Abstract
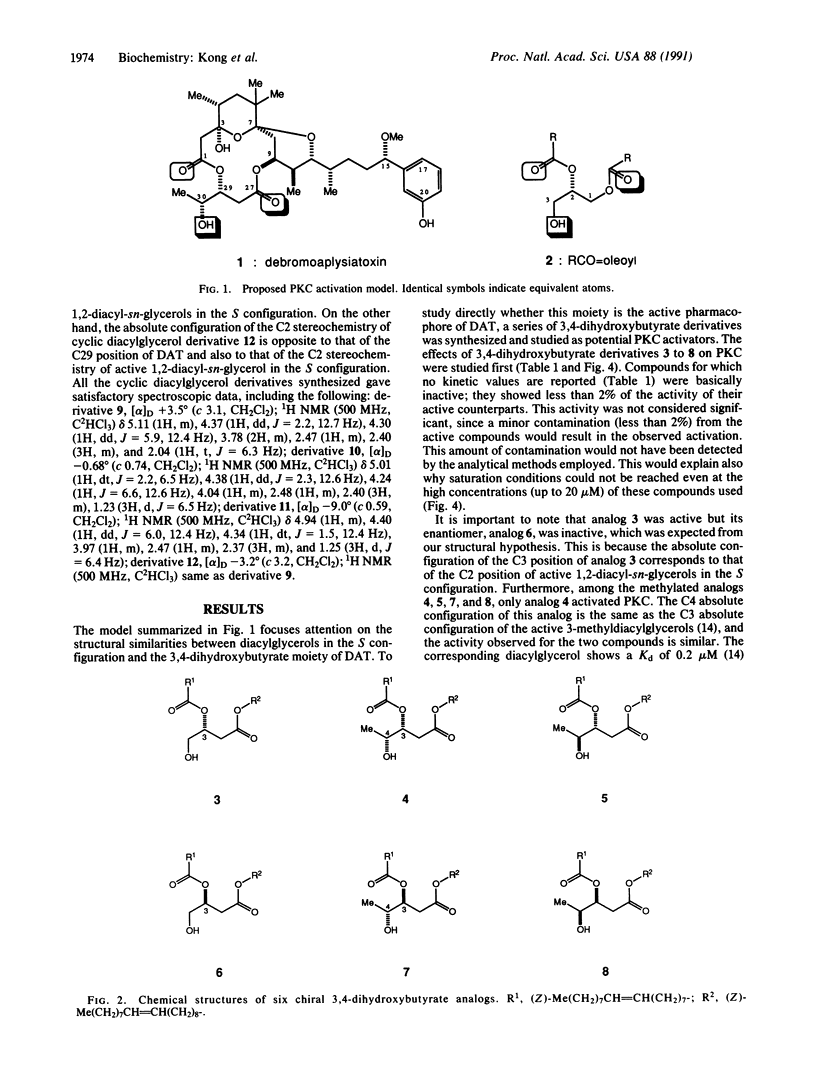
Protein kinase C is physiologically activated by 1,2-diacyl-sn-glycerol in the S configuration. The enzyme is also powerfully activated by structurally diverse tumor promotors. A model has been developed that demonstrates how the various tumor promotors and diacylglycerols can all be accommodated by the same binding site of the kinase. One prediction of this model concerns the structural nature of the pharmacophore in the tumor promotor debromoaplysiatoxin. This prediction is realized by synthesizing the analogs with the deduced pharmacophore and demonstrating that they are potent activators of protein kinase C. These findings provide strong experimental support for our structural model of protein kinase C activation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Boni L. T., Rando R. R. The nature of protein kinase C activation by physically defined phospholipid vesicles and diacylglycerols. J Biol Chem. 1985 Sep 5;260(19):10819–10825. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Ganong B. R., Loomis C. R., Hannun Y. A., Bell R. M. Specificity and mechanism of protein kinase C activation by sn-1,2-diacylglycerols. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1184–1188. doi: 10.1073/pnas.83.5.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go M., Sekiguchi K., Nomura H., Kikkawa U., Nishizuka Y. Further studies on the specificity of diacylglycerol for protein kinase C activation. Biochem Biophys Res Commun. 1987 Apr 29;144(2):598–605. doi: 10.1016/s0006-291x(87)80008-6. [DOI] [PubMed] [Google Scholar]
- Jeffrey A. M., Liskamp R. M. Computer-assisted molecular modeling of tumor promoters: rationale for the activity of phorbol esters, teleocidin B, and aplysiatoxin. Proc Natl Acad Sci U S A. 1986 Jan;83(2):241–245. doi: 10.1073/pnas.83.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U., Nishizuka Y. The role of protein kinase C in transmembrane signalling. Annu Rev Cell Biol. 1986;2:149–178. doi: 10.1146/annurev.cb.02.110186.001053. [DOI] [PubMed] [Google Scholar]
- Kong F., Kishi Y., Perez-Sala D., Rando R. R. The stereochemical requirement for protein kinase C activation by 3-methyldiglycerides matches that found in naturally occurring tumor promoters aplysiatoxins. FEBS Lett. 1990 Nov 12;274(1-2):203–206. doi: 10.1016/0014-5793(90)81364-t. [DOI] [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B., Cooper H. L., Sando J. J. Decrease in cytosolic calcium/phospholipid-dependent protein kinase activity following phorbol ester treatment of EL4 thymoma cells. J Biol Chem. 1982 Nov 25;257(22):13193–13196. [PubMed] [Google Scholar]
- Molleyres L. P., Rando R. R. Structural studies on the diglyceride-mediated activation of protein kinase C. J Biol Chem. 1988 Oct 15;263(29):14832–14838. [PubMed] [Google Scholar]
- Mori T., Takai Y., Yu B., Takahashi J., Nishizuka Y., Fujikura T. Specificity of the fatty acyl moieties of diacylglycerol for the activation of calcium-activated, phospholipid-dependent protein kinase. J Biochem. 1982 Feb;91(2):427–431. doi: 10.1093/oxfordjournals.jbchem.a133714. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Kishi Y., Pajares M. A., Rando R. R. Structural basis of protein kinase C activation by tumor promoters. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9672–9676. doi: 10.1073/pnas.86.24.9672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Rando R. R. Regulation of protein kinase C activity by lipids. FASEB J. 1988 May;2(8):2348–2355. doi: 10.1096/fasebj.2.8.3282960. [DOI] [PubMed] [Google Scholar]
- Rando R. R., Young N. The stereospecific activation of protein kinase C. Biochem Biophys Res Commun. 1984 Jul 31;122(2):818–823. doi: 10.1016/s0006-291x(84)80107-2. [DOI] [PubMed] [Google Scholar]
- Wender P. A., Koehler K. F., Sharkey N. A., Dell'Aquila M. L., Blumberg P. M. Analysis of the phorbol ester pharmacophore on protein kinase C as a guide to the rational design of new classes of analogs. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4214–4218. doi: 10.1073/pnas.83.12.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]



