Abstract
Background:
Menopause is associated with various complications such as depression, sleep disorders, and genitourinary atrophy. Vaginal atrophy occurs due to the loss of steroid hormones, and its major symptoms include vaginal dryness, itching, dyspareunia, and bleeding after intercourse. According to the literature, vitamin E plays a key role in estrogen stability. The aim of this study was to compare the effects of vitamin E suppositories and conjugated estrogen vaginal cream on vaginal atrophy.
Materials and Methods:
In this clinical trial, 52 postmenopausal women, who were referred to a gynecology clinic in 2013, were recruited and randomly divided into two groups (26 cases per group). One group received 100 IU of vitamin E suppositories (n = 26), whereas the other group applied 0.5 g of conjugated estrogen cream for 12 weeks. Vaginal maturation value (VMV) was compared between the two groups before and after the intervention. VMV ≤ 55 was regarded as a cut-off point for vaginal atrophy. Treatment success was defined as a 10-unit increase in VMV, compared to the baseline value. Data were analyzed by Friedman test and Mann-Whitney test. P value less than 0.05 was considered statistically significant.
Results:
The mean VMV in the vitamin E group before the treatment and after 4, 8, and 12 weeks of treatment was 43.78 ± 13.75, 69.07 ± 22.75, 77.86 ± 21.79, and 80.59 ± 19.23, respectively. The corresponding values in the estrogen cream group were 42.86 ± 14.40, 86.98 ± 12.58, 92.65 ± 15, and 91.57 ± 14.10, respectively. VMV significantly improved in both the treatment groups after the intervention, compared to the preintervention period (P < 0.001). Treatment success was reported in both groups, although estrogen cream (100%) appeared to be more effective after 4 weeks of treatment, compared to vitamin E suppositories (76.9%) (P = 0.01).
Conclusions:
Based on the findings, use of vitamin E suppositories could improve the laboratory criteria for vaginal atrophy and treatment success. Therefore, vitamin E suppositories are suggested for relieving the symptoms of vaginal atrophy, especially in women who are unable to use hormone therapy or cope with the associated side effects.
Key words: Conjugated estrogen, vitamin E, vaginal atrophy
INTRODUCTION
Menopause is a natural and important stage in every woman's life. According to statistics, this stage is identified by infertility and amenorrhea among women.[1] According to the World Health Organization, in most countries, the average life expectancy is estimated at 80 years for women. In general, menopause occurs in women either naturally or due to surgery.[2]
Menopause is accompanied by different problems such as hot flashes, sweating, depression, sleep disorders, sexual problems, urinary incontinence, and urogenital atrophy. The signs and symptoms of menopause seem to be universal, despite the differences in their incidence in diverse ethnic groups.[3,4]
Vaginal atrophy is the second most common complaint of menopausal women after hot flashes and is regarded as a bothersome complication. In total, 75% of menopausal women complain of vaginal atrophy, caused by the reduced level of steroid hormones.[5,6,7] Following vaginal atrophy in menopausal women, vaginal lubrication and cervical maturation are changed, resulting in an increase in the number of basal cells and a decline in the number of superficial cells.[6]
Menopause leads to the manifestation of signs and symptoms such as vaginal dryness, itching, dyspareunia, postcoital bleeding, and vaginismus.[5,8,9,10] According to the literature, 40–50% of women complain of dyspareunia due to reduced blood circulation and vaginal dryness.[11,12] In addition, 75% of postmenopausal women experience vaginal dryness.[13]
Despite the differences in the age of menopause between communities, all women experience menopause in the course of their life.[14] Even among women with no sexual activity, vaginal atrophy could cause vaginal itching, sensitivity, and burning.[9] The symptoms of vaginal atrophy could negatively affect the individual's comfort and quality of life.[15]
Vaginal atrophy could be diagnosed based on patient history, clinical examinations, and laboratory tests.[11,16] The laboratory diagnosis is based on the vaginal maturation value (VMV), which can identify vaginal atrophy caused by the reduced number of superficial cells.[17] Several hormonal and nonhormonal methods have been proposed for diminishing the changes caused by vaginal atrophy.[12] So far, estrogen therapy (applied either locally or systemically) is the only effective method through which the collagen content of the skin and its thickness are improved.[17,18]
In this regard, Raymond et al. studied the effect of conjugated estrogen on vaginal atrophy in 150 menopausal women. They reported a considerable increase in VMV. Moreover, the clinical examination of genitalia showed an improvement in each visit, compared to the pre-intervention visits.[19]
Although hormone replacement therapy may improve the quality of life and decrease the associated symptoms and complications in menopausal women,[6] most women are reluctant to receive this therapy due to its disadvantages.[20] Undoubtedly, different hormones in the body can cause problems such as irregular bleeding and thromboembolism.[6] As a result, not only patients but also physicians are interested in alternative medicine to introduce more effective methods and reduce the associated side effects.[21]
Herbal medicine, vaginal lubricants, lifestyle changes, and use of vitamins could be mentioned as nonhormonal methods.[12] Vitamin E plays a key role in maintaining the estrogen level and improving the symptoms of menopause, such as hot flashes, sensitivity, insomnia, dizziness, palpitation, dyspareunia, and vaginal dryness.[22] This vitamin also keeps the arteries flexible and facilitates blood circulation, which consequently increases the metabolism of vaginal connective tissues and enhances the moisture and flexibility of vaginal walls.[23,12]
Ziagham et al. compared the therapeutic effects of hyaluronic acid and vitamin E suppositories on vaginal atrophy in postmenopausal women and found that vitamin E could relieve the symptoms of vaginal atrophy.[24] Moreover, Ziaei et al. evaluated the effects of vitamin E and placebo on hot flashes in menopausal women. As the results indicated, vitamin E and placebo could both reduce the frequency and severity of hot flashes in menopausal women. However, no significant difference was detected between the groups in terms of the percentage of parabasal or superficial cells before and after the experiment.[25]
In previous studies, vitamin E has been used either independently or in combination with other agents. Moreover, some studies have investigated the effect of vitamin E in animal studies. Considering the significant complications of hormone replacement therapy and vaginal estrogens, there is a growing tendency among women to experience alternative medicine for safe treatment.
Considering the high incidence of vaginal atrophy in postmenopausal women, this study was conducted to compare the therapeutic effects of vitamin E suppositories and conjugated estrogen vaginal cream on vaginal atrophy in postmenopausal women.
MATERIALS AND METHODS
This clinical trial was conducted among postmenopausal women, aged 40-65 years, who were referred to the gynecology clinic of Qaem Hospital and healthcare centers of Mashhad, Iran in 2014. The sample size was calculated based on the comparison of mean values between the independent groups (error rate of 0.05). Finally, 30 participants were enrolled in each group through simple random sampling.
The inclusion criteria were as follows: 1) Age range of 40-65 years; 2) amenorrhea for at least 12 months; 3) a 6-week interval since bilateral oophorectomy, accompanied with or without hysterectomy (it is not normally required in menopausal women); 4) normal Pap smear results over the past 3 years; 5) symptoms of vaginal atrophy based on the patient's self-report; and 6) VMV ≤ 55.
On the other hand, the exclusion criteria were as follows: 1) Diagnosis of endometrial or breast cancer or any suspected cancer of the genital system; 2) abnormal vaginal bleeding; 3) conditions such as diabetes, chronic renal disease, arthritis, cardiovascular diseases, and active disease in the liver or gallbladder; 4) use of antihypertensive drugs (considering their effects on reducing vaginal lubrication); 5) vaginal infections; 6) sensitivity to estrogen compounds or vitamin E; and 7) use of hormone replacement therapy 8 weeks before the study.
Women with the symptoms of vaginal atrophy, who met the inclusion criteria, were introduced to the study. After clinical examinations of the vagina and cervix, the subjects were selected in case no symptoms of infection, wounds, abnormal injury, or discharge were detected.
Data were collected by using questionnaires. The demographic questionnaire and the clinical evaluation form were completed for recording information on VMV and the percentage of superficial, intermediate, and basal cells. The researcher was trained to attain the required skills; the training continued until obtaining a gynecologist's approval.
The validity of the clinical evaluation form was approved by the assessors, including scholars and experts. To determine the reliability of the laboratory staff, two samples with different labels were obtained from five patients and sent to the laboratory before the intervention. After calculating the correlation coefficient between the results, reliability was estimated at r = 0.90.
A sample of vaginal discharge was obtained from all eligible subjects by resecting one-third of the upper lateral vaginal wall using a plastic spatula to determine VMV. If the subjects did not have a normal Pap smear over the past 3 years, Pap smear test was performed.
The slides of vaginal maturation index (VMI) were assessed using Papanicolaou's staining method by a pathologist; the results were studied both quantitatively and qualitatively. The pathologist studied 100 cells and determined their type (i.e., superficial, intermediate, and basal cells) and percentage. Afterward, VMI and VMV were calculated as follows:
VMV = (percentage of intermediate cells × 0.5) + (percentage of superficial cells)
Participants with a normal Pap smear and VMV ≤ 55 were introduced to the study and the study objectives were explained to them. Written informed consents were obtained from the participants and they were randomly assigned to one of the groups.
One group received vitamin E vaginal suppositories (100 units), whereas the other group received conjugated estrogen vaginal cream (0.625 mg, equal to one-eighth of the tube), manufactured by Abureyhan Company (Iran). The treatment continued for a period of 12 weeks. The participants were recommended to use the cream or suppositories every night during the first two weeks and twice a week for the following 10 weeks. The subjects were asked not to use any other hormonal or vaginal drugs. In addition, if they experienced any side effects, they were instructed to stop the treatment and contact the researcher.
After 4, 8, and 12 weeks of treatment, Pap smear was performed again. The samples were obtained from the lateral vaginal wall and sent to laboratory for VMI calculation. Successful treatment was defined as VMV ≥ 10, i.e., a 10-unit increase (or more), compared to the preintervention period.
For statistical analysis, descriptive statistics were calculated, and Friedman and Mann-Whitney tests were performed. P value of less than 0.05 was considered statistically significant.
The Ethics Committee of Mashhad University of Medical Sciences approved this study. It should be mentioned that the participants were referred to a gynecologist for treatment and follow-up in case the treatment ended in failure.
RESULTS
In total, eight participants were excluded from the study, i.e., four participants from the vitamin E group (three for vaginal burning and one for increased vaginal discharge) and four participants from the estrogen group (one for vaginal burning, one for sensitivity and enlargement of breasts, and two for uterine hemorrhage).
The two groups showed no significant difference in terms of age (52.88 ± 6 vs. 52.11 ± 4.7), the time interval since amenorrhea (62.76 ± 59.23 vs. 54.46 ± 52.21 months), parity (5 ± 2.48 vs. 5 ± 2.78), or body mass index (28.62 ± 4.46 vs. 27.25 ± 5.03) (P = 0.62, P = 0.11, P = 1.000, and P = 0.38, respectively).
In order to compare VMI at the specified time intervals in the groups (intragroup comparison), Friedman test was applied. The number of superficial cells significantly increased after 4, 8, and 12 weeks of treatment, whereas the mean number of intermediate and basal cells significantly decreased (P < 0.001) [Table 1].
Table 1.
Comparison of the mean and standard deviation of vaginal maturation index and vaginal maturation value before the experiment and after 4, 8, and 12 weeks of treatment in the vitamin E group
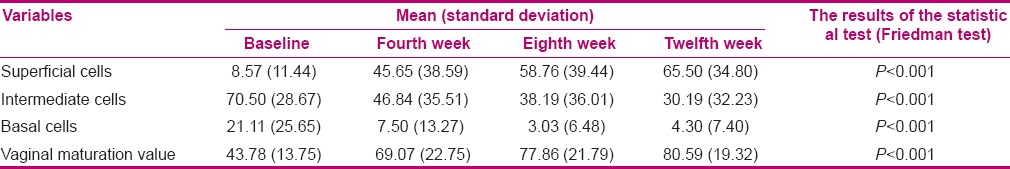
A significant increase was reported in VMV after 4, 8, and 12 weeks of treatment in the vitamin E group (P < 0.001) [Table 1]. In order to compare VMI at the specified time intervals in the estrogen group, Friedman test was applied. The number of superficial cells significantly increased after 4, 8, and 12 weeks of treatment, while the mean number of intermediate and basal cells significantly decreased (P < 0.001) [Table 2].
Table 2.
Comparison of the mean and standard deviation of vaginal maturation index and vaginal maturation value before the experiment and after 4, 8, and 12 weeks of treatment in the estrogen group
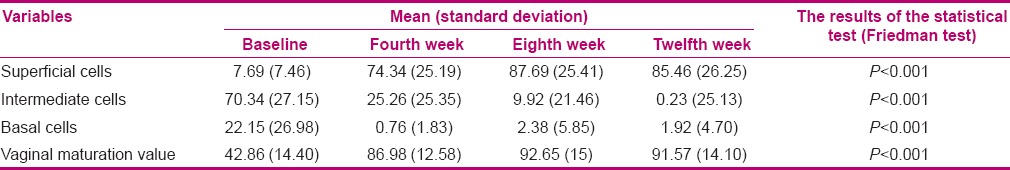
In the vitamin E group, the results showed an improvement in treatment success for vaginal atrophy after 4, 8, and 12 weeks of treatment (76.9, 80.8, and 92.3, respectively); however, the difference was not statistically significant (P = 0.19) [Table 3]. In addition, there was a significant increase in VMV after 4, 8, and 12 weeks of treatment in the estrogen group (P < 0.001) [Table 2].
Table 3.
Comparison of the success rate of vaginal atrophy after 4, 8, and 12 weeks of treatment in the two groups
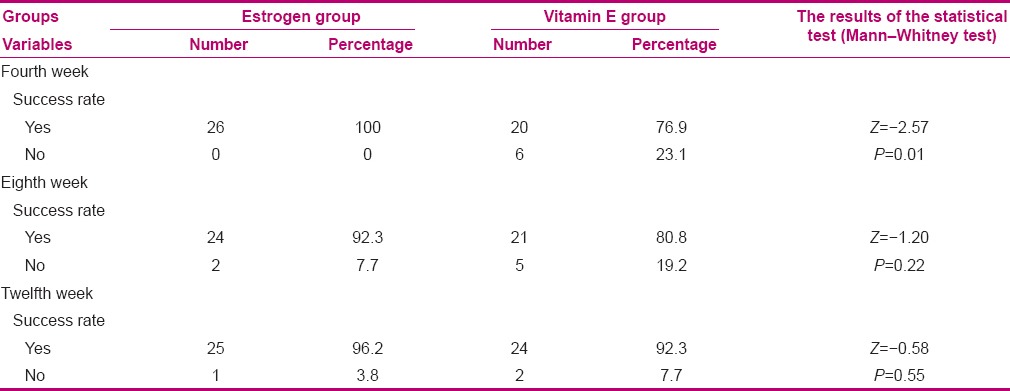
In the estrogen group, the results showed an improvement in treatment success for vaginal atrophy after 4, 8, and 12 weeks of treatment (100, 92.3, and 96.2, respectively); however, the difference was not statistically significant (P = 0.36) [Table 3]. In order to compare the percentage of treatment success at the specified time intervals between the groups (intergroup comparison), Mann-Whitney test was used. Based on the findings, a significant difference was detected only after four weeks of treatment (P = 0.01) [Table 3].
DISCUSSION
In the present study, VMV was significantly different before and after the interventions in the two groups. After 4 weeks of treatment, there was a significant difference between the groups in terms of treatment success. In fact, the rate of treatment success was significantly higher in the conjugated estrogen cream group, compared to the vitamin E group. However, no significant difference was found after 8 and 12 weeks of treatment.
Vitamin E is a fat-soluble compound,[27] which affects the urogenital system by preventing oxide/peroxide damages in cell membranes by inhibiting granula accumulation. This compound also impacts the levels of luteinizing hormone, follicle-stimulating hormone, and testosterone.[28] Raymond et al. conducted a study to investigate the effect of local conjugated estrogen cream on vaginal atrophy in menopausal women. They reported an increase in the percentage of superficial cells.[19]
Moreover, Marx et al. investigated the effect of low-dose conjugated estrogen (0.3 mg) for vaginal atrophy management in menopausal women. They found that the percentage of superficial cells and VMV increased, compared to the preintervention period.[8] Maasoumi et al. studied the therapeutic effect of tibolone and continuous hormone replacement therapy on VMI and VMV in menopausal women. Based on their findings, VMI, VMV, and percentage of superficial cells increased in the hormone therapy group.[6]
Ziaghem et al. compared the effects of hyaluronic acid and vitamin E vaginal suppositories on VMV. They showed a significant difference in VMV before and after 8 weeks of treatment.[5] Their findings were in agreement with the present study, which could be attributed to the evaluation of age-matched groups and similarities in the used instruments. Further, Constantino et al. determined the effectiveness and safety of vitamin E, vitamin A, and hyaluronic acid vaginal suppositories and showed a decline in vaginal dryness, itching, burning, dyspareunia, and inflammation after treatment.[26]
Moreover, Das et al. studied the changes in uterus and ovaries in mice with vitamin E deficiency. They found that the weight of the uterus and ovaries significantly decreased in the vitamin E deficiency group, compared to the control group. In addition, vitamin E deficiency could cause a decline in estrogen-induced enzymes and uterine peroxidase activity.[29]
In the aforementioned study, the serum estrogen level in the vitamin E deficiency group was significantly lower than the control group.[29] According to the literature review, daily use of vitamin E (100–600 units), either orally or locally, caused an increase in vaginal elasticity and improved dryness and burning due to vaginal atrophy.[18]
Vitamin E, which is often known as the “sex vitamin,” is a powerful antiaging antioxidant, which is produced by mucosal membranes and prevents free radical-induced damages. This compound is also necessary for synthesizing hormones and hormone-like substances.[30]
Vitamin E can gradually influence tissue recovery and decrease dyspareunia due to the healing of atrophic wound in the lower urogenital system.[22] Some physicians have recommended vitamin E as an estrogen substitute. Moreover, daily use of vitamin E (100 units) for protecting the cervix has been suggested;[31] this could in fact justify the increase in VMV in the vitamin E vaginal suppository group.
In a study by Ziaee et al. on the effects of vitamin E on hot flashes in menopausal women, the percentage of superficial cells did not significantly increase.[25] Also, McLoren et al. performed a study on the effect of vitamin E during menopause. In the mentioned study, vaginal smear rarely changed and no relationship was detected between VMV and symptom improvement. Moreover, the cervix was not stimulated for secretion;[5] the difference could be related to the use of various forms of drugs.
In vaginal application, some factors such as periodical changes, thickness and opening of the vaginal epithelium, viscosity, and pH of vaginal secretion influence drug absorption.[32] In general, the results of this study indicated a significant difference between the two groups in terms of therapeutic response. The percentage of VMI was higher in the group receiving estrogen cream, compared to the vitamin E group.
According to the literature, estrogen therapy (applied either orally or locally) could increase both tissue collagen and tissue thickness. The major mechanism contributing to this therapeutic effect is the increased level of polysaccharides and hyaluronic acids and maintaining the functional property of vaginal epithelial cells.[6]
Estrogen therapy is the only successful treatment for vaginal atrophy. Considering the presence of estrogen receptors in the urogenital system, the rate of treatment success was higher in the conjugated estrogen group, compared to the vitamin E group after 4 weeks of treatment in the present study. Although estrogen is mostly absorbed locally, some parts are absorbed systemically and stimulate the growth of endometrium.[17]
Vaginal conditions, hemorrhage, irregular menstrual period, dysmenorrhea, amenorrhea, increased risk of breast and endometrial cancers, cervical sensitivity, cervical discharge, and growth of uterine fibroids are complications associated with conjugated estrogen.[10,33] In conclusion, because the administration of estrogen is the only effective treatment for vaginal atrophy and vitamin E is a nonhormonal treatment, VMV was expected to be lower in the vitamin E group compared to the hormone therapy group. This finding was confirmed only after 4 weeks of treatment in this study.
The limitations of this research were the blind design of the study and the limited use of different forms of drugs.
CONCLUSION
Based on the results of this study, there was a significant difference in terms of treatment success between the two groups after 1 week of treatment. According to the findings, the laboratory index for studying vaginal atrophy improved after taking vaginal vitamin E suppositories, and the treatment proved to be successful. Therefore, vitamin E vaginal suppository is recommended for women with vaginal atrophy, who either show contraindication to hormone replacement therapy or are sensitive to such drugs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This study was approved by the Ethics Committee of Mashhad University of Medical Sciences (approval code: 911186) and funded by the Vice-presidency for Research. We would like to thank the research committee and the laboratory staff of Mashhad School of Pharmacy for their sincere cooperation. We also extend our gratitude to the personnel of gynecology clinics at Mashhad Qaem Hospital and healthcare centers No. 1 and 3. (IRCT code: 1N2013060913611).
REFERENCES
- 1.Noroozi E, Kasiri DN, Eslami A, Hassanzadeh A, Davari S. Knowledge and attitude of 40-45 year old women toward menopause. J Health Sys Res. 2011;7:460–8. [Google Scholar]
- 2.Baghdari N. Effect of Flaxseed on hot flashes in women at the climacteric, Mashhad University of Medical Sciences, School of Nursing and Midwifery. 2007 [Google Scholar]
- 3.Berek JS, Novak E. Berek and Novak's Gynecology. 14th ed. Philadelphia: Lippincott Williams & Wilkins; 2012. pp. 522–9. [Google Scholar]
- 4.Blake J. Menopause: Evidence-based practice. Best Pract Res Clin Obstet Gynaecol. 2006;20:799–83. doi: 10.1016/j.bpobgyn.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Ziagham Z, Abbaspoor Z, Abbaspour MR, Rashidi I, Haghighizadeh M. The comparison between the effects of hyaluronic acid vaginal suppository and vitamin E on vaginal maturation index in menopausal women. AJUMS. 2013;11:393–401. [Google Scholar]
- 6.Maasoumi R, Zieaie S, Faghihzade S. Comparing the effects of tibolone and continuous hormone replacement therapy on the vaginal maturation index and value in menopausal women. Koomesh J. 2012;13:397–404. [Google Scholar]
- 7.Panjari M, Davis SR. Vaginal DHEA to treat menopause related atrophy: A review of the evidence. Maturitas. 2011;70:22–5. doi: 10.1016/j.maturitas.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Marx P, Schade G, Wilbourn S, Blank S, Moyer DL, Nett R. Low-dose (0.3 mg) synthetic conjugated estrogens A is effective for managing atrophic vaginitis. Maturitas. 2004;47:47–54. doi: 10.1016/s0378-5122(03)00240-8. [DOI] [PubMed] [Google Scholar]
- 9.Speroff L, Fritz MA. Clinical gynecologic endocrinology and infertility. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 552–99. [Google Scholar]
- 10.Gharekhani P, Sadatiyan A. Women's diseases CMMD. 5th ed. Tehran: Noordanesh publication; 2000. pp. 309–24. [Google Scholar]
- 11.Palacios S. Managing urogenital atrophy. Maturitas. 2009;63:315–8. doi: 10.1016/j.maturitas.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Tadayon M, Rad P, Abbaspour MR, Latifi SM, Rashidi I, Barati M, Delaaviz H. The effect of vitamin D suppository on atrophic vaginal mucosa in menopausal women. Armaghane-danesh. 2012;17:187–195. [Google Scholar]
- 13.Labrie F, Archer D, Bouchard C, Fortier M, Cusan L, Gomez G, et al. Intravaginaldehydroepiandrosterone (Prasterone), a physiological and highly efficient treatment of vaginal atrophy. Menopause. 2009;16:907–22. doi: 10.1097/gme.0b013e31819e8e2d. [DOI] [PubMed] [Google Scholar]
- 14.Rajaeefard A, Mohammad-Beigi A, Mohammad-Salehi N. Estimation of natural age of menopause in Iranian women: A meta-analysis study. Koomesh J. 2011;13:1–7. [Google Scholar]
- 15.Shohani M, Rasouli F, Haji AP, Hasanpoor DA, Mahmoudi M. Evaluation of the urogenital problems of menopausal woman referred to the health care centers of Ilam, Iran. HMJ. 2009;13:189–96. [Google Scholar]
- 16.Greendale G, Zibecchi L, Petersen L, Ouslandert G, kahn B, Ganz P. Development and validation of aphysical examination scale vaginal atrophy and inflammation to assess vaginal atrophy and inflammation. Climacteric Journal. 1999;2:197–204. doi: 10.3109/13697139909038062. [DOI] [PubMed] [Google Scholar]
- 17.Rayan KJ, Berkowitz RS. Kisner's Gynecology & women's Health. 7th ed. Boston: Mosby; 1999. pp. 634–46. [Google Scholar]
- 18.Castelo-Branco C, Cancelo M, Villero G, Nohales F, Juli M. Management of post-menopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52:46–52. doi: 10.1016/j.maturitas.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Raymundo N, Yu-cheng B, Zi-yan H, Lai CH, Leung K, Subramaniam R, et al. Treatment of atrophic vaginitis with topical conjugated equine estrogens in postmenopausal Asian women. Climacteric. 2004;7:312–8. doi: 10.1080/13697130400003147. [DOI] [PubMed] [Google Scholar]
- 20.Gass M, Taylor M. Alternatives for women through menopause. AJOG. 2001;185:47–56. doi: 10.1067/mob.2001.116524. [DOI] [PubMed] [Google Scholar]
- 21.Xu LW, Jia M, Salchow R, Kentsch M, Cui X, Deng HY, et al. Efficacy and side effects of Chinese herbal medicine for menopausal symptoms: A critical review. Evid Based Complement Alternat Med. 2012;2012:568106. doi: 10.1155/2012/568106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziagham S, Abbaspoor Z, Safyari S, Rad P. Effect of vitamin E vaginal suppository on atrophic vaginitis among postmenopausal women. Jundishapur Journal of Chronic Disease Care. 2013;2:11–19. [Google Scholar]
- 23.Vitamin E (tocopherol) Org [Internet] [Last accessed on 2013 Jan 21]. Available from http://www.vitamins.supplements.org .
- 24.Ziagham Z, Abbaspoor Z, Abbaspour MR. The comparison between the effects of hyaluronic acid vaginal suppository and vitamin E on the treatment of atrophic vaginitis in menopausal. AMUJ. 2012;15:57–64. [Google Scholar]
- 25.Ziaei S, Kazemnejad A, Zareai M. The effect of vitamin E on hot flashes in menopausal women. Gynecol Obstet Invest. 2007;64:204–7. doi: 10.1159/000106491. [DOI] [PubMed] [Google Scholar]
- 26.Costantino D, Guaraldi C. Effectiveness and safety of vaginal suppositories for the treatment of the vaginal atrophy in postmenopausal women: An open, non-controlled clinical trial. Eur Rev Med Pharmacol Sci. 2008;12:411–6. [PubMed] [Google Scholar]
- 27.Vitamin E. 2013. [Last accessed on 2013 Jan 21]. Available from: https://fa.wikipedia.org/wiki .
- 28.Mohamadi NI. Vitamin in health and disease. 1st ed. Tehran: Boshrapublisher; 2007. pp. 135–50. [Google Scholar]
- 29.Das P, Chowdhury M. Vitamin E-deficiency induced changes in ovary and Uterus. Molecular and Cellular Biochemistry Journal. 1999;198:151–156. doi: 10.1023/a:1006954032164. [DOI] [PubMed] [Google Scholar]
- 30.Kohi D, Ghaderpanahi M. Nutrition and sexual orientation. [Last accessed on 2005 Nov 22]. Available from: http://www.civilica.com/Paper-NCFSP02-NCFSP02_004.htm2005 .
- 31.Feinstein A. Treatment with vitamins. 1st ed. Tehran: Afarinegan Publisher; 2000. pp. 413–591. [Google Scholar]
- 32.Valenta C. The use of mucoadhesive polymers in vaginal delivery. Adv Drug Deliv Rev. 2005;57:1692–712. doi: 10.1016/j.addr.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Shahraz S, Ghaziani T. Comprehensive text book of drug information. 4th ed. Tehran: Teimourzadeh publisher; 2007. p. 281. [Google Scholar]


