Abstract
Background:
Ventilator-associated pneumonia (VAP) is a common side effect in patients who receive intravenous sedation infusion. In routine care, after starting sedation infusion for patients who receive mechanical ventilation, interruption of sedation starts without protocol. This study aimed to evaluate the effect of daily sedation vacation protocol on the incidence of VAP in mechanically ventilated patients.
Materials and Methods:
In this clinical trial study, 80 patients with intravenous sedation infusion were selected and randomly allocated to intervention and control groups. In the intervention group, daily sedation vacation protocol and in the control group, routine sedation vacation was followed. Modified clinical pulmonary infection score questionnaire was completed before intervention and on the third, fourth, and fifth days after intervention. Data were analyzed by using repeated measures analysis of variance (ANOVA), Chi-square, and independent t-test.
Results:
The results of this study showed that the incidence rate of VAP in the intervention and control groups was 0% versus 15% on the third day of intervention, 12.5% versus 50% on the fourth day, and 27.5% versus 55.3% on the fifth day of intervention in the intervention and control groups, respectively. The incidence of VAP in the intervention group was significantly lower than in the control group (P < 0.05).
Conclusions:
The results of this study showed that in patients with intravenous sedation, infusion of a daily sedation vacation protocol may reduce the incidence of VAP. Therefore, in order to prevent VAP, nurses are recommended to use this daily sedation vacation protocol.
Key words: Iran, ventilator-associated pneumonia, sedation, sedation vacation
INTRODUCTION
The purpose of mechanical ventilation (MV) is to support breathing and provide ventilation to the patient, so that the background cause of the disease is removed and the patient is enabled to breathe.[1] Although MV leads to improving the exchange of oxygen in the lungs, it has certain side effects. These effects include cardiovascular and hemodynamic complications, as well as barotrauma and effects on the renal system and other systems of the body.[2] Besides, the risk of developing pulmonary infection increases as a consequence of distorting the natural condition of air pathways through placing the trachea pipe. One of the most common and lethal effects of MV is the risk of ventilator-associated pneumonia (VAP).[3]
VAP is subdivided into early-onset and late-onset types.[4] Early-onset VAP occurs within 48–96 h after starting invasive MV and is usually caused due to pathogens which are sensitive to antibiotics within a colony (such as Staphylococcus aureus and Moraxella catarrhalis).[5]
VAP is the second common nosocomial infection and also the first common infection within coronary care units (CCUs).[6] In contrast with the infections that engage all systems (like skin and urinary tract) and have a mortality rate of 1–4%, the mortality rate for VAP is 24–50% and it can also reach 76% in some high-risk groups.[7]
The rate of VAP in developing countries varies from 10 to 41%.[8] In the meantime, CCU-related VAP has an incidence rate of 21% in Pakistan, 45% in Lebanon, and 81% in India. In Iran, its rate has been reported to be 46%.[9] In Arabnejad et al.'s study conducted in 2011, the frequency of early-onset VAP was reported to be between 40 and 42%,[8] while it has been reported to be 12.7% at Mashhad Hospital and 9.2% at Semnan Hospital. Such a low rate of incidence for VAP compared with the statistics for all the countries in the region might be due to lack of correct diagnosis of this nosocomial infection.[10] The incidence of nosocomial pneumonia at Al Zahra Hospital in Isfahan, Iran in 2012 was reported to be 24.8%, which is accompanied by a 5% increase in expenditures.[11]
VAP leads to increase in therapeutic expenses, mortality rate, and the time needed for MV and also the days of hospitalization in intensive care unit (ICU).[12,13]
Patients who receive MV require continuous infusion of sedation, so that their discomfort is relieved. These patients face the risk of receiving excessive sedation and, consequently, they are susceptible to get prolonged MV and being afflicted with VAP as a result of the high risk for aspiration and suppression of coughing reflex. Ventilator bundles include the rising of bed up to 30°-45°, preventing ulcer stress, interrupting daily sedation, and preventing deep vein thrombosis, and thus, they decrease VAP. Daily sedation interruption (DSI) is the main component of these measures for preventing the spread of VAP among patients who receive MV.[14] DSI can accelerate extubation and prevent the leakage of secretions behind the cuff and, subsequently, decrease VAP. In addition, another reason for the increase of VAP among intubated patients is the increase in length of MV, which can be reduced through administering sedation interruption protocol.[15] According to studies, DSI brings about positive outcomes for patients and can reduce the rate of pneumonia.[16] DSI is a protocol for reducing daily sedation rate according to special criteria and in case a patient fulfills these criteria, his/her sedation is reduced within a few days and then stopped while being frequently assessed throughout this process.[17]
As mentioned before, one of the methods that can be effective in decreasing the incidence rate of VAP is applying DSI protocol (DSIP) as one of the main caring components of ventilator bundles. However, according to some studies, applying the DSIP did not have any effect on reducing the rate of VAP.[18] Moreover, there is no single protocol for DSI in ICUs and sedation interruption is carried out mentally through the cooperation between physician and nurse. As a result, while most patients fulfill the criteria for sedation interruption, they return to their past condition and become prone to an increase in the rate of VAP.[17] Therefore, with regard to the absence of a special protocol for interrupting sedation and also lack of sufficient evidence for verifying the effect of sedation interruption protocol on VAP, the researchers carried out this study with the aim of exploring the effect of DSIP on the incidence of early-onset VAP among patients hospitalized in the ICUs of Al Zahra Hospital, Isfahan, Iran in 2014.
MATERIALS AND METHODS
This is a single-blind clinical trial study IRCT Registration number IRCT2015052022353N1. Accordingly, none of the subjects was aware of being placed in either the control or the intervention group. The study was carried out in 2014 on patients who were hospitalized in ICUs at Al Zahra Hospital, Isfahan, Iran and were receiving MV.
According to Budama et al.'s study and the probability of type 1 error of 0.05 and a coefficient of 0.80, the sample size was 40 individuals in each of the intervention and control groups. Due to lack of possibility for random selection, the samples were primarily selected from available eligible individuals among patients hospitalized in ICUs at Al Zahra Hospital through continuous convenient sampling method. Accordingly, they were assigned to the control and intervention groups by minimization randomization method. using this method, randomization and homogenization of samples were done and they were equally placed in each group while attending to distributing variables. In this study, a total of 100 eligible samples were enrolled in the intervention group (52 individuals) and the control group (48 individuals). During the study, 20 individuals withdrew from the study; among these, 12 individuals were from the intervention group. Out of these 12 individuals, 8 withdrew due to not being able to tolerate sedation interruption following major discomfort, 3 due to being transferred to other wards, and 1 due to undergoing surgical operation. The remaining eight individuals belonged to the control group, among whom four withdrew due to undergoing surgical operation, three due to major discomfort, and one due to passing away. It finally resulted in 40 individuals for each group.
Informed written consent was obtained from all samples for participating in the research, while the ones from unconscious patients were obtained from their parents.[14] The inclusion criteria included: Patients who had continuous infusion of sedation or tranquilizer; aged more than 18 and less than 65 years; were receiving MV for 24 h; had no pneumonia at the beginning of the research [with a score lower than 5 according to modified clinical pulmonary infection score (MCPIS)]; had not participated in any other studies; did not have intracerebral hypertension attested by their physician, major cerebral damages, and a Glasgow Coma Scale (GCS) lower than 8; and were not hospitalized due to major neurologic problems like cerebrovascular accident (CVA).[18] Meanwhile, the exclusion criteria were: Lack of interest of the patients’ parents in participating in the study, the patients who died or were transferred to other wards or other hospitals, excessive discomfort on the side of patients which could prevent sedation interruption, and undergoing surgical operations during the research.
The samples were randomly selected and allocated to either the control or the intervention group by using minimization randomization method. The pre-specified variables for inserting the samples into the software were gender, age, type of peptic ulcer prophylaxis, past record of smoking, and type of antibiotic received. The researcher extracted patients’ demographic and clinical information from their hospital profile and then inserted them in the first section of the data collection instrument. Before beginning the research, the MCPIS was filled in for all samples and the ones who were suffering from VAP were excluded. Then, the researcher administered DSIP for patients within the intervention group. The third section of instrument included the richmond agitation-sedation scale (RASS) for measuring the amount of discomfort in patients during the study.
The MCPIS is a standard scale which contains the five criteria of body temperature, pulmonary secretions, white blood cell (mm Hg), and chest radiography. Data collection was carried out by the researcher and his colleague who holds a BSc in Nursing, while the interpretation of chest radiography was done by an expert in anesthetics. For each criterion in this scale, a point between 0 and 2 has been considered and the maximum point is 10. Gaining a point above 5 indicates VAP.[19,20] The Persian version of the MCPIS has been used in this research. Sabery et al. calculated its reliability using Cronbach alpha test and an internal correlation coefficient of 91%.[21] According to previous studies, the MCPIS was measured in the third, fourth, and fifth days of the research at 7 a.m.
The RASS scale was used to measure the rate of patient's sedation and discomfort and for finding the amount of applied sedation. Its reliability and validity has been approved in various research papers and books.[22] Moreover, in order to administer sedation interruption, the designed protocol in foreign papers was used, whose validity had been approved by ICU experts and also faculty members of the nursing department.
The protocol designed according to approved papers advised DSI at 7 a.m. for patients who had continuous infusion of sedation with morphine, midazolam, fentanyl, and propofol, while the patient was to be assessed continuously for 5 h by the RASS scale. If there was no major discomfort while there was a RASS scale between 0 and 2, sedation interruption was kept up and if there was a score above 2 or there was major discomfort after injecting bolus to patients, the infusion was done with half the previous dosage. In the following days, DSI was tried again and this protocol was continued for 5 days, which led to the start of early-onset VAP. Sedation interruption was commenced for patients in the control group without any special protocol, according to the ward routine with the infusing sedative based on physician's recommendation or nurse's suggestion, while increasing the amount of drug was done to the point that the patient could tolerate the trachea pipe and would not feel any discomfort. Decrease followed by interruption of sedation was initiated according to nurse's or physician's suggestion routinely and mentally and the dosage of the drug was reduced. In case any discomfort was faced by patients, repeated infusion was carried out and if the patient could tolerate, the reduction and interruption of sedation were done until the complete interruption of the sedative. After completely interrupting the sedation, the patient was prepared for extubation.
SPSS software, version 18, was used for analyzing the data. In order to examine the homogeneity of the samples’ ages within both groups, independent t-test was used and for assessing the homogeneity of samples regarding their gender, cause of hospitalization, record of disease, previous record of smoking, and immune system deficiency, Chi-square test was used. Finally, in order to compare the scores of MCPIS before and after the study in both groups, independent t-test and Repeated Measures Analysis of Variance were used.
Ethical considerations
The research was approved by the Ethics Committee of Isfahan University of Medical Sciences.
RESULTS
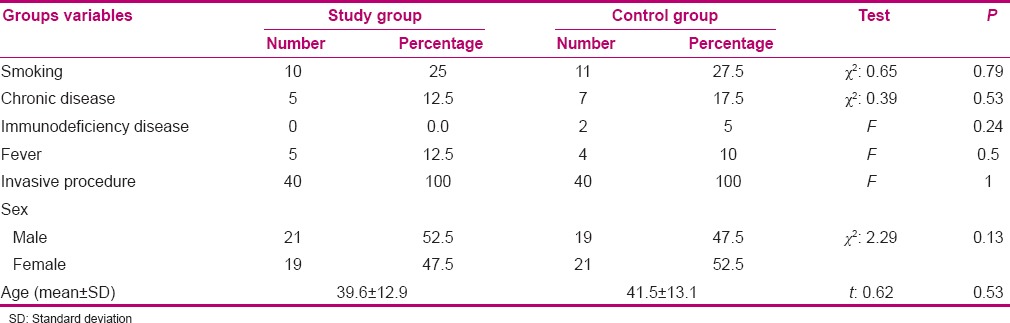
The mean (standard deviation) of age among samples in the intervention and control groups were 39.6 (12.9) and 41.5 (13.1) years, respectively. Fifty percent of the subjects were male, while the remaining subjects were female. Independent t-test, Chi-square, and Fisher's exact test indicated that the subjects in both groups were homogenous regarding age, gender, and underlying problems (P > 0.05) [Table 1].
Table 1.
Comparison of the frequency distribution of subjects regarding age, sex, and underlying problems in the study and control groups
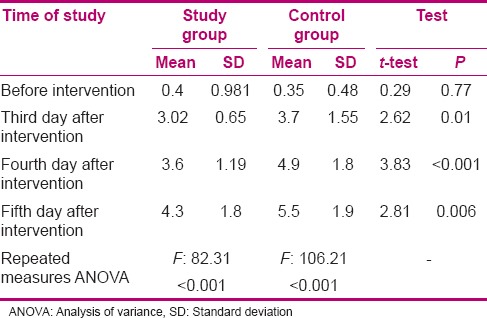
Results of the independent t-test indicated that the mean score of MCPIS did not have any significant difference in the pre-intervention stage between both groups and that both groups were homogenous (P > 0.05). However, throughout the third, fourth, and the fifth days of intervention, the mean score of MCPIS in the intervention groups was significantly lower than in the control group (P < 0.05). This means that on the third, fourth, and the fifth days of the intervention, the incidence rate of VAP was lower in the intervention group [Table 2].
Table 2.
Comparison of modified clinical pulmonary infection score before intervention and on the third, fourth, and fifth days after intervention between study and control groups
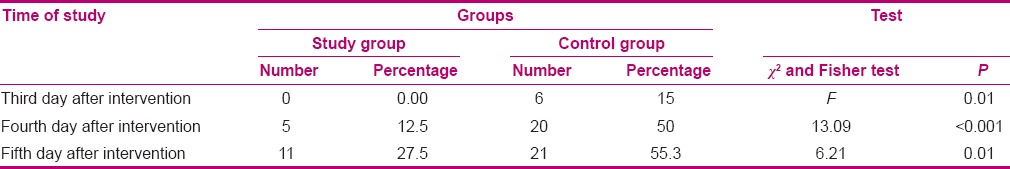
The incidence rate of VAP in the intervention and control groups was 0% versus 15% on the third day of intervention, 12.5% versus 50% on the fourth day, and 27.7% versus 55.3% on the fifth day of intervention. Results of the Fisher's exact test indicated that on the third day after the intervention, the frequency of VAP was significantly higher in the control group (P < 0.05). Moreover, Chi-square test indicated that on the fourth and the fifth days after the intervention, the incidence rate of VAP was significantly higher in the control group (P < 0.05) [Table 3].
Table 3.
Comparison of outbreak of ventilator-associated pneumonia before intervention and on the third, fourth, and fifth days after intervention between the study and control groups
DISCUSSION
Schweickert et al.'s study showed that administering the DSIP leads to reduction of many complications among intubated patients who receive MV. Among them, ventilator-associated infections are most prominent. Nonetheless, the rate of MCPIS was significantly lower in the intervention group (P < 0.05).[23] In Kress et al.'s study, it was also mentioned that administering the DSIP significantly leads to decrease in the length of MV and accordingly reduces the incidence rate of VAP in the intervention group more than in the control group.[24]
With regard to administering the DSIP for patients receiving MV in the intervention group, it was expected that the MCPIS would be lower in this group compared with the control group, which was true in practice. Subsequently, on the third, fourth, and fifth days after the intervention, the MCPIS in the intervention group was significantly lower than in the control group. According to the study of Quenot et al. (2007), administering the DSIP leads to a significant decrease in the rate of MCPIS among the patients in the intervention group 5 days after the first intubation, which is in agreement with the present study (P < 0.001). The results of their study, like the present study, showed that administering the sedation interruption by nurses results in reducing VAP.[25]
So far, few studies have investigated the effect of sedation interruption separately on VAP and most studies have assessed the simultaneous effect of ventilator bundles comprising four components on VAP. In 2012, a study by Smith explored the effect of administering sedation interruption protocol by nurses on ventilator-associated infection at Georgia University. This study was done retrospectively and indicated that administering DSI brings about positive outcomes for patients, including reducing VAP.[26]
In another study, Anifantaki et al. (2009) assessed the effect of continuous sedation infusion DSI on patients hospitalized at ICUs. In this study, the average length of MV did not have significant difference in the two groups (P = 0.7), while it was 8.7 days in the control and 7.7 days in the intervention group. Moreover, no significant difference was observed regarding the length of stay at ICU and the incidence of VAP. The general conclusion is that using sedation interruption protocol by the nurse compared with interruption of routine sedation does not have that much difference by virtue of its effects.[18] Although this research was not congruent with the present research, it is necessary to attend to this point that the protocol was used in the present research as a new and structured method for interruption of sedation. While no protocol has been used with this aim in our context, the presence of a structured protocol is obvious in Anifantaki's study which had been carried out in order to compare two protocols.
Schweickert and Kress carried out a study with the aim to assess the effect of DSIP on reducing the complications of VAP, including decreasing the length of MV, reducing the length of stay at ICU, and cutting down on VAP. The patients who were under MV while sedation interruption protocol was administered for them (intervention group) experienced fewer complications than the patients who received simple sedation (control group) (2.8% for the intervention group and 6.2% for the control group, P = 0.04). The difference of this research with the present study was its retrospective nature. However, its findings are congruent with the present research and approve the positive effect of protocol on VAP.[27] In all the aforementioned studies whose results were congruent with the present study, it was stated that by applying the methods of sedation interruption, the number of days for being attached to ventilator and the amount of its associated infections are reduced. With regard to the point that the presence of trachea pipe is a reason that leads to VAP, it seems that the main reasons for the reduced incidence rate of VAP are the administration of DSIP, early awakening of the patient, and decreased dependence of the patient to the ventilator as a result of faster extubation. Consequently, nurses can reduce the incidence rate of VAP among patients receiving ventilation through administering DSIP. Among the limitations of this study are lack of homogenization of medications received for continuous sedation infusion and the length of the study which did not explore the late-onset infection among these patients.
CONCLUSION
Administering DSIP can lead to reduction in the length of ventilation and subsequently lowers the incidence rate of VAP. Reduction of infection is a major advantage for patients hospitalized at ICUs. Hence, using sedation interruption protocols for patients receiving ventilation is recommended to personnel, especially the nurses at ICU.
Financial support and sponsorship
Isfahan University of Medical Sciences (No. 393699)
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
This article was derived from the Master's thesis of Mehdi Shahabi with project number 393699 in Isfahan University of Medical Sciences, Isfahan, Iran. We appreciate the cooperation of the staff of Nursing and Midwifery Faculty and the Vice Chancelor of Research of Isfahan University of Medical Sciences, and the staff at Critical Care Units at Al Zahra Hospital of Isfahan. We are also grateful to all patients who participated in this study.
REFERENCES
- 1.Marino P, Sutin K. The ICU Book. 3th ed. Philadelphia: Williams & Wilkins Pub.; 2007. [Google Scholar]
- 2.Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): A randomised controlled trial. Lancet. 2008;371:126–34. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 3.Mofrad MN, Shiri H. Intensive Care in ICU. 13th ed. Tehran: Noor-Danesh pub.; 2013. [Google Scholar]
- 4.Roy G. Interventions by critical care nurses reduce VAP. Dynamics. 2007;18:28–31. [PubMed] [Google Scholar]
- 5.Esperatti M, Ferrer M, Theessen A, Liapikou A, Valencia M, Saucedo LM, et al. Nosocomial pneumonia in the Intensive Care Unit acquired by mechanically ventilated versus nonventilated patients. Am J Respir Crit Care Med. 2010;182:1533–9. doi: 10.1164/rccm.201001-0094OC. [DOI] [PubMed] [Google Scholar]
- 6.Augustyn B. Ventilator-associated pneumonia: Risk factors and prevention. Crit Care Nurse. 2007;27:32–6. [PubMed] [Google Scholar]
- 7.Efrati S, Deutsch I, Antonelli M, Hockey PM, Rozenblum R, Gurman GM. Ventilator-associated pneumonia: Current status and future recommendations. J Clin Monit Comput. 2010;24:161–8. doi: 10.1007/s10877-010-9228-2. [DOI] [PubMed] [Google Scholar]
- 8.Arabnejad H, Mohammadbagheri M, Jonidi N, Ahmadinejad M, Tadrisi D, et al. Frequency of ventilator associated pneumonia. Iran J Infect Dis Trop Med. 2011;16:47–53. [Google Scholar]
- 9.Ranjbar H, Jafari S, Kamrani F, Alavi Majd H, Yaghmaei F, Asgari A. Effect of chlorhexidine gluconate oral rinse on preventing of late onset ventilator-associated pneumonia and it's interaction with severityof illness. Iran J Crit Care Nurse. 2010;3:81–6. [Google Scholar]
- 10.Seyedalshohadaee M, Rafii F, Haghani H, Arani FF. Evaluating the effect of mouth washing with chlorhexidine on the ventilator associated pneumonia. Iran J Nurs. 2012;25:34–44. [Google Scholar]
- 11.Khorvash F, Abbasi S, Meidani M, Dehdashti F, Ataei B. Comparison between pantoprazole and sucralfate in Ventilation Associated Pneumonia (VAP) in ICU admitted patients. [Last cited on 2016 Jan 19];Adv Biomed Res. 2014 3:52. doi: 10.4103/2277-9175.125789. Available from: http://www.advbiores.net/text.asp?2014/3/1/52/125789 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbott CA, Dremsa T, Stewart DW, Mark DD, Swift CC. Adoption of a ventilator-associated pneumonia clinical practice guideline. Worldviews Evid Based Nurs. 2006;3:139–52. doi: 10.1111/j.1741-6787.2006.00066.x. [DOI] [PubMed] [Google Scholar]
- 13.Rea-Neto A, Youssef NC, Tuche F, Brunkhorst F, Ranieri VM, Reinhart K, et al. Diagnosis of ventilator-associated pneumonia: A systematic review of the literature. Crit Care. 2008;12:R56. doi: 10.1186/cc6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouadma L, Wolff M, Lucet JC. Ventilator-associated pneumonia and its prevention. Curr Opin Infect Dis. 2012;25:395–404. doi: 10.1097/QCO.0b013e328355a835. [DOI] [PubMed] [Google Scholar]
- 15.Grap MJ, Munro CL, Hummel RS, 3rd, Elswick RK, Jr, McKinney JL, Sessler CN. Effect of backrest elevation on the development of ventilator-associated pneumonia. Am J Crit Care. 2005;14:325–32. [PubMed] [Google Scholar]
- 16.O’Keefe-McCarthy S, Santiago C, Lau G. Ventilator-associated pneumonia bundled strategies: An evidence-based practice. Worldviews Evid Based Nurs. 2008;5:193–204. doi: 10.1111/j.1741-6787.2008.00140.x. [DOI] [PubMed] [Google Scholar]
- 17.Wip C, Napolitano L. Bundles to prevent ventilator-associated pneumonia: How valuable are they? Curr Opin Infect Dis. 2009;22:159–66. doi: 10.1097/QCO.0b013e3283295e7b. [DOI] [PubMed] [Google Scholar]
- 18.Anifantaki S, Prinianakis G, Vitsaksaki E, Katsouli V, Mari S, Symianakis A, et al. Daily interruption of sedative infusions in an adult medical-surgical Intensive Care Unit: Randomized controlled trial. J Adv Nurs. 2009;65:1054–60. doi: 10.1111/j.1365-2648.2009.04967.x. [DOI] [PubMed] [Google Scholar]
- 19.Lauzier F, Ruest A, Cook D, Dodek P, Albert M, Shorr AF, et al. The value of pretest probability and modified clinical pulmonary infection score to diagnose ventilator-associated pneumonia. J Crit Care. 2008;23:50–7. doi: 10.1016/j.jcrc.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Koenig SM, Truwit JD. Ventilator-associated pneumonia: Diagnosis, treatment, and prevention. Clin Microbiol Rev. 2006;19:637–57. doi: 10.1128/CMR.00051-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabery M, Shiri H, Moradiance V, Taghadosi M, Gilasi H, Khamechian M. The frequency and risk factors for early-onset ventilator-associated pneumonia in Intensive Care Units of Kashan Shahid-Beheshti hospital during 2009-2010. Kashan Univ Med Sci J FEYZ. 2013;16:560–9. [Google Scholar]
- 22.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, et al. The richmond agitation-sedation scale: Validity and reliability in adult Intensive Care Unit patients. Am J Respir Crit Care Med. 2002;166:1338–44. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 23.Schweickert WD, Gehlbach BK, Pohlman AS, Hall JB, Kress JP. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med. 2004;32:1272–6. doi: 10.1097/01.ccm.0000127263.54807.79. [DOI] [PubMed] [Google Scholar]
- 24.Kress JP, Gehlbach B, Lacy M, Pliskin N, Pohlman AS, Hall JB. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med. 2003;168:1457–61. doi: 10.1164/rccm.200303-455OC. [DOI] [PubMed] [Google Scholar]
- 25.Quenot JP, Ladoire S, Devoucoux F, Doise JM, Cailliod R, Cunin N, et al. Effect of a nurse-implemented sedation protocol on the incidence of ventilator-associated pneumonia. Crit Care Med. 2007;35:2031–6. doi: 10.1097/01.ccm.0000282733.83089.4d. [DOI] [PubMed] [Google Scholar]
- 26.Smith S. The impact of nurses’ adherence to sedation vacations on ventilator associated pneumonia prevention. Nursing Dissertations. 2012. [Last accessed on 2016 Jun 08]. p. 33. Available from: http://www.scholarworks.gsu.edu/cgi/viewcontent.cgi?article=1035&context=nursing_diss .
- 27.Schweickert WD, Kress JP. Strategies to optimize analgesia and sedation. Crit Care. 2008;12(Suppl 3):S6. doi: 10.1186/cc6151. [DOI] [PMC free article] [PubMed] [Google Scholar]


