Abstract
Recent studies have identified a role for supraphysiological GABA in the regulation of mTOR (mechanistic target of rapamycin), a protein kinase with pleiotropic roles in cellular development and homeostasis, including integration of growth factors and nutrient sensing, and synaptic input in neurons (Lakhani et al 2014; Vogel et al 2015). Aldehyde dehydrogenase 5a1-deficient (aldh5a1−/−) mice, the murine orthologue of human succinic semialdehyde dehydrogenase deficiency (SSADHD), manifest increased GABA that disrupts mitophagy and increases mitochondria number with enhanced oxidant stress. Treatment with the mTOR inhibitor, rapamycin, significantly attenuates these GABA-related anomalies. We extend those studies through characterization of additional rapalog agents including temsirolimus, dual mTOR inhibitors (torin 1 and 2 (Tor1/ Tor 2), Ku-0063794, and XL-765), as well as mTOR-independent autophagy inducers (trehalose, tat-Beclin 1, FK-506, and NF-449) in aldh5a1−/− mice. Rapamycin, Tor1, and Tor2 rescued these mice from premature lethality associated with status epilepticus. XL-765 extended lifespan significantly and induced weight gain in aldh5a1−/− mice; untreated aldh5a1−/− mice fail to increase body mass. Expression profiling of animals rescued with Tor1/Tor2 and XL-765 revealed multiple instances of pharmacological compensation and/or correction of GABAergic and glutamatergic receptors, GABA/glutamate transporters, and GABA/glutamate-associated proteins, with Tor2 and XL-765 showing optimal outcomes. Our studies lay the groundwork for further evaluation of mTOR inhibitors in aldh5a1−/− mice, with therapeutic ramifications for heritable disorders of GABA and glutamate neurotransmission.
Introduction
The inhibitory neurotransmitter GABA derives from excitatory glutamate via the catalytic action of glutamate decarboxylase (GAD). Subsequently, GABA converts to succinic acid via the sequential actions of aminobutyrate aminotransferase (ABAT) and succinic semialdehyde dehydrogenase (ALDH5A1; aldehyde dehydrogenase 5a1=succinic semialdehyde dehydrogenase (SSADH)). Mendelian disorders of each catabolic enzyme exist (Pearl et al. 2015; Lapalme-Remis et al. 2015; Rodan et al. 2015). SSADHD presents with non-specific mild to moderate developmental delay, severe expressive language impairment, variable epilepsy (Parviz et al. 2014; Pearl et al. 2014), and neuropsychiatric problems (i.e., ADHD, OCD, aggression). Metabolically, there is accumulation of glutamate, GABA and the GABA-derivative γ-hydroxybutyrate (GHB; Snead and Gibson 2005), the latter an intermediate with a varied and complex pharmacological history (Maitre et al. 2016) (Fig. 1).
Fig. 1. Interrelationships of GABA metabolism.
Arrows (downward/upward) indicate the direction of metabolite disruption in aldh5a1−/− mice. Abbreviations: Gln, glutamine; Glu, glutamate; GABA, 4-aminobutyrate; SSA, succinic semialdehyde; GHB, gamma-hydroxybutyrate; mTOR, mechanistic target of rapamycin; GAD, glutamic acid decarboxylase; GABA-T, GABA-transaminase; SSR, succinic semialdehyde reductase; GHBDH, gamma-hydroxybutyrate dehydrogenase; SSADH, succinic semialdehyde dehydrogenase (site of the defect in patients with SSADHD); GLS, glutamate synthetase; GLNASE, glutaminase. Vigabatrin (VGB), an antiepileptic and irreversible inhibitor of GABA-T, is a frequently employed therapeutic agent for SSADHD.
Although historically considered a CNS inhibitory neurotransmitter, a growing literature underscores broader implications for GABA in peripheral roles, as well as in mTOR signaling. Mechanistic target of rapamycin (mTOR) regulates cellular development and homeostasis including integration of growth factors and nutrient sensing, and synaptic input in neurons (Lafourcade et al. 2013; Santinon et al. 2015; Han et al. 2016). For example, mTOR mediates synaptic regulation by modulation of synapse number and miniature inhibitory postsynaptic currents (Weston et al. 2012a). Hyperactive mTOR increases evoked synaptic responses in both glutamatergic and GABAergic neurons, and the glutamatergic component is corrected by the mTOR inhibitor rapamycin. Workman and colleagues demonstrated that GABAB receptors can activate mTOR via calcium signaling, and further demonstrated that signaling of the GABAB receptor was necessary for mTOR-dependent protein synthesis (Workman et al. 2013). These few publications highlight the complex synergy that appears to exist between GABA and mTOR.
Lakhani and coworkers recently identified a novel relationship between GABA and autophagy in yeast in which elevated GABA impaired both mitophagy and pexophagy (Lakhani et al. 2014). These findings were extended to the aldh5a1−/− mouse, a model for which intervention with rapamycin resulted in a significant mitigation of hepatic elevations of pS6 (a kinase linked to mTOR function), superoxide dismutase (SOD), and mitochondrial number. Comparable impairments of autophagy have recently been documented for vigabatrin, an antiepileptic agent that irreversibly inactivates ABAT and elevates GABA (Vogel et al. 2015). Accordingly, the mTOR pathway appears to be a viable therapeutic target for disorders featuring dysregulated GABA homeostasis. To further explore this hypothesis, we have examined the effect of various modulators of mTOR and autophagy in aldh5a1−/− mice to further interrogate the preclinical efficacy of this approach. Here, we summarize the outcomes of our studies.
Methods
Reagents and drugs
Rapamycin, Torin 1, Torin 2, Temsirolimus, XL765, Ku-0063794, FK-506, and NF-449 were purchased from Cayman Chemical (Ann Arbor MI) and prepared in DMSO at 25 mg/mL. Trehalose was obtained from Sigma Aldrich (St. Louis MO), and prepared in DMSO at 250 mg/mL. Tat-Beclin 1(tat-Bec1) human recombinant peptide was purchased from EMD Millipore (Billerica MA) and prepared at concentrations of 25 and 125 mg/mL. Cell culture grade dimethyl sulfoxide (DMSO) was obtained from Thermo Fisher (Waltham MA).
Animal studies
All animal procedures were approved by the Washington State University IACUC (protocol 4232 and 4276). Tail biopsy of aldh5a1 mice was performed at DOL 10–12, followed by DNA extraction and genotyping by 3 primer 2 reaction PCR (Hogema et al. 2001). This procedure was repeated at the conclusion of survival studies in order to confirm genotype. For drug treatment, stock solutions were diluted in PBS based on body weight to a total injection volume of 50 μl. Intraperitoneal injections were given daily between 0700–1000 hours. Rapamycin, Tor1, Tor2, temsirolimus, XL765, Ku-0063794, and FK506 were administered at 5 mg/kg/day; Tor 2 was also further characterized at 10 mg/kg/day. Tat-Bec1 was evaluated at 5 and 25 mg/kg/day, and trehalose at 100 mg/kg/day. Large litters of mice were culled to less than six pups after completion of the first round of genotyping. At weaning (21 days of age), mice were housed with 1–2 identical gender litter mates (not singly). Kaplan-Meier plots of survival data were generated with GraphPad Prism 6, which computed survival proportions and log-rank (Mantel-Cox) test p values, with 0.05 set as the threshold for significance.
Expression Studies
RNA was prepared by pooling liver or brain tissues with n=4 for wild-type (Wt) and mutant (Mt) mice, day of life (DOL) 21, n=2 survivors for Tor1 (DOL 50); n=1 survivor each for 5mg/kg Tor 2, tat-Bec1 and XL-765 (DOL 50), and homogenized with TRIzol® (Invitrogen). The aldh5a1−/− model demonstrates uniform lethality by DOL 24 from status epilepticus (Hogema et al. 2001; Gupta et al. 2002), such that survival to DOL 50 is highly significant. Chloroform was added to pooled tissues, and samples were separated by centrifugation to isolate the RNA-enriched aqueous supernatant. RNA was subsequently precipitated and washed with isopropanol and ethanol followed by brief air drying and reconstitution in water. RNA was quantified via absorbance (260 nm) and purity assessed using 260 nm/280 nm absorbance ratio (> 1.8), with storage at −80°C. iScript mastermix (Biorad, Hercules CA) was employed to generate cDNA in an Eppendorf mastercycler per manual instructions. Sybr green (Biorad) reactions (10 μl) were loaded with 50 ng of cDNA/well into prearrayed and validated, per MIQE guidelines (Bustin et al. 2009), GABA/Glutamate analysis plates (M394 PrimePCR™ SABioscience target list). A BioRad CFX384 real-time PCR operated with BioRad CFX manager v3.0 software was employed for data acquisition, normalization, expression and statistical analysis with significance set at 0.05 based upon two replicates per group.
Results
Rescue of aldh5a1−/− mice from premature lethality – mTOR and dual Inhibitors of autophagy
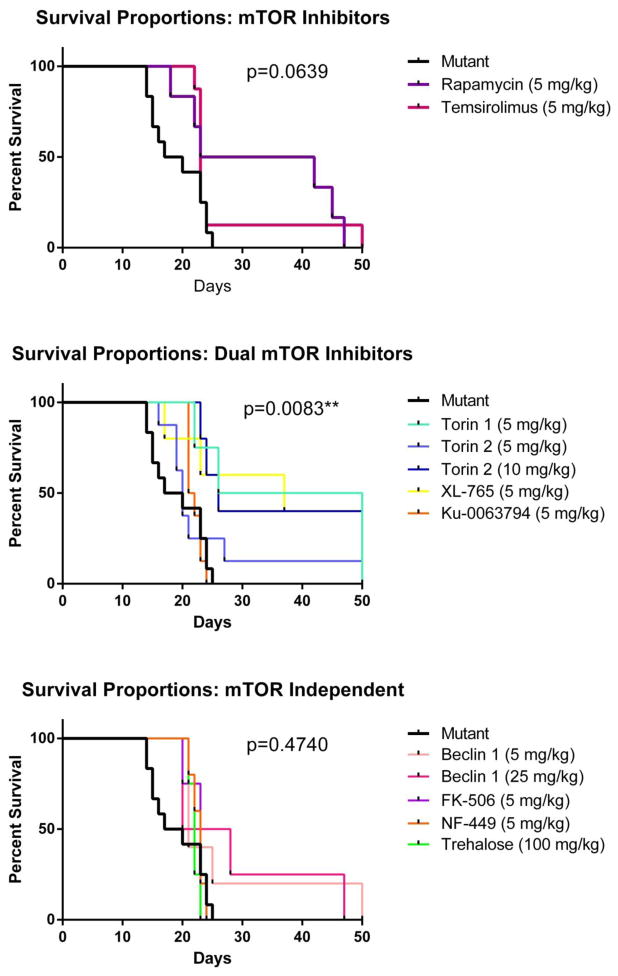
Ald5ha1−/− mice (mixed gender) provided baseline data, with survival to a maximum of 25 days (n=12, 50% at 17 days). Rapamycin, the classic mTOR complex 1 (mTORC1) inhibitor, extended lifespan of 3 aldh5a1−/− mice to 42, 45 and 47 days (n=6, 50% survival to 23 days, 5 mg/kg body weight, p=0.0327*). Temsirolimus, a next generation mTORC1 inhibitor with improved bioavailability, extended lifespan of one mouse to 50 days (n=8, 50% survival at 22–23 days, 5 mg/kg, p=ns) (Fig. 2). Tor1, Tor2 and Ku-0063794 inhibit both complexes of mTOR, mTORC1 and mTORC2. Tor1 extended lifespan for two aldh5a1−/− mice to 50 days of life (n=4, 50% survival to 26 days, 5 mg/kg dose, p=0.0116*). Adverse effects observed in aldh5a1−/− mice with Tor1 included development of fibrotic tissue at the injection site, ataxia and distended limbs. Tor2 manifested none of these side effects. Tor2 extended lifespan of aldh5a1−/− mice, including one mouse at the 5 mg/kg dose (n=8, 50% survival to 19–20 days, p=ns) and two mice at 10 mg/kg (n=5, 50% survival to 25 days, p=0.0074**). Ku-0063794 had no effect on longevity of aldh5a1−/− mice (n=8, 50% survival to 22 days, 5 mg/kg, p=ns). Aldh5a1−/− mice are essentially static for, or lose, body weight from two weeks of age until expiration (Gupta et al. 2002; Nylen et al. 2008a). XL-765 is a potent dual inhibitor of PI3K and mTOR known to permeate the blood-brain barrier, and its use resulted in extended survival and modest improvement to body weight of aldh5a1−/− mice out to 50 days (n=4, 40% survival to 37 days, 5 mg/kg, p=0.0194*).
Fig. 2. Modified Kaplan-Meier survival curves for aldh5a1−/− mice.
Top, mTOR inhibitors; middle, mTOR-independent inhibitors of autophagy; bottom, mixed (dual) mTOR inhibitors. P-values indicated in this figure correspond to the overall data plotted on each pane for each class of inhibitor. For drug specific statistical evaluations, please see text.
Rescue of aldh5a1−/− mice from premature lethality – mTOR-independent inducers of autophagy
FK-506 (tacrolimus), an inhibitor of calcineurin and an mTOR-independent inducer of autophagy, extended lifespan of aldh5a1−/− mice up to 23 days (n=4, 75% survival to 20 days, 5 mg/kg, p=ns). NF-449, a suramin analog and P2X1 inhibitor, induces autophagy through impaired binding of the G protein Gsα-s NF-449did not extend lifespan (n=5, 60% survival to 22 days, 5 mg/kg, p=ns). Trehalose, a disaccharide which induces autophagy independent of mTOR, extended lifespan of aldh5a1−/− mice up to 23 days of life (n=4, 50% survival to 21–22 days, 100 mg/kg, p=ns). Tat-Bec1, an autophagy inducing peptide potentially acting through GAPR-1 (Shoji-Kawata et al. 2013), extended the lifespan of one aldh5a1−/− mouse to 50 days (n=5, 50% survival to 17–20 days, 5 mg/kg, p=ns) and one aldh5a1−/− mouse to 47 days of life (n=4, 50% survival to 20 days, 25 mg/kg, p=ns) (Fig. 2).
Improved body weight in aldh5a1−/− mice via mTOR
In addition to early lethality, aldh5a1−/− mice are runted and essentially show little capacity for weight gain from birth until death. The only effective mechanism thus far for induction of weight gain in this animal has been the ketogenic diet (Nylen et al. 2008b). In our study, we monitored weight gain as a function of drug intake (Fig. 3). Fig. 3 presents body mass for the longest lived individual mouse as a function of drug intervention. In our hands, weight gain (best to worst) revealed the following trend: XL-765 >Tor1~ Tor 2~tat-Bec1 > rapamycin.
Fig. 3. Anthropormorphic characteristics (body weight) of longest surviving animals undergoing therapy.
Smoothed trendline characteristics of daily body weight with treatment intervention (RM, rapamycin; Tor1, torin 1; Tor2, torin 2).
Gene expression profiling
We assessed the pharmacologic effects of Tor1/ Tor 2, XL-765 and tat-Bec1 on GABA and glutamate receptor subunits and transporters, as well as GABA and glutamate related enzymes and proteins, in the brain. The pooled tissues represented n=4 each untreated aldh5a1+/+ mice and untreated aldh5a1−/− animals (DOL 21), n=2 Tor1 treated aldh5a1−/− mice, and one each Tor2, XL-765 and tat-Bec1 treated aldh5a1−/− animal. Statistical analysis for the arrays employed a one-way ANOVA with post-hoc Bonferroni t-test correction. Aldh5a1−/− mice (treated or untreated) were compared to the expression level detected for untreated, control aldh5a1+/+ mice (expression set to 1.0).
We initially focused attention on GABA receptor subunits and transporters (both vesicular and non-vesicular; Fig. 4). As predicted, for all subunits and transporters, the direction of expression was down-regulation in untreated aldh5a1−/− mice, expected with use-dependent effects of elevated GABA. Intervention with only Tor1 led to correction of Gabrb1 and Gabrg expression level. Tor 2, XL-765 and tat-Bec1 were effective in leading to correction of subunit expression, normalizing the levels of Gabrb3, Gabre, Slc32a1 and Slc6a11. Both Tor1 and Tor 2 resulted in the correction of Gabra1, Gabra2 and Slc6a12 expression. Tor1, XL-765 and tat-Bec1 normalized Gabbr1, and XL-765 and tat-Bec1 also normalized Gabbr2. It was of interest that Tor2 did not correct GABAB receptor (metabotropic) anomalies, while numerous corrections of GABAA receptors (ionotropic) were observed.
Fig. 4. Gene array expression data for GABA receptors and transporters in the brain.
GABAA and GABAB receptor subunits and names, as well as GABA transporters, are shown above individual graphs. Expression in wild-type mice is set to 1.0. For methodological and statistical details, see text.
We next turned our attention to glutamate receptors and transporters. The former included kainate, NMDA, AMPA and metabotropic glutamate receptors, while the latter included both vesicular glutamate as well as excitatory amino acid transporters (Fig. 5). As was the case for GABA receptor subunits and transporters, the direction for expression in untreated aldh5a1−/− mice was down-regulation, as expected since glutamate is also elevated in this model (Gupta et al. 2004). The sole exception was the up-regulation of the transporter Slc7a11 (Fig. 5). Intervention with Tor1 corrected the expression of Gria3, Grik5, Grin2b, and Slc1a1. Intervention with Tor 2 resulted in the corrected expression of Gria1, Grm7, Slc17a6, Slc17a8, and Slc38a1. Both interventions lead to the correction of Gria2, Gria4, Grik2, Grin2b, Grin2c, Grm1, Grm2, Grm4, Grm5, Grm8, and Slc1a2. XL-765 corrected Grin2b, Grm1, Grm2, Grm5, Slc17a6, Slc17a8, Slc1a1, Slc1a2, and Slc38a1. Tat-Bec1 corrected Grik5, Grin2b, Grm1, Grm6, Slc17a6, Slc17a8 and Slc1a2.
Fig. 5. Gene array expression data for glutamate receptors and transporters in the brain.
Glutamate receptor (AMPA, kainate, NMDA and metabotropic) subunits and names are shown above individual graphs. Expression in wild-type mice is set to 1.0. For methodological and statistical details, see text.
Finally, we extended the preceding studies to include GABA- and glutamate-related enzymes and proteins (Fig. 6). As for the GABA and glutamate receptors and transporters, the direction of expression in untreated aldh5a1−/− mice was down-regulation, with only a single instance of upregulation (B2M). Tor2 corrected the aberrant expression of App, Cln3, Gls, Glul, Nsf and Snca (see Fig. 5 for abbreviations). Tor1 corrected the aberrant expression of B2M. All the treatments (Tor1, Tor2, XL-765, and tat-Bec1) corrected Abat, Cln3, Gad1, Gls, Glul, Gphn, Hsp90ab1, Nsf, Pla2g6 and Srr. In some instances, the relationship of these genes to GABA/glutamate metabolism is readily apparent (e.g., Gls, glutamate synthetase; Glul, glutaminase; Abat, aminobutyrate aminotransferase (GABA-transaminase); Gad1, glutamic acid decarboxylase), but the relationship of other genes is not immediately obvious. Fig. 7 provides a schematic overview of the interactions for these various proteins and enzymes between glial cells and the inhibitory GABAergic and excitatory glutamatergic synapses.
Fig. 6. Gene array expression data for GABA and glutamate related enzymes and proteins in the brain.
Gene names are shown above individual graphs. Expression in wild-type mice is set to 1.0. For methodological and statistical details, see text. Fig. 7 provides a schematic that clarifies the role of these genes/receptors/enzymes (putative and proven) in both glutamatergic and GABAergic signaling.
Fig. 7. Schematic of the interralationships of glial cell function with that of glutamatergic and GABAergic synapses.
The roles of Aldh5a1 (localized to the mitochondria, not shown), Abat (4-Aminobutyrate Aminotransferase), Gad1 (glutamate decarboxylase 1), Gls (glutaminase) and Glul (ferredoxin-dependent glutamate synthase 1) are recognizable. Other genes characterized in array expression (Fig. 4) data and whose role(s) are less readily apparent in glial-neuronal signaling include (circled in the figure): Adora2a (adenosine A2A receptor), a heteroreceptor modulating glutamate/GABA release; App (amyloid beta precursor protein), a soluble oligomeric protein interacting with NMDA receptors; Avp (arginine vasopressin), a hormone active in stimulation of regional GABAergic neurons; Cln3 (ceroid lipofuscinosis 3), a gene shown to perturb glutamine and glutamate levels in cortex and cerebellum; Gphn (gephyrin), a scaffold protein stabilizing GABAA receptors; Itpr1 (inositol 1,4,5-trisphosphate receptor1), a phospholipase receptor with interactions targeting the metabotropic glutamate receptor (mGluR); Nsf (N-ethylmaleimide-sensitive factor), a protein interacting with AMPA-type glutamate receptors; Pla2g6 (phospholipase A2 group VI), one of multiple phospholipases regulating the release of aspartate glutamate, glycine and GABA; Snca (synuclein alpha), a secreted protein interacting with ATP-sensitive potassium channels modulating GABA transmission; and Srr (serine racemase), an activator of NMDA receptors. Not shown are β-2-microglobulin (B2M) and heat shock protein 90kDa alpha class B member 1 (Hsp90ab1), both of which may exert chaperone-type roles in scaffolding of oligomeric glutamatergic and GABAergic receptors.
Discussion
The current report expands the link between supraphysiological GABA and mTOR signaling described in earlier studies (Lakhani et al. 2014; Vogel et al. 2015). Until now, drugs that have extended the lifespan of aldh5a1−/− mice have included taurine, vigabatrin (an irreversible inhibitor of GABA-transaminase), CGP-35348 (a GABAB receptor antagonist), NCS-382 (a GHB receptor antagonist), and the ketogenic diet (Hogema et al. 2001; Gupta et al. 2002; Nylen et al. 2008b). With the exception of taurine and the ketogenic diet, all target either GABA metabolism, or GABAergic/GHBergic neurotransmission. Taurine was evaluated because of its high content in dam’s milk associated with premature lethality of ald5ha1−/− mice in the weaning period. Taurine also possesses diffuse GABAA and GABAB receptor modulatory effects (Gupta et al. 2002). An open-label trial of taurine in SSADHD patients was, however, without clinical benefit (Pearl et al. 2014). Here, we present new evidence of attenuation of premature lethality in our murine model using mTOR inhibitors, supported by expression array data on GABAergic/glutamatergic systems revealing pharmacological correction with mTOR inhibitors (Donarum et al. 2006).
We found Tor 2, XL-765 and tat-Bec1 to be optimal therapeutic agents for aldh5a1−/− mice and that dual inhibition of mTORC1/2 was more effective than mTORC1 inhibition alone. Similar outcomes for survival occurred for tat-Bec1, emphasizing the importance of autophagy activation in the aldh5a1−/− mice. Tat-Bec1, not to be confused with exogenous beclin 1 that is an inducer of apoptosis and autophagy (Kang et al. 2011), was the only mTOR-independent autophagy inducer tested (including trehalose, tat-Bec1, FK-506, and NF-449) that increased survival. Similarity of the expression profiles between the dual mTORC inhibitors (Tor2, XL-765) and tat-Bec1 may be related to the feedback mechanism between autophagy and mTOR and inhibitory effects on mTOR during autophagy initiation by the tat-Bec 1peptide. The adverse effect profile for Tor1 was substantial, even with multiple gene expression corrections (Figs. 4–6). In our hands, these three treatments (Tor 2, XL-765, tat-Bec 1) had no visible effects on the aldh5a1−/− phenotype, other than the expected neurological morbidity normally encountered (mild ataxia). Substantial metabolic and neurophysiological data, from our laboratory and others, has previously documented disruptions of the glutamate/glutamine/GABA axis in aldh5a1−/− mice, in addition to abnormalities of GABAA and GABAB receptors. Early metabolic studies verified elevations of GHB, GABA, and glutamate (Gupta et al. 2002; Gupta et al. 2004) that correlated with alterations of glutamate/glutamine cycling in the CNS (Chowdhury et al. 2007). Neurophysiological and neurochemical characterization of aldh5a1−/− mice verified central, as well as localized (hippocampal, cortical) disruption of GABAergic neurotransmission that manifested as down-regulated GABAB and GABAA receptors (Cortez et al. 2004; Wu et al. 2006; Buzzi et al. 2006; Drasbek et al. 2008; Dósa et al. 2010; Vardya et al. 2010; Errington et al. 2011). The data in mice have been confirmed in SSADHD patients using transcranial magnetic stimulation for GABAB receptors (Reis et al. 2012) and 11C-flumazenil binding for GABAA receptors (Pearl et al. 2009), as well as magnetic resonance spectroscopy (Ethofer et al. 2004; Wang et al. 2015) with spectral editing for GABA and Glx (Glx=glutamine and glutamate combined). The current results, indicating significant down-regulation of receptor subunits and transporters, is consistent with the mouse and human neurophysiological/neurochemical data, but the pharmacological compensation with mTOR inhibitors, is quite novel and clinically relevant (Figs. 4–6).
An exciting finding in our study was the effect of Tor1 and Tor 2 on glutamatergic systems (Fig. 5), which was unexpected despite our previous demonstration of glutamate increase in the CNS of aldh5a1−/− mice (Gupta et al. 2004). A link between glutamatergic signaling and mTOR activation is beginning to emerge (Weston et al. 2012b; Theilhaber et al. 2013; Chen et al. 2015; Rosenberg et al. 2015). These observations are relevant to aldh5a1−/− embryonic mice, in which neurotransmitter imbalances are evident as early as E13, a developmental period in which GABAergic neurotransmission is still excitatory with the potential for development of a heightened excitatory state that may predispose neural networks to epilepsy in the embryonic period (Jansen et al. 2008). Our results for pharmacological compensation of glutamatergic systems with Tor1 and Tor 2 suggest it will be worthwhile to investigate glutamatergic neurophysiology in aldh5a1−/− mice as a readout of treatment efficacy, an area which has been underexplored in this animal model.
We found that mTOR-independent activators of autophagy were far less effective in rescue of aldh5a1−/− mice from premature lethality (Fig. 2), with the exception of tat-Bec1. Conversely, dual mTOR pathway inhibitors appeared to be superior to mTOR complex 1 (mTORC1) inhibitors in facilitating weight gain and longevity (Fig. 3). Moreover, the data of Fig. 6 were of interest with respect to broader pathway compensation for aldh5a1−/− mice treated with Tor1 and 2, revealing robust correction of the down-regulated expression of Abat, Gad1, Gls and Glul (Fig. 4). The relationship(s) of many of the GABA- and glutamate-associated proteins and genes to GABAergic/glutamatergic signaling is schematically diagrammed in Fig. 7. Two genes quantified in the array data of Fig. 5 were of additional interest. The down-regulation of both Adora2a and Avp were significantly exacerbated in Tor1 animals by ~ 50- to 60-fold. The adenosine A2A receptor (Adora2a) broadly functions in GABA and glutamate release (heteroreceptors), while arginine vasopressin (Avp) is the precursor of antidiuretic hormone (Vaz et al. 2015). This observation in the Tor1 group on Avp may have underpinned some of the adverse effects we observed with this mTORC1/2 inhibitor. Nevertheless, these two results would strengthen the argument for not using Tor1 intervention in aldh5a1−/− mice. It is of interest that the accumulation of GABA in both aldh5a1−/− mice alters arginine metabolism and induces accumulation of guanidinobutyrate and guanidinoacetate (Jansen et al. 2006), which may provide some insight into alterations of arginine vasopressin (Avp).
In summary, the results presented provide evidence for pharmacological compensation of GABAergic/glutamatergic networks in aldh5a1−/− mice treated with the mTOR inhibitors Tor1 and Tor 2. The clinical relevance of these findings for treatment of SSADHD are significant, since it is likely that multiple treatment approaches will be needed to gain incremental improvements in the phenotype. Nonetheless, the potential of mTOR inhibitors to compensate transcriptional abnormalities in central GABAergic/glutamatergic pathways is an attractive consideration in the therapeutics of SSADHD. The translational relevance of this finding is high, since mTOR inhibitors (primarily rapamycin and temsirolimus) are actively under study in clinical trials (www.clinicaltrials.gov). More broadly, our data supports consideration of mTOR inhibitors in other Mendelian disorders, including GABA-transaminase deficiency (Pearl et al. 2015), an expanding disorder of GABA metabolism, as well as disorders of glutamate receptors (Morice et al. 2013; Soto et al. 2014). Future studies in aldh5a1−/− mice will further gauge correction of the phenotype of aldh5a1−/− mice with mTOR inhibitors using electrocorticographic assessment (Cortez et al. 2004). These efforts will likel include more complete metabolic profiling of key metabolites (e.g., GABA, SSA, succinate, γ-hydroxybutyrate ) as well as further evaluation of candidate clinical biomarkers.
Synopsis.
Dual mTOR complex 1/2 and PI3K inhibitors extend lifespan, improve bodyweight and correct GABA/glutamate receptor expression profiles in SSADH-deficient mice.
Acknowledgments
This work was supported in part by R21 NS 85369
Abbreviations
- GABA
gamma-aminobutyric acid
- Glu
glutamate
- Tor1
Torin 1
- Tor 2
Torin 2
- SSADH
succinic semialdehyde dehydrogenase
- SSADHD
succinic semialdehyde dehydrogenase deficiency
- aldh5a1 (identical to succinic semialdehyde dehydrogenase)
aldehyde dehydrogenase 5a1
Footnotes
Conflict of interest:
Kara R. Vogel declares that she has no conflict of interest.
Garrett R. Ainslie declares that he has no conflict of interest
K. Michael Gibson declares that he has no conflict of interest.
Compliance with Ethics Guidelines
Animal Rights:
All institutional and national guidelines of the care and use of laboratory animals were followed.
Author contributions
Contributed to experimental design and execution: Kara R. Vogel, Garrett R. Ainslie
Data analysis and interpretation: Kara R, Vogel, K. Michael Gibson
Contribution to preparation of the manuscripts: Kara R. Vogel, Garrett R. Ainslie, K. Michael Gibson
References
- Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Buzzi A, Wu Y, Frantseva MV, et al. Succinic Semialdehyde Dehydrogenase Deficiency: GABAB receptor-mediated function. Brain Res. 2006;1090:15–22. doi: 10.1016/j.brainres.2006.02.131. [DOI] [PubMed] [Google Scholar]
- Chen W-Y, Cheng Y-H, Hsieh N-H, et al. Physiologically based pharmacokinetic modeling of zinc oxide nanoparticles and zinc nitrate in mice. Int J Nanomedicine. 2015;10:6277–6292. doi: 10.2147/IJN.S86785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury GMI, Gupta M, Gibson KM, et al. Altered cerebral glucose and acetate metabolism in succinic semialdehyde dehydrogenase-deficient mice: evidence for glial dysfunction and reduced glutamate/glutamine cycling. J Neurochem. 2007;103:2077–2091. doi: 10.1111/j.1471-4159.2007.04887.x. [DOI] [PubMed] [Google Scholar]
- Cortez MA, Wu Y, Gibson KM, Snead OC. Absence seizures in succinic semialdehyde dehydrogenase deficient mice: a model of juvenile absence epilepsy. Pharmacol Biochem Behav. 2004;79:547–553. doi: 10.1016/j.pbb.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Donarum EA, Stephan DA, Larkin K, et al. Expression profiling reveals multiple myelin alterations in murine succinate semialdehyde dehydrogenase deficiency. J Inherit Metab Dis. 2006;29:143–156. doi: 10.1007/s10545-006-0247-6. [DOI] [PubMed] [Google Scholar]
- Dósa Z, Nieto-Gonzalez JL, Korshoej AR, et al. Effect of gene dosage on single-cell hippocampal electrophysiology in a murine model of SSADH deficiency (gamma-hydroxybutyric aciduria) Epilepsy Res. 2010;90:39–46. doi: 10.1016/j.eplepsyres.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasbek KR, Vardya I, Delenclos M, et al. SSADH deficiency leads to elevated extracellular GABA levels and increased GABAergic neurotransmission in the mouse cerebral cortex. J Inherit Metab Dis. 2008;31:662–668. doi: 10.1007/s10545-008-0941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington AC, Gibson KM, Crunelli V, Cope DW. Aberrant GABA(A) receptor-mediated inhibition in cortico-thalamic networks of succinic semialdehyde dehydrogenase deficient mice. PloS One. 2011;6:e19021. doi: 10.1371/journal.pone.0019021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethofer T, Seeger U, Klose U, et al. Proton MR spectroscopy in succinic semialdehyde dehydrogenase deficiency. Neurology. 2004;62:1016–1018. doi: 10.1212/01.wnl.0000115385.45515.df. [DOI] [PubMed] [Google Scholar]
- Gupta M, Greven R, Jansen EEW, et al. Therapeutic intervention in mice deficient for succinate semialdehyde dehydrogenase (gamma-hydroxybutyric aciduria) J Pharmacol Exp Ther. 2002;302:180–187. doi: 10.1124/jpet.302.1.180. [DOI] [PubMed] [Google Scholar]
- Gupta M, Polinsky M, Senephansiri H, et al. Seizure evolution and amino acid imbalances in murine succinate semialdehyde dehydrogenase (SSADH) deficiency. Neurobiol Dis. 2004;16:556–562. doi: 10.1016/j.nbd.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Han C, Wei S, Song Q, et al. Insulin Stimulates Goose Liver Cell Growth by Activating PI3K-AKT-mTOR Signal Pathway. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2016;38:558–570. doi: 10.1159/000438650. [DOI] [PubMed] [Google Scholar]
- Hogema BM, Gupta M, Senephansiri H, et al. Pharmacologic rescue of lethal seizures in mice deficient in succinate semialdehyde dehydrogenase. Nat Genet. 2001;29:212–216. doi: 10.1038/ng727. [DOI] [PubMed] [Google Scholar]
- Jansen EEW, Struys E, Jakobs C, et al. Neurotransmitter alterations in embryonic succinate semialdehyde dehydrogenase (SSADH) deficiency suggest a heightened excitatory state during development. BMC Dev Biol. 2008;8:112. doi: 10.1186/1471-213X-8-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen EEW, Verhoeven NM, Jakobs C, et al. Increased guanidino species in murine and human succinate semialdehyde dehydrogenase (SSADH) deficiency. Biochim Biophys Acta. 2006;1762:494–498. doi: 10.1016/j.bbadis.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafourcade CA, Lin TV, Feliciano DM, et al. Rheb activation in subventricular zone progenitors leads to heterotopia, ectopic neuronal differentiation, and rapamycin-sensitive olfactory micronodules and dendrite hypertrophy of newborn neurons. J Neurosci Off J Soc Neurosci. 2013;33:2419–2431. doi: 10.1523/JNEUROSCI.1840-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhani R, Vogel KR, Till A, et al. Defects in GABA metabolism affect selective autophagy pathways and are alleviated by mTOR inhibition. EMBO Mol Med. 2014;6:551–566. doi: 10.1002/emmm.201303356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapalme-Remis S, Lewis EC, De Meulemeester C, et al. Natural history of succinic semialdehyde dehydrogenase deficiency through adulthood. Neurology. 2015 doi: 10.1212/WNL.0000000000001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitre M, Klein C, Mensah-Nyagan AG. Mechanisms for the Specific Properties of γ-Hydroxybutyrate in Brain. Med Res Rev. 2016 doi: 10.1002/med.21382. [DOI] [PubMed] [Google Scholar]
- Morice E, Farley S, Poirier R, et al. Defective synaptic transmission and structure in the dentate gyrus and selective fear memory impairment in the Rsk2 mutant mouse model of Coffin-Lowry syndrome. Neurobiol Dis. 2013;58:156–168. doi: 10.1016/j.nbd.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Nylen K, Velazquez JLP, Likhodii SS, et al. A ketogenic diet rescues the murine succinic semialdehyde dehydrogenase deficient phenotype. Exp Neurol. 2008a;210:449–457. doi: 10.1016/j.expneurol.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylen K, Velazquez JLP, Likhodii SS, et al. A ketogenic diet rescues the murine succinic semialdehyde dehydrogenase deficient phenotype. Exp Neurol. 2008b;210:449–457. doi: 10.1016/j.expneurol.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parviz M, Vogel K, Gibson KM, Pearl PL. Disorders of GABA metabolism: SSADH and GABA-transaminase deficiencies. J Pediatr Epilepsy. 2014;3:217–227. doi: 10.3233/PEP-14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl PL, Gibson KM, Quezado Z, et al. Decreased GABA-A binding on FMZ-PET in succinic semialdehyde dehydrogenase deficiency. Neurology. 2009;73:423–429. doi: 10.1212/WNL.0b013e3181b163a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl PL, Koenig M, Riviello J, et al. Novel intervention in GABA-transaminase deficiency 2015 [Google Scholar]
- Pearl PL, Parviz M, Vogel K, et al. Inherited disorders of gamma-aminobutyric acid metabolism and advances in ALDH5A1 mutation identification. Dev Med Child Neurol. 2014 doi: 10.1111/dmcn.12668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Cohen LG, Pearl PL, et al. GABAB-ergic motor cortex dysfunction in SSADH deficiency. Neurology. 2012;79:47–54. doi: 10.1212/WNL.0b013e31825dcf71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan LH, Gibson KM, Pearl PL. Clinical Use of CSF Neurotransmitters. Pediatr Neurol. 2015;53:277–286. doi: 10.1016/j.pediatrneurol.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Niglio SA, Salehomoum N, et al. Targeting Glutamatergic Signaling and the PI3 Kinase Pathway to Halt Melanoma Progression. Transl Oncol. 2015;8:1–9. doi: 10.1016/j.tranon.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santinon G, Pocaterra A, Dupont S. Control of YAP/TAZ Activity by Metabolic and Nutrient-Sensing Pathways. Trends Cell Biol. 2015 doi: 10.1016/j.tcb.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Shoji-Kawata S, Sumpter R, Leveno M, et al. Identification of a candidate therapeutic autophagy-inducing peptide. Nature. 2013;494:201–206. doi: 10.1038/nature11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snead OC, Gibson KM. Gamma-hydroxybutyric acid. N Engl J Med. 2005;352:2721–2732. doi: 10.1056/NEJMra044047. [DOI] [PubMed] [Google Scholar]
- Soto D, Altafaj X, Sindreu C, Bayés A. Glutamate receptor mutations in psychiatric and neurodevelopmental disorders. Commun Integr Biol. 2014;7:e27887. doi: 10.4161/cib.27887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilhaber J, Rakhade SN, Sudhalter J, et al. Gene expression profiling of a hypoxic seizure model of epilepsy suggests a role for mTOR and Wnt signaling in epileptogenesis. PloS One. 2013;8:e74428. doi: 10.1371/journal.pone.0074428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardya I, Drasbek KR, Gibson KM, Jensen K. Plasticity of postsynaptic, but not presynaptic, GABAB receptors in SSADH deficient mice. Exp Neurol. 2010;225:114–122. doi: 10.1016/j.expneurol.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz SH, Lérias SR, Parreira S, et al. Adenosine A2A receptor activation is determinant for BDNF actions upon GABA and glutamate release from rat hippocampal synaptosomes. Purinergic Signal. 2015;11:607–612. doi: 10.1007/s11302-015-9476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel KR, Ainslie GR, Jansen EEW, et al. Torin 1 partially corrects vigabatrin-induced mitochondrial increase in mouse. Ann Clin Transl Neurol. 2015;2:699–706. doi: 10.1002/acn3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KY, Barker PB, Lin DDM. A case of acute onset succinic semialdehyde dehydrogenase deficiency: neuroimaging findings and literature review. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg. 2015 doi: 10.1007/s00381-015-2942-9. [DOI] [PubMed] [Google Scholar]
- Weston MC, Chen H, Swann JW. Multiple roles for mammalian target of rapamycin signaling in both glutamatergic and GABAergic synaptic transmission. J Neurosci Off J Soc Neurosci. 2012a;32:11441–11452. doi: 10.1523/JNEUROSCI.1283-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston MC, Chen H, Swann JW. Multiple roles for mammalian target of rapamycin signaling in both glutamatergic and GABAergic synaptic transmission. J Neurosci Off J Soc Neurosci. 2012b;32:11441–11452. doi: 10.1523/JNEUROSCI.1283-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman ER, Niere F, Raab-Graham KF. mTORC1-dependent protein synthesis underlying rapid antidepressant effect requires GABABR signaling. Neuropharmacology. 2013;73:192–203. doi: 10.1016/j.neuropharm.2013.05.037. [DOI] [PubMed] [Google Scholar]
- Wu Y, Buzzi A, Frantseva M, et al. Status epilepticus in mice deficient for succinate semialdehyde dehydrogenase: GABAA receptor-mediated mechanisms. Ann Neurol. 2006;59:42–52. doi: 10.1002/ana.20686. [DOI] [PubMed] [Google Scholar]