Abstract
A long-standing goal of psychopathology research is to develop objective markers of symptomatic states, yet progress has been far slower than expected. While prior reviews have attributed this state of affairs to diagnostic heterogeneity, symptom comorbidity, and phenotypic complexity, little attention has been paid to the implications of intra-individual symptom dynamics and inter-relatedness for biomarker study designs. In this critical review, we consider the impact of short-term symptom fluctuations on widely-used study designs that regress the “average level” of a given symptom against biological data collected at a single time-point, and summarize findings from ambulatory assessment studies suggesting that such designs may be sub-optimal to detect symptom-substrate relationships. While such designs play a crucial role in advancing our understanding of biological substrates related to more stable, longer-term changes (e.g., grey matter thinning during a depressive episode), they may be less optimal for the detection of symptoms that exhibit show high frequency fluctuations, are susceptible to common reporting biases, or may be heavily influenced by the presence of other symptoms. We propose that a greater emphasis on intra-individual symptom chronometry may be useful for identifying subgroups of patients with a common, proximal pathological indicators. Taken together, these three recent developments in the areas of symptom conceptualization and measurement raise important considerations for future studies attempting to identify reliable biomarkers in psychiatry.
Introduction
A major goal of psychiatry research is to develop objective tests of illness.1-3 In recent decades, these efforts have been largely focused on the identification of biomarkers that may establish the presence, risk, or stage of a particular disorder.4 Advances have been slower than expected, however, as many of the most promising biomarker candidates have been found to lack requisite sensitivity and specificity. As has been articulated previously,3, 5 causes for this delayed progress include the vast heterogeneity of diagnostic categories, significant co-morbidity across disorders, and the sheer complexity of the phenotypes, all of which have hindered the identification of disorder-specific pathophysiology necessary to develop meaningful objective diagnostic or prognostic tests in psychiatry.
These factors do not, however, explain why the field still lacks proximal bio-signatures of symptom expression. As compared to the complex developmental trajectories that may hamper the discovery of ultimate biological diatheses, proximal, ‘in-the-moment’ correlates of psychiatric symptoms should be more easily identified. Panic attacks, for example, have a number of established biological sequelae that may be objectively measured to corroborate a subjective report, and these are much easier to detect than, say, genetic risk factors for the development of panic disorder. Indeed, in the related field of cognitive neuroscience, the ability to decipher the neural code associated with a given experience has become so advanced that neuroimaging data can be used to reconstruct perceived images,6 enable direct brain-to-brain interaction,7 and command remote-controlled machines.8 Despite this progress, we still have no reliable biological indicator for most of the core symptoms of our field – the onset of a dysphoric mood, an intrusive negative thought, or a sudden craving.
This absence of markers for symptomatic states can be difficult to reconcile with the ever-growing number of reliable group-level findings in psychiatric patient populations. For example, numerous studies and meta-analyses have confirmed that anxiety is associated with increased amygdala responsivity,9, 10 patients with major depression exhibit structural reductions in prefrontal and hippocampal areas,11-14 and striatal dopamine levels are altered in schizophrenia.15 Even more recent work has begun to uncover a number of broadly transdiagnostic markers.16, 17 Unfortunately, however, such effects tend to only emerge on average, and fail to provide meaningful information at the level of an individual patient.18 The promise of biomarkers is to bridge the gap between group-average differences and positive or negative predictive power for individuals, which is critical for the deployment of ‘precision science’ in psychiatry.19 To date, however, this promise remains largely unfulfilled.
There have been a number of excellent reviews on the challenges inherent to biomarker discovery in psychiatry, 3, 5, 20, 21 and proposed solutions have included a shift towards targeting of particular circuits and symptoms rather than whole disorders (e.g., the RDoC initiative), substantial increases in power–especially in genetic studies17, 22-24–and ever-increasing sophistication in the acquisition and analysis of biological data (e.g., graph-theoretical approaches to processing neuroimaging data).25, 26 Issues of symptom assessment and interrelatedness, however, have received comparatively less attention; this represents an important oversight, as recent developments in the measurement and conceptualization of mood, affect and well-being raise important questions regarding methods for biomarker identification.
In this review, we focus on three core issues surrounding the accurate assessment of psychiatric symptoms that may undermine the detectability of biomarkers for symptom states in some cases: dynamic variation, reporting biases, and symptom inter-relatedness. While numerous reviews exist on these topics in the contexts of clinical assessment,27, 28 personality and well-being research,29 and affective science,30 the implications for the field of biological psychiatry have not, to our knowledge, been critically examined. Here, we suggest ways in which enhanced measurement and characterization of symptoms may be improved, thereby augmenting statistical power without the added expense of increasing sample sizes. While our points emphasize relationships spanning the level of individual biology and specific symptoms, we note that many of the issues raised are not limited to these two particular levels of analysis. However, we have focused on symptom-substrate relationships in part due to the substantial emphasis that has been placed on biomarker discovery in psychiatric research in recent years.
Symptom-substrate dynamics and ‘average level’ symptom inventories
When attempting to identify a biomarker, one critical question that must be addressed in advance is the hypothesized relationship between symptom and substrate variability. Most symptoms and substrates show periodic and/or stochastic fluctuations over time; if it is presumed that these oscillations are mainly a product of situational factors, measurement error, or other forms of noise, then it would make sense to utilize central tendency statistics that may help reduce such noise distortion. In contrast, if one believes such fluctuations in both symptom and substrate levels are meaningfully coupled, than the process of averaging may remove critical signal, and a time series design may be required. In practice, a substantial number of studies employ cross sectional designs in which measures of symptom severity are assessed using a retrospective report instrument that prompts patients to report their “average-level” of symptoms over various periods of time. These measures will then typically be regressed against a biological measure collected at a single time point. For ease of reference, we will refer to these as “average-level” study designs. Such studies have played a critical role in biological psychiatry to date, and have yielded a number of important discoveries. The appropriateness of this design should not go unexamined, however, and may depend on the dynamic nature of both the symptom and target substrate, as well as their respective sampling rates.
The easiest biomarkers to detect will be those with either minimal variance or highly regular patterns of expression. Consider the example of visual processing deficits following damage to area V1, which is associated with object misperception. While such lesions may prompt an initial period of cortical reorganization, afterwards the substrate (V1 lesion) and symptom (object perception) are relatively stable and therefore readily detectable using average-level designs.31 Similar situations arise when symptom/substrate fluctuations are slow-moving relative to the sampling-rate of the measures used. For example, a number of studies suggest that hippocampal grey matter volume varies over time as a marker of current or remitted depression,11, 12, 14, 32-34 and is differentially impacted by the number of past depressive episodes.35-38 Importantly, these clinical findings are buttressed by a large animal literature suggesting that hippocampal atrophy may gradually occur after a period of sustained chronic stress.39, 40 A key contribution of these preclinical data is to provide an estimate of the period frequency of hippocampal volume changes, suggesting that the temporal dynamics of a depressive episode and structural change may be approximately synchronized. Therefore, while neither depressive episodes nor hippocampal volume changes are as stable as a lesion, their oscillations may be slow enough that a symptom measure that averages over the past week of experience (such as the BDI or HRSD) is suitable to detect a relationship. Consequently, structural changes in this region have been successfully identified as a marker for both depressive state as well as risk for relapse.41, 42
In many ways, the importance of this type of symptom chronometry has been previously recognized by the classic state vs. trait distinction in psychiatry. However, the maximum temporal window for a given symptom state is often not well-characterized empirically,43 and many clinical symptom measures used in ”average level” designs assume, at least in practice, that symptom states show relatively little meaningful variation over time periods as long as a few weeks or more44-53. The growing availability of daily and multi-day assessment data (referred to herein as Ambulatory Assessment; “AA”) suggests that many symptom domains–especially those related to mood, anxiety and stress–show significant day-to-day27, 30, 54-56 and even within-day57-59 variation in both clinical and non-clinical populations. Similarly, various classes of biomarkers, including hormone levels, gene expression and functional connectivity, exhibit dynamic patterns over multiple timescales,60, 61 for which possible relationships to symptomatic mental states are only beginning to be uncovered.60, 62, 63 In rodent models, cellular rhythms involving transcriptional and translational and post-translational feedback mechanisms have been shown to predict the development of depressive symptoms 64, as well as antidepressant response to SSRIs.65 Consequently, to the extent that these fluctuations are meaningfully correlated, average-level designs may be sub-optimal for detecting and/or interpreting these relationships. For example, while average severity levels of common symptoms related to mood, anxiety, and distress may differ significantly between healthy controls and psychiatric patient populations, there is nevertheless substantial overlap in these distributions.27, 28, 55, 66 An average-level design relying on a single time-point for assessment of a biological variable (e.g., an MRI scan session) may include a subset of patients that were scanned on a relatively “low-symptom” day as well as controls who were scanned on a comparatively “high symptom” day, despite robust differences when averaging over time for each individual (e.g., by using a retrospective report).
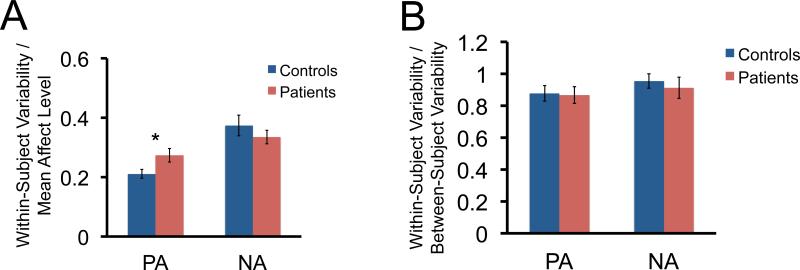
To better quantify this issue, we conducted a pubmed search to identify papers that have used AA measures of mood, affect and stress in healthy controls and various patient groups. The studies included a total of 9,628 healthy/low-symptom subjects and 2,815 patients with various disorders (please see Supplementary Information and Table S1). Importantly, these studies reported both the mean and standard variation for group level of positive affect (PA) and negative affect (NA) ratings averaged individually within-subjects over time, but also the mean and standard variation for variability of affect.67, 68 This allowed us to first examine the magnitude of within subject variability (average of the standard deviation for daily, within subject ratings) relative to mean affect level for healthy controls and different patient groups. Across studies, values for group within-subject variability (WSD) were first divided by group average level to standardize values across the different instruments used. This provides a simple index of the proportion of within-subject variability that was observed relative to affect level, with zero indicating no within-subject variability. We found that for both patients and controls, within subject variability ranged from 24%-37% of the mean level for PA and NA (Figure 1A). The effect was significantly higher for PA in patients compared to controls (Mann-Whitney, p = 0.003), but not for NA (Mann-Whitney, p = 0.785) (see Supplemental Materials). In addition to examining within-subject variability relative to mean affect level, we also examine within-subject variability relative to between subject variability. For both positive and negative affect, within/between variability ratios were close to 1 (NA: 0.94; PA: 0.87) suggesting that within-subject variability in both positive and negative affect over time is almost as large as between-subject variability (Figure 1B).
Figure 1.
Summary of within-subject variability in positive affect (PA) and negative affect (NA) relative to mean affect level. A. Depictions of EMA-based measures of within-subject variability in PA and NA relative to mean affect in patients and controls. Larger values indicate greater change over time relative to mean. B. Depictions of EMA-based measures of within-subject variability in PA and NA relative to mean affect in patients and controls. Values ≥ 1 indicate within-subject variability in PA and NA over time is as large or larger than between-subject variability. * Indicates a p<0.05 (Mann-Whitney).
In sum, contrary to prior studies positing that psychiatric disorders were associated with extremely low levels of within-subject variability,69 this analysis of the existing AA literature on affect in psychopathology suggests that daily lability in both negative and positive affect is relatively high compared to the differences in average level commonly found between patients and controls, which may result in “average” experience ratings are significantly different from “day-of” experiences during biological measurement. Additionally, this variability appeared to be consistent across both clinical and non-clinical samples.
A closely related challenge is the dynamic fluctuations of biomarkers themselves. While some sources of variance may be known and controlled for (e.g., diurnal variation), many are likely unknown. When single ‘basal’ measures are taken, as in a single measure of a target protein or imaging of the brain “at rest”, these sources of variability may significantly attenuate potential relationships. For example, many fMRI studies using functional connectivity techniques have identified networks that appear to be remarkably stable across different individuals and cognitive/emotional states, suggesting a trait-like nature; 70, 71 yet other studies have reported significant changes in network connectivity as a consequence of short-term (e.g., 10-30 minutes) dynamic state change. 72-74 Indeed, one recent paper using a large (n = 575) imaging sample of healthy individuals found that different cognitive states accounted for almost half of the variance in functional connectivity networks. 75 Many studies seek to control this issue by using repeated laboratory assessments of a target biomarker in response to conditions of interest (e.g., change following cognitive or emotional task conditions, a lab stressor, or a pharmacological challenge). While a significant improvement, without some extended characterization of a biomarker's normal range within an individual, such assessments may still suffer from intra-individual variability across different days. Additionally, it is often unknown the extent to which the dynamic range of a target biomarker within the lab matches relevant external environments. Finally, some biomarker relationships may not be readily observable without prolonged, high-density sampling, similar to how ambulatory blood-pressure monitoring studies were necessary to identify cardiovascular disease risks associated with so-called “non-dippers”–individuals with a flattened diurnal variation–that could not be detected using average-level designs 76. In some cases, the relative stability of target biomarkers is unclear.
Taken together, the short-term temporal structure of both symptoms and candidate biomakers is under-studied, and may exert significant impact on the measurement of symptom-substrate relationships. While the classic trait-state distinction has long been recognized in psychiatry, in practice, ‘states’ are often operationalized to extend from several weeks to several months. Available data from the AA literature suggests that variability may exist in this window, highlighting the importance of alternative approaches to data collection.
The Effects of Symptom-Specific Measurement Bias on Symptom-Substrate Relationships
A related concern for ‘average-level’ designs is the use retrospective measures that call upon the individual patient to perform a “mental averaging” of their daily experience. A substantial amount of AA data has emerged in the last decade to suggest that, contrary to expectations, such retrospective measures correlate only moderately with average experience sampled using AA approaches.28, 55 This lack of strong agreement between retrospective and AA reports of the same experiences has led researchers to posit the existence of two distinct “selves”; the “experiencing self” and the “believing self”. 28-30, 77-79 The former reflects an aggregate of reported “in-the-moment” experiences, while the latter is influenced by retrospective reporting biases.
The potential biases that arise from retrospective report, including “peak-and-end effects”, mood-congruent recall, focusing illusions and heuristic-based reconstruction, have been thoroughly reviewed elsewhere.28-30, 79, 80 Here, we raise the question of how these different “selves” may influence symptom reports in average-level designs, and, in turn, biomarker detection. As most symptom inventories are retrospective in nature, they will be susceptible to some reporting biases that more strongly reflect personal narratives about experience rather than experience itself. Importantly, the effect size of these biases may differ both across disorders, as well as across symptom domains within a disorder. For example, a substantial amount of evidence now supports the presence of significant discrepancies among patients with schizophrenia regarding their believed and experienced negative symptoms; patients report significantly less expected enjoyment to laboratory stimuli as compared to their actual enjoyment;81-85 are found to have difficulty reporting consistently about their preferences86-88 and appear unable to translate reported anticipation of pleasure into goal directed behavior.89 Consequently, retrospective reports on rewarding experiences might be expected to substantially diverge from ‘in-the-moment’ reports, reducing observed relationships between average-level symptom scores and biological measures. Similarly, while panic attacks have often been described as occurring unexpectedly, AA data suggest clear alterations across multiple physiological domains prior to onset,90 and studies using actigraphy have identified clear inconsistencies between recorded and retrospectively reported levels of physical activity,91 which may be relevant for predicting the onset of depressive symptoms.92 In short, these studies illustrate that asking patients to report retrospectively on certain types of experiences may access self-related beliefs that are unlikely to be predictive of “in-the-moment” experiences or their neurobiological correlates. Further, the extent to which retrospective reports may be more or less accurate is likely to depend on the individual, the symptom and the disorder.
Conversely, there may be other symptom domains for which isolated assessment of the ‘believing self’ and its associated biomarkers are especially relevant. For example, repeated studies have shown the presence of a persistent negative bias in disorders such as depression,93-96 leading to affective forecasting predictions that are often worse than experienced.97, 98 Consequently, neuroimaging studies seeking to identify the mechanisms of such biases may do better to avoid “in-the-moment” measures of negative affect –which may be less differentiated from controls than reported–and focus on markers of negative forecasting judgments.
It should be noted that while AA measures are potentially helpful against retrospective biases, they may be equally vulnerable to other forms of bias, including focusing effects, demand and social desirability biases, individual differences in item comprehension, and reporting effort among others 29, 30, 99. Additionally, to the extent that momentary assessments clash with important self-narratives (e.g., one who is depressed but feeling ok in a given moment), cognitive dissonance and self-beliefs may still influence AA reports. In other cases, AA measures may introduce sources of bias that retrospective measures help avoid; for example, many assessments of interest can require a significant amount of mental or emotional effort to report on, which may confound their measurement as they unfold experientially. Indeed, it can at times be easier to report accurately on the nature of a particularly distressing experience after the fact.
These limitations aside, the growing evidence that AA and retrospective symptom measures often paint very different portrayals of subjective experience–even over relatively short time periods–should raise important questions about the most appropriate symptom measures selected average level-designs. As discussed in greater detail below, one solution is to increase the use of hybrid designs, that may compare measures of “in-the moment” neurobiological responses to laboratory stimuli with AA data,100 which have helped identify predictive markers of behavior in both clinical and non-clinical populations.101, 102 While such designs do not eliminate sources of bias for either AA or retrospective measures, they do offer a potential means of examining their shared and unshared variance in relationship to biological measures of interest.
Individual differences in symptom inter-relatedness
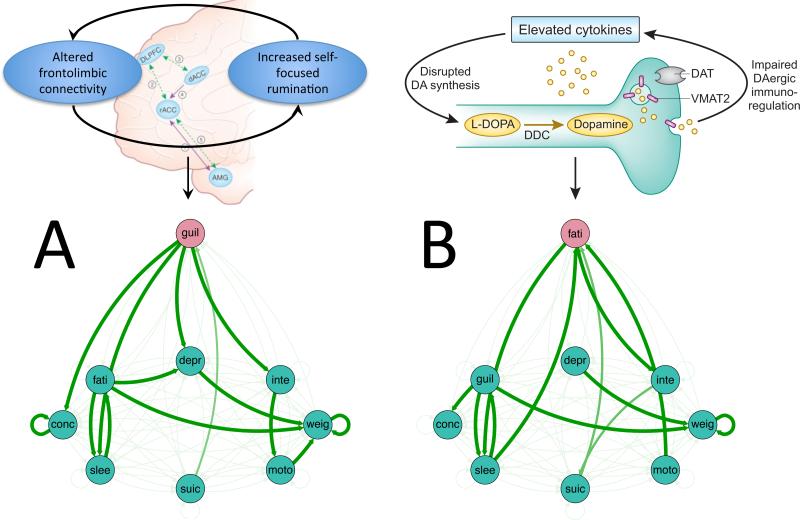
As noted in the introduction, one common explanation for the lack of biomarkers is the heterogeneity of diagnostic categories, case-control designs have largely failed to identify “final common pathways” for psychiatric disorders. The NIMH's Research Domain Criteria (RDoC) initiative has sought to address this issue in part by focusing on markers for specific symptoms rather than diagnostic entities as whole. However, it has long been recognized that like disorders, even a single symptom can reflect different pathologies.103,104 One factor that may impede identification common of pathways at the individual symptom level is the potential for individuals differences in the interactions among symptoms. Consider an example of a hypothetical average-level study design seeking to identify resting functional connectivity relationships with depression severity using individual BDI scores. Patient A is highly self-critical, and often fails to enjoy things because of an active self-critical rumination process, which has frequently been associated with altered medial prefrontal activity.105, 106 For her, anhedonic and fatigue symptoms of depression are not highly central, but are downstream in her symptom network from rumination, guilt and low self-esteem. Patient B, however, experiences chronic inflammation, which has been shown to induce hypodopaminergia and subsequent symptoms of anhedonia and fatigue. 107-113 For him, the severity of immuno-linked anhedonic symptoms may be a primary factor that drives subsequent symptoms related to guilt, self-esteem and others. As result, patients A and B could theoretically present with near-identical scores across all symptoms on the BDI, but with markedly divergent biosignatures stemming from distinct patterns of causality within symptoms (Figure 2). While immuno-related effects on depressive symptoms are themselves heterogenous and complex, identifying patients for whom fatigue and apathy are driving symptoms may significantly enhance the ability to identify inflammation-related and non-inflammation related forms of depressive symptoms. Accomplishing this, however, will require more time-series assessment of symptom inter-relationships. Indeed, recent efforts to characterize intra-individual changes in personal omics 114 and neuroimaging data 115 and their relationship to mood and illness highlight the complexity of such relationships as they unfold over time.
Figure 2.
Schematic of how symptom inter-relationships may result in different symptom networks that produce similar scores on a dimensional measure of depressive symptom severity despite unique pathophysiologies. A. Patient A has altered connectivity patterns in corticolimibic circuitry that underlie and reinforce self-focused rumination,106 leading to frequent experiences of guilt and low self-esteem.131, 132 B. For patient B, high-inflammation disrupts dopamine synthesis, leading to a chroming hypodopaminergic state and feelings of fatigue and anergia107-113, which in turn precipitates social withdrawal, feelings of failure and subsequent other depressive symptoms. In both examples, the activation of a single symptom with differing pathologies can activate interconnected depressive symptoms, resulting in similar levels of symptom expression.
Fortunately, a number of analytical approaches for analyzing the influences of symptoms on other symptoms as they unfold through time have begun to emerge. One such approach has emerged from dynamical systems theory. Given the hypothesis that symptoms may be influenced by each other, it would be expected that increasing inter-correlation among symptoms may indicate a “tipping point” at which symptom convergence results in a transition to a clinical state116. One could easily imagine adopting a similar strategy of identifying such “tipping point” periods and then assessing biomarkers within this time, similar to the strategy recently adopted by Rahdar and Galvan 117. A second approach to such time-series data is the study of symptom networks and network dynamics. 118, 119 Network analysis has received growing attention across a number of closely related fields, and a variety of software tools for the purposes of analysis and visualization of networks have been developed (120, 121 that can help characterize the ebb and flow of individual symptom expression in a variety of ways 122, 123. While most network analyses have been applied at the group level, recent studies have begun to focus on using individual networks to capture multi-level phenotypes over time (e.g., 115).
Future Directions
As summarized above, the combination of symptom fluctuation, well-established reporting biases and individual differences in symptom inter-relationships can all pose challenges for biomarker detection. These issues are not insuperable, however, and in this final section we point to several approaches through which they may be addressed. As mentioned in the introduction, these challenges are also relevant for other aspects of measurement in psychopathology. Indeed, some of these recommendations may be useful for improving the measurement of psychopathology without the end goal of identifying biomarkers, and could help developing novels means of predicting onset or recurrence (e.g., 116).
First, we wish to reiterate that while we have focused on some of the limitations of average-level designs, this critique should not to be taken to imply that such designs are without substantial merit. As noted at the outset, average level designs can have important advantages by reducing noisy fluctuations in symptom expression that may be unrelated to biological variables of interest as well as measurement noise in the assessment of biological measures themselves. Here, we suggest that for some target biomarker relationships, such designs may “average-over” important intra-individual variance. To address this issue, one approach will be an increased use of intra-individual designs with repeated assessments for both symptoms and target biomarkers. While AA measures of symptom severity are not without bias, the collection of both AA and retrospective measures provides a means of potentially identifying the magnitude of these discrepancies for different symptoms and different individuals. This strikes us as an important starting place for improving our understanding of how “believing” and “experiencing” selves may impact the identification of relevant biomarkers. Such data will also help better characterize the short-term temporal structure of various symptoms. While our literature search of available AA studies suggested that there may significant daily variability in positive and negative affect, other symptoms may show greater stability, and it would be useful for future studies to be able to select AA or retrospective measures on this basis. In some cases, AA measures may allow for the comparison of objective and subjective measures, as metrics such as actigraphy, cardiovascular physiology, or estimates of social contact, all of which can be used to assess symptomatic states without some of the biases of self-report. Optimally one could collect, subjective, objective and target biomarkers at a comparable sampling rate; the necessary technology for real-time analysis of saliva, EEG measures and movement is increasingly rapidly (e.g. 124), and this provides new possibilities for measuring symptoms and substrates at an heretofore unprecedented temporal resolution.
There are, however, some limitations to this strategy that will need to be addressed. The first is that for many biomarkers, inexpensive, wearable technology remains some ways off, and is simply not possible at present. The increase in cost is partially offset by the significant increases in statistical power that may be achieved as has been evident by the success of multi-session imaging studies in identifying neural mechanisms underlying dynamic cognitive processes over time (e.g.,125, 126). However, such studies are still difficult and expensive to run. A second challenge is that many biomarkers of interest may only be detectable in particular contexts (e.g., extreme stress or negative affect), and may show relatively little or no association during euthymic periods, requiring long periods of passive data collection. A third option is a hybrid approach that would use repeated-measures assessments of both symptom and substrate measures over a brief amount of time, such as a single 3-4 hour laboratory visit, and then examine how these fluctuations relate to “real world” fluctuations during an extended AA follow-up period.127-129 Ongoing AA can also be used as an alternative to random sampling to help characterize their intra-individual variance patterns prior to biologic assessment, which can help ensure that such assessments are performed when everyone is in a comparable state relative to an individualized baseline. 117
Using AA data to classify symptom relationships prior to biological measurement may also increase the likelihood of identifying subgroups of patients with a shared biological diathesis. For example, by collecting daily symptom data for a period of time prior to biological assessment, one may be able to identify individuals who share common “driving symptoms” who are therefore more likely to exhibit common circuit-level abnormalities than individuals who merely exhibit similar symptom severity. As outlined in figure 2, a group of patients with fatigue as a common symptom of high centrality may be more likely to exhibit abnormalities in inflammation and striatal circuitry than patients for whom fatigue is a consequence of anxious rumination and insomnia. Conversely, one can also take the approach of stratifying patients along a particular candidate biomarker to identify symptom clusters that differentiate between high and low marker expression levels.130
In sum, this review has focused on how recent developments in the conceptualization and measurement of symptoms have raised important caveats for the detection of symptom-substrate relationships. Adoption of recently developed symptom measurement and analysis techniques will help increase power to detect reliable markers of symptom expression, thereby facilitating the development of objective tests for symptoms of psychiatric disorders.
Supplementary Material
Acknowledgements
This work was funded in part by NIMH grant R00MH10355. The authors wish to thank David Zald, Scott Lilienfeld, Irwin Waldman and Joshua Buckholtz for insightful discussions and invaluable criticism. The authors also thank Nicole Treadway, Andrew Teer and Daniel Cole for helpful comments.
Footnotes
Financial Disclosures:
The authors report no conflicts of interest, financial or otherwise. M. Treadway has previously consulted for the Boston Consulting Group and Avanir Pharmaceuticals. No funding or sponsorship was provided by these companies for the current work, and all views expressed herein are solely those of authors.
Supplementary information is available at Molecular Psychiatry's website.
REFERENCES
- 1.Kraemer HC, Schultz SK, Arndt S. Biomarkers in psychiatry: methodological issues. The American journal of geriatric psychiatry. 2002;10(6):653–659. [PubMed] [Google Scholar]
- 2.Meehl PE. Clinical vs. statistical prediction. University of Minnesota Press; Minneapolis: 1954. [Google Scholar]
- 3.Singh I, Rose N. Biomarkers in psychiatry. Nature. 2009;460(7252):202–207. doi: 10.1038/460202a. [DOI] [PubMed] [Google Scholar]
- 4.Davis J, Maes M, Andreazza A, McGrath JJ, Tye SJ, Berk M. Towards a classification of biomarkers of neuropsychiatric disease: from encompass to compass. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.139. [DOI] [PubMed] [Google Scholar]
- 5.Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry. 2012;17(12):1174–1179. doi: 10.1038/mp.2012.105. [DOI] [PubMed] [Google Scholar]
- 6.Naselaris T, Prenger RJ, Kay KN, Oliver M, Gallant JL. Bayesian reconstruction of natural images from human brain activity. Neuron. 2009;63(6):902–915. doi: 10.1016/j.neuron.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao RPN, Stocco A, Bryan M, Sarma D, Youngquist TM, Wu J, et al. A direct brain-to-brain interface in humans. PloS one. 2014;9(11):e111332. doi: 10.1371/journal.pone.0111332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaFleur K, Cassady K, Doud A, Shades K, Rogin E, He B. Quadcopter control in three-dimensional space using a noninvasive motor imagery-based brain–computer interface. Journal of neural engineering. 2013;10(4):046003. doi: 10.1088/1741-2560/10/4/046003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. 2007. [DOI] [PMC free article] [PubMed]
- 10.Ipser JC, Singh L, Stein DJ. Meta-analysis of functional brain imaging in specific phobia. Psychiatry Clin Neurosci. 2013;67(5):311–322. doi: 10.1111/pcn.12055. [DOI] [PubMed] [Google Scholar]
- 11.Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: A meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bora E, Fornito A, Pantelis C, Yucel M. Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2011;138(1-2):9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 13.Bora E, Fornito A, Yucel M, Pantelis C. Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. Biol Psychiatry. 2010;67(11):1097–1105. doi: 10.1016/j.biopsych.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Kempton MJ, Salvador Z, Munafo MR, Geddes JR, Simmons A, Frangou S, et al. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68(7):675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 15.Fusar-Poli P, Meyer-Lindenberg A. Striatal presynaptic dopamine in schizophrenia, Part II: meta-analysis of [18F/11C]-DOPA PET studies. Schizophr Bull. 2012:sbr180. doi: 10.1093/schbul/sbr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. Identification of a Common Neurobiological Substrate for Mental Illness. JAMA psychiatry. 2015;72(4):305–315. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Consortium C-DGotPG Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. The Lancet. 2013;381(9875):1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMahon FJ. Prediction of treatment outcomes in psychiatry—where do we stand? Dialogues Clin Neurosci. 2014;16:455–464. doi: 10.31887/DCNS.2014.16.4/fmcmahon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Insel TR, Cuthbert BN. Brain disorders? Precisely. Science. 2015;348(6234):499–500. doi: 10.1126/science.aab2358. [DOI] [PubMed] [Google Scholar]
- 20.Savitz JB, Rauch SL, Drevets WC. Clinical application of brain imaging for the diagnosis of mood disorders: the current state of play. Mol Psychiatry. 18(5):528–539. doi: 10.1038/mp.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubinov M, Bullmore E. Fledgling pathoconnectomics of psychiatric disorders. Trends in cognitive sciences. 2013;17(12):641–647. doi: 10.1016/j.tics.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Schizophrenia Working Group of the Psychiatric Genomics C Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Consortium SWGotPG Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cross-Disorder Group of the Psychiatric Genomics C Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nature genetics. 2013;45(9):984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience. 2009;10(3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 26.Deco G, Kringelbach ML. Great Expectations: Using Whole-Brain Computational Connectomics for Understanding Neuropsychiatric Disorders. Neuron. 2014;84(5):892–905. doi: 10.1016/j.neuron.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 27.Telford C, McCarthy-Jones S, Corcoran R, Rowse G. Experience sampling methodology studies of depression: the state of the art. Psychol Med. 2012;42(06):1119–1129. doi: 10.1017/S0033291711002200. [DOI] [PubMed] [Google Scholar]
- 28.Solhan MB, Trull TJ, Jahng S, Wood PK. Clinical assessment of affective instability: comparing EMA indices, questionnaire reports, and retrospective recall. Psychol Assess. 2009;21(3):425–436. doi: 10.1037/a0016869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahneman D, Krueger AB, Schkade D, Schwarz N, Stone AA. Would you be happier if you were richer? A focusing illusion. Science. 2006;312(5782):1908–1910. doi: 10.1126/science.1129688. [DOI] [PubMed] [Google Scholar]
- 30.Conner TS, Barrett LF. Trends in ambulatory self-report: the role of momentary experience in psychosomatic medicine. Psychosom Med. 2012;74(4):327–337. doi: 10.1097/PSY.0b013e3182546f18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dilks DD, Serences JT, Rosenau BJ, Yantis S, McCloskey M. Human adult cortical reorganization and consequent visual distortion. The Journal of Neuroscience. 2007;27(36):9585–9594. doi: 10.1523/JNEUROSCI.2650-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frodl T, Jager M, Smajstrlova I, Born C, Bottlender R, Palladino T, et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33(5):423–430. [PMC free article] [PubMed] [Google Scholar]
- 33.Frodl T, Meisenzahl EM, Zetzsche T, Hohne T, Banac S, Schorr C, et al. Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1-year follow-up. J Clin Psychiatry. 2004;65(4):492–499. doi: 10.4088/jcp.v65n0407. [DOI] [PubMed] [Google Scholar]
- 34.Arnone D, McKie S, Elliott R, Juhasz G, Thomas EJ, Downey D, et al. State-dependent changes in hippocampal grey matter in depression. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.150. [DOI] [PubMed] [Google Scholar]
- 35.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19(12):5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34(1):41–54. [PMC free article] [PubMed] [Google Scholar]
- 37.MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proceedings of the National Academy of Sciences. 2003;100(3):1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Treadway MT, Waskom ML, Dillon DG, Holmes AJ, Park MTM, Chakravarty MM, et al. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry. 2015;77(3):285–294. doi: 10.1016/j.biopsych.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22(1):105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 40.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933(1):265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 41.MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research&quest. Mol Psychiatry. 2010;16(3):252–264. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- 42.Dunlop BW, Mayberg HS. Neuroimaging-based biomarkers for treatment selection in major depressive disorder. Dialogues Clin Neurosci. 2014;16(4):479. doi: 10.31887/DCNS.2014.16.4/bdunlop. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davidson RJ. Comment: Affective chronometry has come of age. Emotion Review. 2015;7(4):368–370. [Google Scholar]
- 44.Becker JT, Chang YF, Lopez OL, Dew MA, Sweet RA, Barnes D, et al. Depressed mood is not a risk factor for incident dementia in a community-based cohort. Am J Geriatr Psychiatry. 2009;17(8):653–663. doi: 10.1097/jgp.0b013e3181aad1fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruhl AB, Rufer M, Kaffenberger T, Baur V, Herwig U. Neural circuits associated with positive and negative self-appraisal. Neuroscience. 2014;265:48–59. doi: 10.1016/j.neuroscience.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 46.Fanous AH, Neale MC, Aggen SH, Kendler KS. A longitudinal study of personality and major depression in a population-based sample of male twins. Psychol Med. 2007;37(8):1163–1172. doi: 10.1017/S0033291707000244. [DOI] [PubMed] [Google Scholar]
- 47.Goldberg SB, Manley AR, Smith SS, Greeson JM, Russell E, Van Uum S, et al. Hair cortisol as a biomarker of stress in mindfulness training for smokers. J Altern Complement Med. 2014;20(8):630–634. doi: 10.1089/acm.2014.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kendler KS, Gardner CO. A longitudinal etiologic model for symptoms of anxiety and depression in women. Psychol Med. 2011;41(10):2035–2045. doi: 10.1017/S0033291711000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naudin M, Carl T, Surguladze S, Guillen C, Gaillard P, Belzung C, et al. Perceptive biases in major depressive episode. PLoS ONE. 2014;9(2):e86832. doi: 10.1371/journal.pone.0086832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nikolova YS, Bogdan R, Brigidi BD, Hariri AR. Ventral striatum reactivity to reward and recent life stress interact to predict positive affect. Biol Psychiatry. 2012;72(2):157–163. doi: 10.1016/j.biopsych.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 51.Tadayonnejad R, Yang S, Kumar A, Ajilore O. Clinical, cognitive, and functional connectivity correlations of resting-state intrinsic brain activity alterations in unmedicated depression. J Affect Disord. 2014;172c:241–250. doi: 10.1016/j.jad.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei J, Sun G, Zhao L, Yang X, Liu X, Lin D, et al. Analysis of hair cortisol level in first-episodic and recurrent female patients with depression compared to healthy controls. J Affect Disord. 2015;175:299–302. doi: 10.1016/j.jad.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 53.Ormel J, Oldehinkel AJ, Nolen WA, Vollebergh W. Psychosocial disability before, during, and after a major depressive episode: a 3-wave population-based study of state, scar, and trait effects. Arch Gen Psychiatry. 2004;61(4):387–392. doi: 10.1001/archpsyc.61.4.387. [DOI] [PubMed] [Google Scholar]
- 54.Edmondson D, Shaffer JA, Chaplin WF, Burg MM, Stone AA, Schwartz JE. Trait anxiety and trait anger measured by ecological momentary assessment and their correspondence with traditional trait questionnaires. Journal of research in personality. 2013;47(6):843–852. doi: 10.1016/j.jrp.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trull TJ, Ebner-Priemer U. Ambulatory assessment. Annual review of clinical psychology. 2013;9:151. doi: 10.1146/annurev-clinpsy-050212-185510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gruber J, Kogan A, Quoidbach J, Mauss IB. Happiness is best kept stable: Positive emotion variability is associated with poorer psychological health. Emotion. 2013;13(1):1. doi: 10.1037/a0030262. [DOI] [PubMed] [Google Scholar]
- 57.Myin-Germeys I, Oorschot M, Collip D, Lataster J, Delespaul P, van Os J. Experience sampling research in psychopathology: opening the black box of daily life. Psychol Med. 2009;39(09):1533–1547. doi: 10.1017/S0033291708004947. [DOI] [PubMed] [Google Scholar]
- 58.Bowen R, Baetz M, Hawkes J, Bowen A. Mood variability in anxiety disorders. J Affect Disord. 2006;91(2):165–170. doi: 10.1016/j.jad.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 59.Bowen R, Clark M, Baetz M. Mood swings in patients with anxiety disorders compared with normal controls. J Affect Disord. 2004;78(3):185–192. doi: 10.1016/S0165-0327(02)00304-X. [DOI] [PubMed] [Google Scholar]
- 60.Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, et al. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yosef N, Regev A. Impulse control: temporal dynamics in gene transcription. Cell. 2011;144(6):886–896. doi: 10.1016/j.cell.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robinson LF, Atlas LY, Wager TD. Dynamic functional connectivity using state-based dynamic community structure: Method and application to opioid analgesia. Neuroimage. 2015;108:274–291. doi: 10.1016/j.neuroimage.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 63.Kinnison J, Padmala S, Choi J-M, Pessoa L. Network analysis reveals increased integration during emotional and motivational processing. The Journal of Neuroscience. 2012;32(24):8361–8372. doi: 10.1523/JNEUROSCI.0821-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Logan RW, Edgar N, Gillman AG, Hoffman D, Zhu X, McClung CA. Chronic Stress Induces Brain Region-Specific Alterations of Molecular Rhythms that Correlate with Depression-like Behavior in Mice. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee M, Kim Y, Park W, Park O, Kwon S, Hong K, et al. Temporal variability of glucocorticoid receptor activity is functionally important for the therapeutic action of fluoxetine in the hippocampus. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hall DP, Sing HC, Romanoski AJ. Identification and characterization of greater mood variance in depression. Am J Psychiatry. 1991;148(10):1341–1345. doi: 10.1176/ajp.148.10.1341. [DOI] [PubMed] [Google Scholar]
- 67.Eid M, Diener E. Global judgments of subjective well-being: Situational variability and long-term stability. Social Indicators Research. 2004;65(3):245–277. [Google Scholar]
- 68.Trull TJ, Solhan MB, Tragesser SL, Jahng S, Wood PK, Piasecki TM, et al. Affective instability: Measuring a core feature of borderline personality disorder with ecological momentary assessment. J Abnorm Psychol. 2008;117(3):647. doi: 10.1037/a0012532. [DOI] [PubMed] [Google Scholar]
- 69.Cowdry RW, Gardner DL, O'Leary KM, Leibenluft E, Rubinow DR. Mood variability: A study of four groups. The American journal of psychiatry. 1991 doi: 10.1176/ajp.148.11.1505. [DOI] [PubMed] [Google Scholar]
- 70.Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp. 2008;29(7):828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kitzbichler MG, Henson RNA, Smith ML, Nathan PJ, Bullmore ET. Cognitive effort drives workspace configuration of human brain functional networks. The Journal of Neuroscience. 2011;31(22):8259–8270. doi: 10.1523/JNEUROSCI.0440-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22(1):158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hermans EJ, van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, van Kesteren MT, et al. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334(6059):1151–1153. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- 75.Geerligs L, Rubinov M, Henson RN. State and Trait Components of Functional Connectivity: Individual Differences Vary with Mental State. The Journal of Neuroscience. 2015;35(41):13949–13961. doi: 10.1523/JNEUROSCI.1324-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24(6):793–801. doi: 10.1161/01.hyp.24.6.793. [DOI] [PubMed] [Google Scholar]
- 77.Robinson MD, Clore GL. Belief and feeling: evidence for an accessibility model of emotional self-report. Psychol Bull. 2002;128(6):934–960. doi: 10.1037/0033-2909.128.6.934. [DOI] [PubMed] [Google Scholar]
- 78.Robinson MD, Clore GL. Episodic and semantic knowledge in emotional self-report: evidence for two judgment processes. J Pers Soc Psychol. 2002;83(1):198–215. [PubMed] [Google Scholar]
- 79.Schwarz N. Retrospective and concurrent self-reports: The rationale for real-time data capture. The science of real-time data capture: Self-reports in health research. 2007:11–26. [Google Scholar]
- 80.Schwarz N. Feelings as information: Informational and motivational functions of affective states. In: Higgins ET, Sorrentino R, editors. Handbook of motivation and cognition. Guilford Press; New York, NY: 1990. [Google Scholar]
- 81.Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34(5):835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Strauss GP, Gold JM. A New Perspective on Anhedonia in Schizophrenia. Am J Psychiatry. 2012 doi: 10.1176/appi.ajp.2011.11030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Reserch in Personality. 2006;40:1086–1102. [Google Scholar]
- 84.Heerey EA, Gold JM. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. J Abnorm Psychol. 2007;116(2):268. doi: 10.1037/0021-843X.116.2.268. [DOI] [PubMed] [Google Scholar]
- 85.Burbridge JA, Barch DM. Anhedonia and the experience of emotion in individuals with schizophrenia. J Abnorm Psychol. 2007;116(1):30. doi: 10.1037/0021-843X.116.1.30. [DOI] [PubMed] [Google Scholar]
- 86.Strauss GP, Robinson BM, Waltz JA, Frank MJ, Kasanova Z, Herbener ES, et al. Patients with schizophrenia demonstrate inconsistent preference judgments for affective and nonaffective stimuli. Schizophr Bull. 2011;37(6):1295–1304. doi: 10.1093/schbul/sbq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brown JK, Waltz JA, Strauss GP, McMahon RP, Frank MJ, Gold JM. Hypothetical decision making in schizophrenia: the role of expected value computation and “irrational” biases. Psychiatry Res. 2013;209(2):142–149. doi: 10.1016/j.psychres.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strauss GP. The emotion paradox of anhedonia in schizophrenia: or is it? Schizophr Bull. 2013:sbs192. doi: 10.1093/schbul/sbs192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gard DE, Sanchez AH, Cooper K, Fisher M, Garrett C, Vinogradov S. Do People With Schizophrenia Have Difficulty Anticipating Pleasure, Engaging in Effortful Behavior, or Both? 2014. [DOI] [PMC free article] [PubMed]
- 90.Meuret AE, Rosenfield D, Wilhelm FH, Zhou E, Conrad A, Ritz T, et al. Do unexpected panic attacks occur spontaneously? Biol Psychiatry. 2011;70(10):985–991. doi: 10.1016/j.biopsych.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5(1):56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van de Leemput IA, Wichers M, Cramer AO, Borsboom D, Tuerlinckx F, Kuppens P, et al. Critical slowing down as early warning for the onset and termination of depression. Proceedings of the National Academy of Sciences. 2014;111(1):87–92. doi: 10.1073/pnas.1312114110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol. 2004;113(1):121–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- 94.Joormann J, Gotlib IH. Is this happiness I see? Biases in the identification of emotional facial expressions in depression and social phobia. J Abnorm Psychol. 2006;115(4):705–714. doi: 10.1037/0021-843X.115.4.705. [DOI] [PubMed] [Google Scholar]
- 95.Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. 2008. [DOI] [PubMed]
- 96.Korn C, Sharot T, Walter H, Heekeren H, Dolan R. Depression is related to an absence of optimistically biased belief updating about future life events. Psychol Med. 2014;44(03):579–592. doi: 10.1017/S0033291713001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Strunk DR, Lopez H, DeRubeis RJ. Depressive symptoms are associated with unrealistic negative predictions of future life events. Behav Res Ther. 2006;44(6):861–882. doi: 10.1016/j.brat.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 98.Strunk DR, Adler AD. Cognitive biases in three prediction tasks: A test of the cognitive model of depression. Behav Res Ther. 2009;47(1):34–40. doi: 10.1016/j.brat.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 99.Kagan J. A trio of concerns. Perspectives on Psychological Science. 2007;2(4):361–376. doi: 10.1111/j.1745-6916.2007.00049.x. [DOI] [PubMed] [Google Scholar]
- 100.Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166(1):64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Telzer EH, Fuligni AJ, Lieberman MD, Galván A. Neural sensitivity to eudaimonic and hedonic rewards differentially predict adolescent depressive symptoms over time. Proceedings of the National Academy of Sciences. 2014;111(18):6600–6605. doi: 10.1073/pnas.1323014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. The Journal of Neuroscience. 2012;32(16):5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weinberger DR, Glick ID, Klein DF. Whither Research Domain Criteria (RDoC)?: The Good, the Bad, and the Ugly. JAMA psychiatry. 2015:1161–1162. doi: 10.1001/jamapsychiatry.2015.1743. [DOI] [PubMed] [Google Scholar]
- 104.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35(3):537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9(3):471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 106.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36(1):183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2011;37(1):137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol. 2014 doi: 10.1016/j.yfrne.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, et al. Dopaminergic mechanisms of reduced Basal Ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry. 2012;69(10):1044–1053. doi: 10.1001/archgenpsychiatry.2011.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9(11):853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 113.Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105(1):83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- 114.Chen R, Mias GI, Li-Pook-Than J, Jiang L, Lam HY, Chen R, et al. Personal omics profiling reveals dynamic molecular and medical phenotypes. Cell. 2012;148(6):1293–1307. doi: 10.1016/j.cell.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Poldrack RA, Laumann TO, Koyejo O, Gregory B, Hover A, Chen M-Y, et al. Long-term neural and physiological phenotyping of a single human. Nature communications. 2015;6 doi: 10.1038/ncomms9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.van de Leemput IA, Wichers M, Cramer AOJ, Borsboom D, Tuerlinckx F, Kuppens P, et al. Critical slowing down as early warning for the onset and termination of depression. Proceedings of the National Academy of Sciences. 2014;111(1):87–92. doi: 10.1073/pnas.1312114110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rahdar A, Galván A. The cognitive and neurobiological effects of daily stress in adolescents. Neuroimage. 2014;92:267–273. doi: 10.1016/j.neuroimage.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 118.Barabasi A-L. Linked: How everything is connected to everything else and what it means. Plume Editors. 2002 [Google Scholar]
- 119.Cramer AO, Waldorp LJ, van der Maas HL, Borsboom D. Comorbidity: A network perspective. Behav Brain Sci. 2010;33(2-3):137–150. doi: 10.1017/S0140525X09991567. [DOI] [PubMed] [Google Scholar]
- 120.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 121.Sacha Epskamp AOJC, Waldorp Lourens J., Schmittmann Verena D. Denny Borsboom qgraph: Network Visualizations of Relationships in Psychometric Data. Journal of Statistical Software. 2012;48(4):1–18. [Google Scholar]
- 122.Bringmann L, Lemmens L, Huibers M, Borsboom D, Tuerlinckx F. Revealing the dynamic network structure of the Beck Depression Inventory-II. Psychol Med. 2014:1–11. doi: 10.1017/S0033291714001809. [DOI] [PubMed] [Google Scholar]
- 123.Bringmann LF, Vissers N, Wichers M, Geschwind N, Kuppens P, Peeters F, et al. A network approach to psychopathology: new insights into clinical longitudinal data. PLoS ONE. 2013;8(4):e60188. doi: 10.1371/journal.pone.0060188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Debener S, Minow F, Emkes R, Gandras K, Vos M. How about taking a low-cost, small, and wireless EEG for a walk? Psychophysiology. 2012;49(11):1617–1621. doi: 10.1111/j.1469-8986.2012.01471.x. [DOI] [PubMed] [Google Scholar]
- 125.McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, et al. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323(5915):800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- 126.Dux PE, Tombu MN, Harrison S, Rogers BP, Tong F, Marois R. Training improves multitasking performance by increasing the speed of information processing in human prefrontal cortex. Neuron. 2009;63(1):127–138. doi: 10.1016/j.neuron.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hasler G, Fromm S, Carlson PJ, Luckenbaugh DA, Waldeck T, Geraci M, et al. Neural response to catecholamine depletion in unmedicated subjects with major depressive disorder in remission and healthy subjects. Arch Gen Psychiatry. 2008;65(5):521–531. doi: 10.1001/archpsyc.65.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Homan P, Drevets WC, Hasler G. Neural correlates of free T3 alteration after catecholamine depletion in subjects with remitted major depressive disorder and in controls. Psychopharmacology (Berl) 2013:1–9. doi: 10.1007/s00213-013-3250-2. [DOI] [PubMed] [Google Scholar]
- 129.Silk JS, Stroud LR, Siegle GJ, Dahl RE, Lee KH, Nelson EE. Peer acceptance and rejection through the eyes of youth: pupillary, eyetracking and ecological data from the Chatroom Interact task. Soc Cogn Affect Neurosci. 2012;7(1):93–105. doi: 10.1093/scan/nsr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Miller AH, Raison CL. Are Anti-inflammatory Therapies Viable Treatments for Psychiatric Disorders? Where the Rubber Meets the Road. JAMA Psychiatry. 2015;72(6) doi: 10.1001/jamapsychiatry.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Watkins E, Teasdale JD. Rumination and overgeneral memory in depression: effects of self-focus and analytic thinking. J Abnorm Psychol. 2001;110(2):353. doi: 10.1037/0021-843x.110.2.333. [DOI] [PubMed] [Google Scholar]
- 132.Kim S, Thibodeau R, Jorgensen RS. Shame, guilt, and depressive symptoms: a meta-analytic review. Psychol Bull. 2011;137(1):68. doi: 10.1037/a0021466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.