pack-years smoked, which represents lifetime tobacco exposure. Patients reported occupational information such as job title of usual occupation (“occupation in which the patient was employed for the majority of their working years”), industry of usual occupation, and chemical exposures (“any chemical exposure the patient had during their working years in their primary occupation”). Publicly available software (http://soccer.nci.nih.gov) was used to code this free-text job information to two-digit level groups within the 2010 Standard Occupational Classification (SOC) system (http://www.bls.gov/soc) [5]. Some job descriptions assigned SOC codes with lower certainty (n=79) received expert review (S.J.L) to determine the most appropriate group assignment. Subjects were further dichotomized into high-risk occupation group (no/yes) based on employment in an occupation with a priori bladder cancer risk in the literature (Online Resource 1). Approximately 19% (69/358) of subjects with data on recurrence were employed in a high-risk occupation. An additional variable was created to indicate exposure to diesel exhaust, as it was the most prevalent high-risk occupational exposure in the data set and a known carcinogen. This was dichotomized (no/yes) based on expert knowledge of jobs with either moderate or high probability of occupational diesel exposure [6]. Approximately 14% (51/358) of subjects with data on recurrence were probably exposed to diesel exhaust. Among these subjects, 33 were also employed in an a priori high-risk occupation and 18 were employed in other jobs.
Statistical Analysis
We plotted the Schoenfeld residuals to detect violations of proportional hazard assumptions for the exposures and potential covariates, and no major violations were observed. We calculated both unadjusted and adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) using Cox proportional hazard models, with separate models for each independent exposure variable of interest: smoking, employment in a high-risk occupation, diesel exhaust exposure, and each individual major two-digit SOC grouping. A competing risks model was also considered, which produced similar results when compared to the Cox proportional hazards model (data not shown). The following characteristics were considered as potential confounders in the adjusted models: smoking status (never, former smoker <30 pack-years, former smoker ≥30 pack-years, current smoker <40 pack-years, current smoker ≥40 pack-years), age at diagnosis (<55, 56–65, 66–75, >75), race (White, Black, Asian), body mass index (14–24, 25–29, ≥30), gender, country of residence (U.S. or other), history of other malignancy, history of non-muscle invasive bladder cancer, histologic subtype (papillary, non-papillary), stage of disease based on the AJCC cancer staging system (II, III, IV), and treatment (immunotherapy, chemotherapy, radiation, combination, unknown). The pack-year cut points for the categorical analysis were selected based on the median pack-year exposure within each stratum. The model of diesel exhaust exposure was also adjusted for employment in high-risk occupations other than those involving diesel exhaust exposure. Models for each independent exposure variable retained covariates that impacted the parameter estimates by more than 10%. For models evaluating smoking, employment in a high-risk occupation, and diesel exposure, we also conducted stratified analyses by the above listed variables. Likelihood ratio tests were used to assess differences between strata (P-interaction). All tests were two-sided and conducted at the α=0.05 level.
RESULTS
In this study, 133 out of 358 (37.2%) patients experienced a recurrence. The median follow-up time was 15 months (IQR 8-27) and the median time to recurrence was 11 months (IQR 7-20). The characteristics of patients by recurrence status are presented in Table 1. Patients who experienced a recurrence presented with primary tumors that were of a higher stage than patients who did not experience a recurrence (47% vs. 24% in stage IV, respectively). In addition, patients who experienced a recurrence more commonly presented with the non-papillary bladder cancer subtype compared to patients who did not experience a recurrence (76% vs. 62%, respectively). No differences were detected when comparing the distribution of these characteristics to patients with missing data (n=47) on recurrence (data not shown).
Table 1.
Characteristics of bladder cancer recurrences
| Characteristic | No Recurrence N=226 | Recurrence N=133 | P-value |
|---|---|---|---|
| Age at diagnosis | |||
| <55 | 36 (15.9) | 10 (7.5) | |
| 56–65 | 53 (23.5) | 47 (35.3) | |
| 66–75 | 71 (31.4) | 48 (36.1) | |
| >75 | 66 (29.2) | 28 (21.1) | 0.05 |
| Country | |||
| United States | 159 (70.4) | 100 (75.2) | |
| Other | 67 (29.7) | 33 (24.8) | 0.52 |
| Race | |||
| White | 176 (77.9) | 107 (80.5) | |
| Black | 10 (4.4) | 10 (7.5) | |
| Asian | 34 (15.0) | 9 (6.8) | |
| Missing | 6 (2.7) | 7 (5.3) | 0.51 |
| Gender | |||
| Male | 172 (76.1) | 97 (72.9) | |
| Female | 54 (23.9) | 36 (27.1) | 0.65 |
| Smoking | |||
| Never | 63 (27.9) | 33 (24.8) | |
| Former (<30 pack-years) | 40 (17.7) | 22 (16.5) | |
| Former (≥30 pack-years) | 34 (15.0) | 40 (30.1) | |
| Current (<40 pack-years) | 22 (9.7) | 9 (6.8) | |
| Current (≥40 pack-years) | 15 (6.6) | 14 (10.5) | |
| Missing | 52 (23.0) | 15 (11.3) | 0.02 |
| Pathologic stage (AJCC) | |||
| Stage II | 93 (41.2) | 31 (23.3) | |
| Stage III | 79 (35.0) | 39 (29.3) | |
| Stage IV | 54 (23.9) | 63 (47.4) | <.0001 |
| Histologic subtype | |||
| Non-papillary | 141 (62.4) | 101 (75.9) | |
| Papillary | 85 (37.6) | 32 (24.1) | 0.07 |
| History of NMI | |||
| No | 156 (69.0) | 74 (55.6) | |
| Yes | 34 (15.0) | 28 (21.1) | |
| Missing | 36 (15.9) | 31 (23.3) | 0.52 |
| History of other malignancy | |||
| No | 174 (77.0) | 89 (66.9) | |
| Yes | 52 (23.0) | 44 (33.1) | 0.35 |
| High-risk occupation | |||
| No | 104 (46.0) | 55 (41.4) | |
| Yes | 33 (14.6) | 36 (27.1) | |
| Missing | 89 (39.4) | 42 (31.6) | 0.01 |
Abbreviations: American Joint Committee on Cancer (AJCC); Non-muscle invasive (NMI). P-values calculated using log-rank tests.
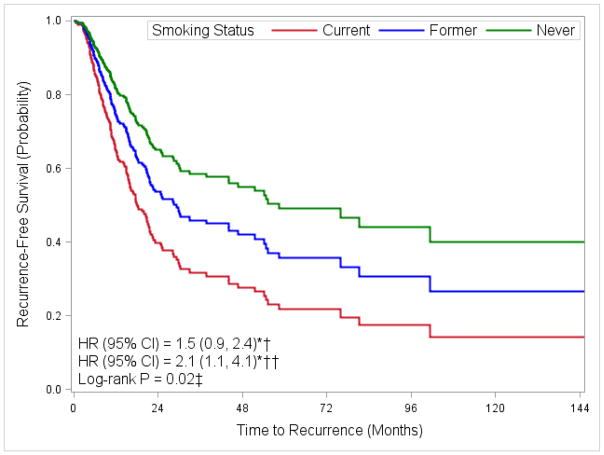
Figure 1 shows the association of smoking status with time to recurrence. Current smokers with over 40 pack-years of exposure had a two-fold increased risk of recurrence when compared to never smokers (HR 2.1, 95% CI 1.1, 4.1). Former smokers with over 30 pack-years of exposure also had an increased risk of recurrence when compared to never smokers, although this finding was not statistically significant (HR 1.5, 95% CI 0.9, 2.4).
Fig. 1. Adjusted Kaplan-Meier plot for time to first recurrence by smoking status.
* Adjusted for age at diagnosis (<55, 56–65, 66–75, >75), stage of disease (II, III, IV), and treatment (immunotherapy, chemotherapy, radiation, combination, unknown). † Former smokers with ≥30 pack-years compared to never smokers. †† Current smokers with ≥40 pack-years compared to never smokers. ‡ Comparing all smoking levels (never, former with <30 pack-years, former with ≥30 pack-years, current with <40 pack-years, current with ≥40 pack-years).
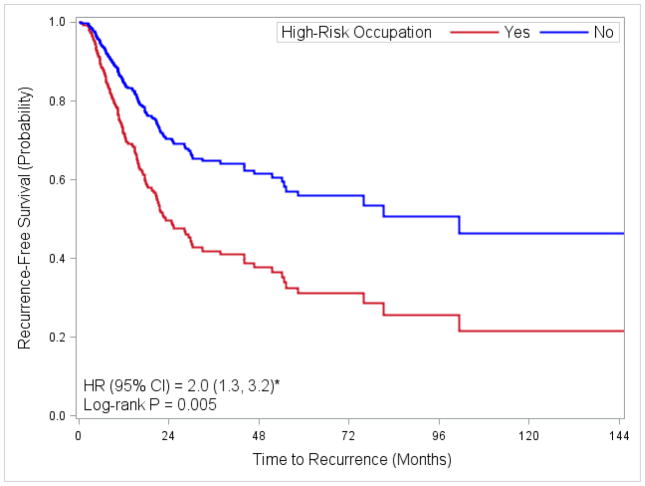
Table 2 shows the association between high-risk occupation, diesel exhaust exposure, and major SOC group with time to recurrence. Employment in a high-risk occupation was associated with a shorter time to recurrence (log-rank P=0.005, Figure 2). In addition, probable diesel exposure was associated with an 80% increased risk of recurrence (HR: 1.8, 95% CI 1.1, 2.8). Among the major SOC groups, employment in production occupations was associated with an increased risk of recurrence (HR: 2.0, 95% CI 1.1, 3.6). In univariate analysis, employment in construction and extraction occupations was associated with a two-fold increased risk of recurrence (HR: 2.1, 95% CI 1.2, 3.6), however, this became only borderline non-significant after adjusting for age, stage and treatment.
Table 2.
Hazard ratios for time to recurrence by high-risk occupation, diesel exposure and major SOC group
| Exposure | Non-Recurrent/Recurrent | Unadjusted HR 95% CI | Adjusted HR* 95% CI |
|---|---|---|---|
| High-Risk Occupation** | |||
| No | 104/55 | Ref | Ref |
| Yes | 33/36 | 1.9 (1.2, 2.9) | 2.0 (1.3, 3.2) |
| Diesel Exhaust Exposure§¶ | |||
| No/Low Probability | 109/68 | Ref | Ref |
| Moderate/High Probability | 28/23 | 1.5 (0.9, 2.3) | 1.8 (1.1, 3.0) |
| Major SOC Group | |||
| 11-Management Occupations †,†† | 8/6 | 0.9 (0.4, 2.1) | 0.6 (0.2, 1.6) |
| 13-Business and Financial Operations Occupations | 6/6 | 1.2 (0.6, 2.8) | 1.1 (0.5, 2.6) |
| 17-Architecture and Engineering Occupations | 6/4 | 0.9 (0.4, 2.5) | 0.9 (0.3, 2.5) |
| 23-Legal Occupations †,†† | 3/2 | 1.3 (0.3, 5.1) | 0.8 (0.2, 3.5) |
| 25-Education, Training, and Library Occupations | 6/3 | 1.0 (0.3, 3.1) | 1.0 (0.3, 3.2) |
| 29-Healthcare Practitioners and Technical Occupations †,††,‡ | 5/4 | 1.1 (0.4, 2.9) | 1.1 (0.4, 3.3) |
| 35-Food Preparation and Serving Related Occupations ††‡ | 4/2 | 0.8 (0.2, 3.3) | 0.7 (0.2, 2.8) |
| 37-Building and Grounds Cleaning and Maintenance Occupations † | 2/1 | 1.0 (0.1, 7.0) | 1.3 (0.2, 9.6) |
| 41-Sales and Related Occupations | 14/6 | 0.7 (0.3, 1.6) | 0.8 (0.3, 1.8) |
| 43-Office and Administrative Support Occupations | 12/4 | 0.5 (0.2, 1.2) | 0.6 (0.2, 1.5) |
| 45-Farming, Fishing, and Forestry Occupations | 12/3 | 0.6 (0.2, 1.9) | 0.7 (0.2, 2.4) |
| 47-Construction and Extraction Occupations | 8/14 | 2.1 (1.2, 3.6) | 1.7 (0.97, 3.1) |
| 49-Installation, Maintenance, and Repair Occupations | 8/2 | 0.4 (0.1, 1.7) | 0.7 (0.2, 2.7) |
| 51-Production Occupations | 12/12 | 1.5 (0.8, 2.7) | 2.0 (1.1, 3.6) |
| 53-Transportation and Material Moving Occupations††, § | 6/9 | 1.7 (0.8, 3.3) | 1.2 (0.6, 2.4) |
| 55-Military Specific Occupations | 4/2 | 1.1 (0.3, 4.4) | 1.2 (0.3, 4.8) |
| 99-Housewives †† | 6/6 | 1.4 (0.6, 3.1) | 1.1 (0.4, 2.5) |
Adjusted for age at diagnosis (<55, 56–65, 66–75, >75), stage of disease (II, III, IV), and treatment (immunotherapy, chemotherapy, radiation, combination, unknown).
Additionally adjusted for gender.
Additionally adjusted for body mass index.
Additionally adjusted for employment in high-risk occupations other than those involving diesel exhaust exposure
Additionally adjusted for smoking.
Additionally adjusted for history of non-muscle invasive bladder cancer.
Additionally adjusted for history of other malignancy.
Fig. 2. Adjusted Kaplan-Meier plot for time to recurrence by high-risk occupation.
* Adjusted for age at diagnosis (<55, 56–65, 66–75, >75), gender, stage of disease (II, III, IV), and treatment (immunotherapy, chemotherapy, radiation, combination, unknown).
No additional noteworthy findings were observed in stratified models and no significant interactions were observed.
DISCUSSION
Smoking status and usual adult occupation were both found to be associated with time to UBC recurrence for patients with MIBC. Current smoking of over 40 pack-years was associated with an increased risk of recurrence. In addition, employment in a high-risk occupation, occupations with probable diesel exhaust exposure, and production occupations were each found to be associated with an increased risk of recurrence.
There is some evidence in the literature of the effect of smoking on UBC recurrence, although most studies focus on the impact of smoking on recurrence in NMI disease, given the high rate of recurrence in this subgroup. Five studies have included MIBC patients when examining this association [7–11]. Two of them found no effect of smoking status on risk of recurrence [10,11], while one did observe a difference [9]; these studies, however, had limited information on smoking (ever/never). Of the two studies with more detailed data on smoking history, one found no association among current smokers compared to non-smokers (HR: 0.91, 95% CI: 0.63, 1.31) [7] while the other study found an increased risk of recurrence for heavy long-term smokers compared to light short-term smokers (HR: 2.22, 95% CI: 1.62, 3.02) [8]. Our findings are consistent with the latter study in that we found an increased risk of recurrence among current smokers of over 40 pack-years when compared to never smokers (HR: 2.1, 95% CI: 1.1, 4.1). The exact mechanism underlying the effect of smoking on UBC recurrence is not known, although it is theorized that exposure to carcinogens in cigarettes, such as aromatic amines, may have cancer-promoting effects [12]. The potential influence of the timing of smoking cessation on risk of UBC recurrence is also not known.
There is strong evidence of an increased risk of bladder cancer incidence among certain occupations, such as dyestuffs workers, aromatic amine manufacturing workers, rubber workers, leather workers, painters, truck drivers, and aluminum workers [3]. There has also been some evidence of an increased risk among metal workers, printers, chemical workers, construction workers, miners, and textile workers, among others [3]. Our study found an increased risk of recurrence among people who held some of these high-risk jobs as their usual adult occupation. In addition, we found a similar increased risk of recurrence for those employed in production occupations, which include job titles such as: textile industry worker, chemical machine operator, and machinist. There is a causal relationship between occupation and bladder cancer risk; our study suggests that occupation may also be related to adverse outcomes after diagnosis. Some of these high-risk occupations have exposures that are known to be related to UBC incidence [13,14], others have exposures that are likely highly heterogeneous. More work is needed to identify the specific exposures that drive these associations and how these exposures may promote UBC.
There is growing evidence in the literature of an association between occupational exposure to diesel exhaust and risk of bladder cancer. A meta-analysis examining this association found effect estimates to suggest an overall relative risk of approximately 1.1–1.3 [15]. Additionally, the summary relative risk for high diesel exposure was 1.44 (95% CI: 1.18, 1.76) [15]. Our findings indicate an increased risk of recurrence among those employed in an occupation with probable diesel exhaust exposure. No association was found, however, for employment in transportation and material moving occupations, which may have exposure to diesel. This disparity is likely due to the fact that diesel exhaust exposure spans many different job titles with heterogeneous exposures. Although the mechanism by which diesel exhaust exposure might influence recurrence is unknown, a p53-dependent mechanism has been suggested [16,17] and is a common feature of MI disease [18].
This study is the first to our knowledge to evaluate the association between usual occupation and UBC recurrence. Although we were able to categorize occupation according to standardized groupings, information on tasks performed within a broad occupational grouping was lacking and limited the ability to characterize specific exposures associated with high-risk occupations. There was also minimal data available on duration of employment and on industry for many subjects, however the use of the usual adult occupation is likely to be most representative of the subjects’ job history and exposure circumstance. In addition, the data on exposure to diesel exhaust does not reflect the changes over time in diesel engines and in emissions. A strength of this study was the availability of information on both smoking status and cumulative exposure in terms of pack-years. The smoking data was limited, however, by the fact that changes in smoking status over time or smoking cessation were not collected. Other limitations of this study are the relatively small sample size, limited power, and missing data on the exposures of interest. The lack of a representative sample may also limit the ability to compare the results of this study to that of other population-based studies. Despite this limitation, we observed an association between high-risk occupation and recurrence that is comparable in magnitude to studies of bladder cancer incidence [19], which lends credence to our findings. In addition, the effect observed here with smoking is similar to the largest of only two studies which have evaluated more detailed smoking information and risk of recurrence [8]. Lastly, data collected in TCGA only indicate the occurrence of death. Disease-specific survival was unavailable and thus we were unable to assess whether smoking and occupation are associated with bladder cancer survival, in addition to recurrence.
It will be important for future studies to evaluate the association between smoking and occupational exposures with UBC recurrence in patients with NMIBC because of the high cost of long-term medical surveillance of large numbers of survivors resulting from high recurrence rate in this subgroup. Work is also needed to determine whether these important bladder cancer risk factors are associated with certain molecular tumor characteristics that influence progression of disease.
Supplementary Material
References of high-risk occupations associated with bladder cancer in the literature
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, NCI, Division of Cancer Epidemiology and Genetics (Z01CP010119).
Footnotes
Ethics approval: TCGA is a public resource and all approvals were acquired by the original study.
Conflict of Interest: The authors declare that they have no conflict of interest.
Informed consent: Informed consent was obtained from all individual participants included in each participating TCGA study.
References
- 1.Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. Jama. 2011;306(7):737–745. doi: 10.1001/jama.2011.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverman DT, Levin LI, Hoover RN, Hartge P. Occupational risks of bladder cancer in the United States: I. White men. Journal of the National Cancer Institute. 1989;81(19):1472–1480. doi: 10.1093/jnci/81.19.1472. [DOI] [PubMed] [Google Scholar]
- 3.Silverman DT, Devesa SS, Moore LE, Rothman N. Bladder Cancer. In: Schottenfeld D, Fraumeni JFJ, editors. Cancer Epidemiology and Prevention. 3. Oxford University Press; New York: 2006. pp. 1101–1127. [Google Scholar]
- 4.Crivelli JJ, Xylinas E, Kluth LA, Rieken M, Rink M, Shariat SF. Effect of smoking on outcomes of urothelial carcinoma: a systematic review of the literature. European urology. 2014;65(4):742–754. doi: 10.1016/j.eururo.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Russ DE, Ho KY, Colt JS, Armenti KR, Baris D, Chow WH, Davis F, Johnson A, Purdue MP, Karagas MR, Schwartz K, Schwenn M, Silverman DT, Johnson CA, Friesen MC. Computer-based coding of free-text job descriptions to efficiently identify occupations in epidemiological studies. Occupational and environmental medicine. 2016;73(6):417–424. doi: 10.1136/oemed-2015-103152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pronk A, Coble J, Stewart PA. Occupational exposure to diesel engine exhaust: a literature review. Journal of exposure science & environmental epidemiology. 2009;19(5):443–457. doi: 10.1038/jes.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee C, Kim KH, You D, Jeong IG, Hong B, Hong JH, Ahn H, Kim CS. Smoking and survival after radical cystectomy for bladder cancer. Urology. 2012;80(6):1307–1312. doi: 10.1016/j.urology.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Rink M, Zabor EC, Furberg H, Xylinas E, Ehdaie B, Novara G, Babjuk M, Pycha A, Lotan Y, Trinh QD, Chun FK, Lee RK, Karakiewicz PI, Fisch M, Robinson BD, Scherr DS, Shariat SF. Impact of smoking and smoking cessation on outcomes in bladder cancer patients treated with radical cystectomy. European urology. 2013;64(3):456–464. doi: 10.1016/j.eururo.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 9.Yafi FA, Aprikian AG, Chin JL, Fradet Y, Izawa J, Estey E, Fairey A, Rendon R, Cagiannos I, Lacombe L, Lattouf JB, Bell D, Drachenberg D, Kassouf W. Contemporary outcomes of 2287 patients with bladder cancer who were treated with radical cystectomy: a Canadian multicentre experience. BJU international. 2011;108(4):539–545. doi: 10.1111/j.1464-410X.2010.09912.x. [DOI] [PubMed] [Google Scholar]
- 10.Baumann BC, Guzzo TJ, He J, Keefe SM, Tucker K, Bekelman JE, Hwang WT, Vaughn DJ, Malkowicz SB, Christodouleas JP. A novel risk stratification to predict local-regional failures in urothelial carcinoma of the bladder after radical cystectomy. International journal of radiation oncology, biology, physics. 2013;85(1):81–88. doi: 10.1016/j.ijrobp.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Boorjian SA, Kim SP, Weight CJ, Cheville JC, Thapa P, Frank I. Risk factors and outcomes of urethral recurrence following radical cystectomy. European urology. 2011;60(6):1266–1272. doi: 10.1016/j.eururo.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Escudero DO, Shirodkar SP, Lokeshwar VB. Bladder Carcinogenesis and Molecular Pathways. In: Lokeshwar VB, Merseburger AS, Haurmann SH, editors. Bladder Tumors: Molecular Aspects and Clinical Management. Humana Press; New York: 2011. pp. 23–41. [Google Scholar]
- 13.Colt JS, Friesen MC, Stewart PA, Donguk P, Johnson A, Schwenn M, Karagas MR, Armenti K, Waddell R, Verrill C, Ward MH, Freeman LE, Moore LE, Koutros S, Baris D, Silverman DT. A case-control study of occupational exposure to metalworking fluids and bladder cancer risk among men. Occupational and environmental medicine. 2014;71(10):667–674. doi: 10.1136/oemed-2013-102056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naito S, Tanaka K, Koga H, Kotoh S, Hirohata T, Kumazawa J. Cancer occurrence among dyestuff workers exposed to aromatic amines. A long term follow-up study. Cancer. 1995;76(8):1445–1452. doi: 10.1002/1097-0142(19951015)76:8<1445::aid-cncr2820760823>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 15.Boffetta P, Silverman DT. A meta-analysis of bladder cancer and diesel exhaust exposure. Epidemiology (Cambridge, Mass) 2001;12(1):125–130. doi: 10.1097/00001648-200101000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder JC, Conway K, Li Y, Mistry K, Bell DA, Taylor JA. p53 mutations in bladder cancer: evidence for exogenous versus endogenous risk factors. Cancer research. 2003;63(21):7530–7538. [PubMed] [Google Scholar]
- 17.Yun YP, Lee JY, Ahn EK, Lee KH, Yoon HK, Lim Y. Diesel exhaust particles induce apoptosis via p53 and Mdm2 in J774A.1 macrophage cell line. Toxicology in vitro : an international journal published in association with BIBRA. 2009;23(1):21–28. doi: 10.1016/j.tiv.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nature reviews Cancer. 2015;15(1):25–41. doi: 10.1038/nrc3817. [DOI] [PubMed] [Google Scholar]
- 19.Figueroa JD, Koutros S, Colt JS, Kogevinas M, Garcia-Closas M, Real FX, Friesen MC, Baris D, Stewart P, Schwenn M, Johnson A, Karagas MR, Armenti KR, Moore LE, Schned A, Lenz P, Prokunina-Olsson L, Banday AR, Paquin A, Ylaya K, Chung JY, Hewitt SM, Nickerson ML, Tardon A, Serra C, Carrato A, Garcia-Closas R, Lloreta J, Malats N, Fraumeni JF, Jr, Chanock SJ, Chatterjee N, Rothman N, Silverman DT. Modification of Occupational Exposures on Bladder Cancer Risk by Common Genetic Polymorphisms. Journal of the National Cancer Institute. 2015;107(11) doi: 10.1093/jnci/djv223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
References of high-risk occupations associated with bladder cancer in the literature