Menkes disease is an X-linked multisystem disorder with epilepsy, kinky hair, and neurodegeneration caused by mutations in the copper transporter ATP7A. Other ATP7A mutations have been linked to juvenile occipital horn syndrome and adult-onset hereditary motor neuropathy.1,2 About 5%–10% of the patients present with “atypical Menkes disease” characterized by longer survival, cerebellar ataxia, and developmental delay.2 The intracellular copper transport is regulated by 2 P type ATPase copper transporters ATP7A and ATP7B. These proteins are expressed in the trans-Golgi network that guides copper to intracellular compartments, and in copper excess, it relocates copper to the plasma membrane to pump it out from the cells.3 ATP7B mutations cause Wilson disease with dystonia, ataxia, tremor, and abnormal copper accumulation in the brain, liver, and other organs.4
Here, we report an ATP7A mutation, manifesting with an unusual complex phenotype resembling Wilson disease.
Methods.
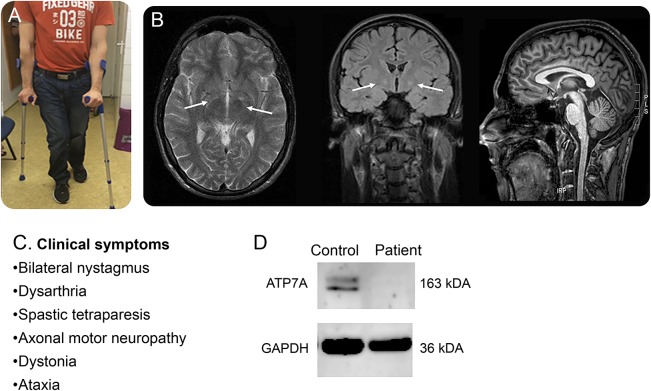
A 29-year-old man was born to a nonconsanguineous family; his father and paternal uncle suffer from genetically confirmed X-linked Kennedy disease. He achieved normal developmental milestones and manifested with progressive gait ataxia and proximal and distal leg weakness with early teens onset. Four limb spasticity evolved with extrapyramidal movement disorder, and he started using wheelchair at the age of 20. Clinical examination detected normal stature with no skeletal and joint changes and no connective tissue, cardiovascular, or hepatic abnormalities. He had normal vision and no evidence of Kayser-Fleischer rings, but bilateral nystagmus was present. He had severe spasticity and dystonia in all four extremities. Deep tendon reflexes were increased (4+) except for absent ankle jerks; clonus was present; and Babinski sign was positive. Cerebellar symptoms associated include intention tremor, dysmetria, and dysdiadochokinesis, and Romberg test was positive. His gait was spastic-ataxic (figure, A and C). He had dysarthria but preserved cognition and no mental illness. Routine laboratory investigations were normal. Metabolic tests including coeruloplasmin (0.19 g/L) and copper in serum and urine were repeatedly normal. EMG of the left tibial anterior muscle revealed increased insertional activity with fibrillations and larger motor units. Nerve conduction velocities were normal, but amplitudes were reduced in the peroneal and medial nerves, suggesting axonal motor neuropathy. Initial brain MRI at 9 years of age indicated high signal intensity of bilateral globus pallidus on T2-weighed images. Follow-up scan at age 29 years showed mildly increased signal intensity of bilateral globus pallidus on fluid-attenuated inversion recovery (FLAIR) sequences but not on T2-weighed images and mild cerebellar atrophy (figure, B).
Figure. Clinical presentation, neuroimaging, and immunoblotting.
(A) Photograph of the patient illustrates spasticity. (B) Neuroimages indicate bilateral abnormal signal intensity in the globus pallidus (T2, fluid-attenuated inversion recovery) and mild cerebellar atrophy (T1). (C) Leading clinical symptoms. (D) Immunoblot analysis detected severely reduced ATP7A protein in the patient's fibroblasts.
Genetic testing was negative for Kennedy disease and common ataxias. Illumina TruSeq 62 Mb exome capture, sequencing (100 bp paired-end reads, HiSeq 2000; Illumina, San Diego, CA), and alignment (UCSC hg19) was performed in the patient. Potentially deleterious recessive or X-linked variants were identified using QIAGEN Ingenuity Variant Analysis and validated by Sanger sequencing. Immunoblotting was performed using standard protocols.1
Results.
The patient carried the hemizygous c.2279A>G, p.(Tyr760Cys) variant in ATP7A. His healthy mother was heterozygous for the sequence change which was absent in her healthy brother. The variant was rare (Exome Aggregation Consortium: 4 in 87,766 heterozygous X chromosomes, no hemizygous), predicted highly deleterious by 5 different prediction tools, and affected a highly conserved residue in the third transmembrane domain of ATP7A. The neighboring p.(Ser761Pro) has been associated with the moderate Menkes phenotype.2 Immunoblotting confirmed severely reduced ATP7A protein in the patient's fibroblasts compared with the control (figure, D).
Discussion.
We identified the c.2279A>G, p.(Tyr760Cys) ATP7A variant in a patient with complex neurologic signs of spastic tetraparesis, ataxia, dystonia, and axonal motor neuropathy. The mutation segregated with the disease in the family and resulted in reduced ATP7A protein. Smaller amounts of functional ATP7A have been reported as sufficient to cause milder phenotypes.1 However, the association of spastic tetraparesis, ataxia, dystonia, and axonal motor neuropathy observed in our patient is remarkably different from any of the phenotypes reported with mutations in ATP7A. Wilson disease presents with heterogeneous hepatic and/or neurologic presentation, including variable combinations of dystonia, cerebellar, extrapyramidal, or psychiatric symptoms.4 White matter lesions and cerebral atrophy are seen in mild Menkes disease, but T2-weighted high signal intensities, indicating abnormal copper deposition in the globus pallidus, are more characteristic for Wilson disease, a copper retention disorder caused by ATP7B mutations.4 ATP7A variants as modifiers have been studied in Wilson disease based on a recent canine model carrying mutations in either ATP7A or ATP7B.5 The 2 proteins share sequence homology for residues involved in copper translocation, regardless of their directionally different trafficking. A 38 amino acid segment within the third transmembrane domain is implicated in the trans-Golgi retention of ATP7A.6 This same region is mutated in our patient suggesting subsequent ATP7A mislocalisation and misfolding in the disease mechanism. It is possible that the mutation triggers conformational changes and induces aberrant protein-protein interactions leading to impaired ATP7A trafficking.3
Our case supports the large phenotypic variability of ATP7A mutations and highlights that deficiency of the two copper transporter ATPases may cause overlapping phenotypes. ATP7A seem to be a human disease gene with very variable clinical presentations, and better understanding of these phenotypes may point to mechanistic overlap with other copper metabolism disorders, e.g., aceruloplasminemia. We recommend genetic screening for ATP7A mutations in patients who manifest clinical symptoms of Wilson disease without mutations in ATP7B.
Acknowledgments
Acknowledgment: The authors thank Agnes Sebok, MD, for some follow-up examinations of the patient.
Footnotes
Author contributions: Boglarka Bansagi: drafting the manuscript; study concept and design; and acquisition, analysis, and interpretation of data. David Lewis-Smith: analysis and interpretation of data. Endre Pal: acquisition of data. Jennifer Duff, Helen Griffin, Angela Pyle, and Juliane S. Müller: analysis and interpretation of data. Gabor Rudas and Zsuzsanna Aranyi: acquisition of data. Hanns Lochmüller and Patrick F. Chinnery: critical revision of manuscript for intellectual content. Rita Horvath: drafting/revision the manuscript; study concept and design; and study supervision.
Study funding: Supported by the Medical Research Council (MRC) Centre for Neuromuscular Diseases Biobank Newcastle and the EuroBioBank. R.H. was supported by the MRC (UK) (G1000848) and the European Research Council (309548) and is a Wellcome Trust Investigator (109915/Z/15/Z). B.B. is an MRC-funded Clinical Research Fellow. Z.A. was supported by the National Brain Research Program (NAP B) of the Hungarian government (KTIA_NAP_13-2-2014-0012). P.F.C. is a Wellcome Trust Senior Fellow in Clinical Science (101876/Z/13/Z) and a UK National Institute for Health Research (NIHR) Senior Investigator who receives support from the MRC Mitochondrial Biology Unit (MC_UP_1501/2), the Wellcome Trust Centre for Mitochondrial Research (096919Z/11/Z), the MRC (UK) Centre for Translational Muscle Disease (G0601943), EU FP7 TIRCON, and the NIHR Biomedical Research Centre based at Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Disclosure: Boglarka Bansagi has received research support from the MRC Centre for Neuromuscular Diseases. David Lewis-Smith, Endre Pal, Jennifer Duff, Helen Griffin, Angela Pyle, Juliane S. Müller, and Gabor Rudas report no disclosures. Zsuzsanna Aranyi has received research support from the Hungarian National Brain Research Program. Hanns Lochmüller has served on the scientific advisory board of the German Duchenne parents project, the IRDiRC Interdisciplinary Scientific Committee, the German Muscular Dystrophy Network, the Myotubular Trust Patient Registry, the Action Duchenne Patient Registry, and the German Patient Registries on DMD and SMA; has served on the editorial boards of the Journal of Neuromuscular Disease and the Journal of Neurology; has been a consultant for Roche Pharmaceuticals, ASD Therapeutics Partners LLC, IOS Press, Alexion Pharmaceuticals Inc., Ultragenyx Pharmaceutical Inc., and Fondazione Cariplo; and has received research support from Marigold Foundation Ltd, Ultragenyx Pharmaceutical Inc, PTC Therapeutics Inc, Eli Lilly and Co., Action Benni & Co e.v, GSK (GlaxoSmithKline), Trophos SA, European Commission, Medical Research Council (MRC), NIH, Action Duchenne, Association Francaise Contre les Myopathies, the British Heart Foundation, Muscular Dytrophy UK, National Cancer Institute, Spinal Muscular Atrophy Support UK, Wellcome Trust, Jennifer Trust, and Duchenne Parent Project. Patrick F. Chinnery has served on the editorial board of BRAIN and has received research support from Medical Research Council (UK), NIHR (England), and Wellcome Trust. Rita Horvath reports no disclosures. Go to Neurology.org/ng for full disclosure forms. The Article Processing Charge was paid by the Wellcome Trust.
References
- 1.Møller LB. Small amounts of functional ATP7A protein permit mild phenotype. J Trace Elem Med Biol 2015;31:173–177. [DOI] [PubMed] [Google Scholar]
- 2.Tümer Z. An overview and update of ATP7A mutations leading to Menkes disease and occipital horn syndrome. Hum Mutat 2013;34:417–429. [DOI] [PubMed] [Google Scholar]
- 3.Telianidis J, Hung YH, Materia S, Fontaine SL. Role of the P-Type ATPases, ATP7A and ATP7B in brain copper homeostasis. Front Aging Neurosci 2013;5:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coffey AJ, Durkie M, Hague S, et al. . A genetic study of Wilson's disease in the United Kingdom. Brain 2013;136:1476–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fieten H, Gill Y, Martin AJ, et al. . The Menkes and Wilson disease genes counteract in copper toxicosis in Labrador retrievers: a new canine model for copper-metabolism disorders. Dis Model Mech 2016;9:25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francis MJ, Jones EE, Levy ER, Ponnambalam S, Chelly J, Monaco AP. A Golgi localization signal identified in the Menkes recombinant protein. Hum Mol Genet 1998;7:1245–1252. [DOI] [PubMed] [Google Scholar]