Abstract
Background
The aim of this study was to investigate which surgical method is better in lymph node (LN) dissection of lung cancer.
Methods
A comprehensive search of PubMed, Ovid MEDLINE, EMBASE, Web of Science, ScienceDirect, the Cochrane Library, Scopus, and Google Scholar was performed to identify studies comparing thoracoscopic lobectomy (video-assisted thoracic surgery (VATS) group) and thoracotomy (open group) in LN dissection.
Results
Twenty-nine articles met the inclusion criteria and involved 2763 patients in the VATS group and 3484 patients in the open group. The meta-analysis showed that fewer total LNs (95% confidence interval [CI] −1.52 to −0.73, p < 0.0001) and N2 LNs (95% CI −1.25 to −0.10, p = 0.02) were dissected in the VATS group. A similar number of total LN stations, N2 LN stations, and N1 LNs were harvested in both groups. Only one study reported that fewer N1 LN stations were dissected in the VATS group (1.4 ± 0.5 vs. 1.6 ± 0.6, p = 0.04).
Conclusions
Open lobectomy could achieve better LN dissection efficacy than thoracoscopic lobectomy in the treatment of lung cancer, especially in the N2 LNs dissection. These findings require validation by high-quality, large-scale randomized controlled trials.
Keywords: Video-assisted thoracic surgery, Thoracotomy, Meta-analysis, Lung cancer, Lymph node dissection
Background
Lung cancer is the leading cause of cancer deaths in many countries [1, 2]. Surgical treatment is the preferred treatment for early-stage non-small cell lung cancer (NSCLC). D’Cunha’s research showed that N1 and N2 lymph nodes (LNs) were positive in 27.5% of patients with lung cancer under lobectomy [3]. However, non-invasive examinations, such as computed tomography (CT) and positron emission tomography-computed tomography (PET-CT), are not sensitive and specific for the clinical staging of lung cancer. Video-assisted thoracic surgery (VATS) is the preferred surgical procedure, with fewer incidences of postoperative complications and a higher survival rate compared with thoracotomy [4–7]. However, whether VATS can achieve the same LN dissection efficacy is controversial, and there remains a lack of high-quality, large-scale clinical research.
To determine whether VATS can achieve the same LN dissection efficacy as thoracotomy in lung cancer, we performed a systemic review and meta-analysis.
Methods
Search strategy
MEDLINE and manual searches were performed by two investigators independently and in duplicate to identify all relevant scientific articles published from January 1990 to May 2016. The MEDLINE search was performed using PubMed, Ovid MEDLINE, EMBASE, Web of Science, ScienceDirect, The Cochrane Library, Scopus, and Google Scholar. The MeSH terms “lung cancer or lung neoplasm”, “thoracotomy or open surgery”, and “video-assisted thoracic surgery or VATS” and comparative study were used.
Inclusion criteria and exclusion criteria
The following inclusion criteria were applied: (1) published in English, (2) compared the LN dissection of thoracoscopic lobectomy with thoracotomy in treating patients with lung cancer, and (3) the most recent study was chosen when duplication of data is in more than one article.
Reviews without original data, case reports, meta-analyses, letters, expert opinions, and animal studies were excluded. Studies on robotic-assisted VATS were also excluded.
Data extraction
Two investigators independently extracted data from the eligible studies. The extracted data included first author, year of publication, geographical area, study design, duration of enrollment, information on preoperative staging, number of patients per group, LN number (LNN), and LN station number (LNS).
Quality assessment for included studies
Two investigators independently assessed the quality of each included study using the Newcastle-Ottawa Scale (NOS) for non-randomized studies and the Jadad scale for randomized controlled trials (RCTs).
The NOS evaluates the quality of studies by analyzing three items: selection, comparability, and exposure. The scale assigns a maximum of nine points to each study: a maximum of four points for selection, two points for comparability, and three points for exposure. Therefore, the highest quality study would score nine points. In our analysis, high-quality studies were defined as those that scored nine or eight points; medium-quality studies were those that scored seven or six points [8].
The Jadad scale (five points) contained questions for three main parts: randomization, masking, and accountability of all patients (withdrawals and dropouts). Studies scored ≥3 points were considered as high quality [9].
Statistical analysis
Meta-analysis was conducted by Review Manager 5.3 and SPSS 18.0, p value < 0.05 suggested statistically significant. The differences were compared between the two groups using analysis of variance for continuous variables and pooled relative risk (RR) with 95% confidence interval (CI) for categorical variables. We used I 2 and Cochran Q to evaluate the between-study heterogeneity. A random-effects model was adopted when the heterogeneity was significant (p ≤ 0.10 and I 2 > 50%); otherwise, a fixed-effects model was used. Rank correlation test of funnel plot asymmetry was used to assess the potential publication bias.
Results
Search results and quality assessment of the included studies
We initially identified 2341 publications from the database and reference list searches and reviewed 29 articles for final analysis (Fig. 1). The articles involved a total of 6247 patients, of whom 2763 underwent VATS and 3484 underwent thoracotomy. Of these 29 publications, three studies were RCTs and 26 were retrospective studies. According to the NOS and Jadad scales assessment scores, 23 articles were of good quality and the remaining six were medium quality. The baseline characteristics of these articles are listed in Table 1.
Fig. 1.
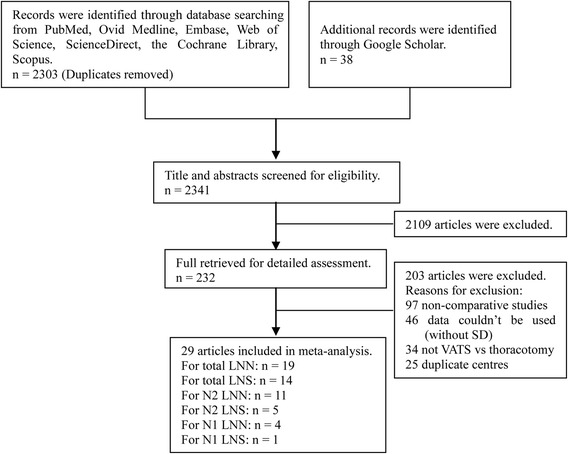
Flow diagram of screened and included papers
Table 1.
Summary of the 29 trials included in the present meta-analysis
| Study | Institution | Enrolled year | No. of patients | Clinical stage | Outcomes | Design | Quality | |||
|---|---|---|---|---|---|---|---|---|---|---|
| VATS | Open | VATS | Open | |||||||
| 1995 | Kirby [28] USA | Single | 1991.10–1993.12 | 1991.10–1993.12 | 25 | 30 | I | ① | RCT | 3 |
| 1998 | Morikawa [29] JPN | Single | 1996.04–1996.12 | 1995.01–1996.03 | 39 | 41 | I–II | ③ | Retrospective | 9 |
| 2000 | Luketich [30] USA | Single | Not mentioned | Not mentioned | 31 | 31 | I | ① | Retrospective | 7 |
| 2000 | Sugi [31] JPN | Single | 1993.01–1994.06 | 1993.01–1994.06 | 48 | 52 | Ia | ③, ⑤ | RCT | 3 |
| 2001 | Nomori [32] JPN | Single | 1999.08–2000.12 | 1998.04–1999.07 | 33 | 33 | I | ①,⑦,⑧ | Retrospective | 7 |
| 2005 | Watanabe [33] JPN | Single | 1997–2004 | 1997–2004 | 221 | 190 | I | ①, ③, ⑦, ⑧ | Retrospective | 8 |
| 2006 | Petersen [34] USA | Single | 2001–2005 | 1996–2005 | 12 | 85 | I–IV | ② | Retrospective | 8 |
| 2006 | Shigemura [35] JPN | Multi | 1999.01–2004.01 | 1999.01–2004.01 | 50 | 55 | Ia | ① | Retrospective | 9 |
| 2008 | Shiraishi [36] JPN | Single | 1994.11–2005.10 | 1994.11–2005.10 | 20 | 55 | I | ③, ⑤ | Retrospective | 8 |
| 2008 | Watanabe [37] JPN | Single | 1997–2006 | 1997–2006 | 37 | 32 | I | ①, ②, ③, ④ | Retrospective | 8 |
| 2008 | Whitson [38] USA | Single | Not mentioned | Not mentioned | 6 | 7 | Not mentioned | ① | Retrospective | 6 |
| 2009 | Nakanishi [39] JPN | Single | 2000.04–2007.01 | 2000.04–2007.01 | 13 | 14 | I–IV | ③ | Retrospective | 8 |
| 2009 | Okur [40] TR | Single | 2007.01–2007.11 | 2007.01–2007.11 | 20 | 28 | I | ② | Retrospective | 8 |
| 2010 | Denlinger [21] USA | Single | 2000.01–2008.08 | 2000.01–2008.08 | 79 | 464 | I | ①, ③, ⑤ | Retrospective | 8 |
| 2011 | D’Amico [41] USA | Single | 2007.01–2010.09 | 2007.01–2010.09 | 199 | 189 | I–III | ②,④ | Retrospective | 7 |
| 2012 | Bu [42] CHN | Single | 2001.05–2011.04 | 2001.05–2011.04 | 46 | 87 | Not mentioned | ①, ② | Retrospective | 7 |
| 2012 | Li [43] CHN | Single | 2006.09–2009.12 | 2006.09–2009.12 | 29 | 47 | I | ③, ④ | Retrospective | 8 |
| 2012 | Licht [44] DNK | Multi | 2007.01–2011.12 | 2007.01–2011.12 | 717 | 796 | I | ② | Retrospective | 8 |
| 2013 | Fan [45] CHN | Single | 2005.01–2010.12 | 2005.01–2010.12 | 79 | 77 | I–II | ①, ② | Retrospective | 8 |
| 2013 | Lee [22] USA | Single | 1990.05–2011.12 | 1990.05–2011.12 | 141 | 115 | Not mentioned | ①, ②, ③, ④, ⑤, ⑥ | Retrospective | 8 |
| 2013 | Palade [46] GER | Single | 2008.05–2011.12 | 2008.05–2011.12 | 32 | 32 | I | ①, ⑦, ⑧ | RCT | 3 |
| 2013 | Zhong [47] CHN | Single | 2006.03–2011.08 | 2006.03–2011.08 | 67 | 90 | I | ①, ②, ③, ④ | Retrospective | 8 |
| 2014 | Li [48] CHN | Single | 2011.02–2013.02 | 2011.02–2013.02 | 21 | 32 | I–II | ① | Retrospective | 8 |
| 2014 | Stephens [4] USA | Single | 2002.01–2011.12 | 2002.01–2011.12 | 307 | 307 | I | ② | Retrospective | 8 |
| 2015 | Cai [49] CHN | Single | 2010.01–2012.05 | 2010.01–2012.05 | 71 | 67 | I–II | ①, ② | Retrospective | 9 |
| 2015 | Kuritzky [50] USA | Single | 2007–2012 | 2007–2012 | 74 | 224 | I | ② | Retrospective | 8 |
| 2015 | Murakawa [51] JPN | Single | 2001–2010 | 2001–2010 | 101 | 101 | I | ①, ② | Retrospective | 8 |
| 2015 | Nwogu [6] USA | Multi | 2004.10–2010.06 | 2004.10–2010.06 | 175 | 175 | I–II | ①, ② | Retrospective | 7 |
| 2015 | Zhang [52] CHN | Single | 2012.10–2013.11 | 2012.10–2013.11 | 70 | 28 | I | ① | Retrospective | 9 |
① total lymph node number, ② total lymph node station number, ③ N2 LNN, ④ N2 LNS, ⑤ N1 LNN, ⑥ N1 LNS, ⑦ left-side LNN, ⑧ right-side LNN
CHN China, DNK Denmark, JPN Japan, GER Germany, TR Turkey, USA United States of America, RCT randomized controlled trial
Comparison of total LNN and LNS
We identified 19 articles for total LNN comparison. They involved 1297 patients in the VATS group and 1731 patients in the open group (thoracotomy). The heterogeneity between these studies was acceptable (p = 0.02, I 2 = 44%). Fewer total LNs were dissected in the VATS group as compared with the open group (95% CI −1.52 to −0.73, p < 0.00001, Fig. 2a).
Fig. 2.
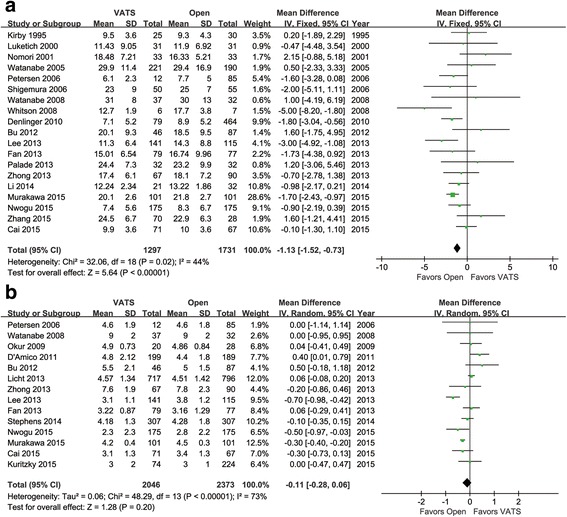
Forest plot of the mean difference in total LNN (a) and LNS (b) in the VATS group vs. the open group
Fourteen articles were identified for total LNS comparison. They involved 2046 patients in the VATS group and 2373 patients in the open group. The mean difference in total LNS between the two groups was not significant (95% CI −0.28 to 0.06, p = 0.20), with significant heterogeneity across studies (p < 0.00001, I 2 = 73%, Fig. 2b).
Comparison of N2 LNN and LNS
Eleven articles were identified for N2 LNN comparison. They involved 726 patients in the VATS group and 1132 patients in the open group. The heterogeneity between these studies was acceptable (p = 0.08, I 2 = 41%). Fewer N2 LNs were dissected in the VATS group as compared with the open group (95% CI −1.38 to −0.49, p < 0.0001, Fig. 3a).
Fig. 3.
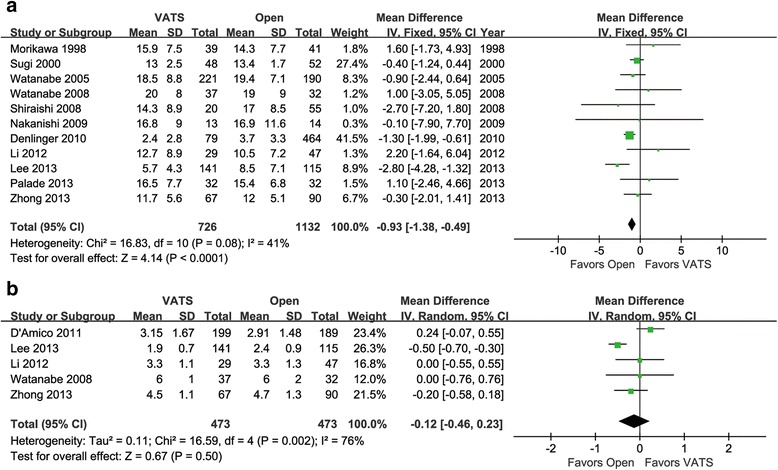
Forest plot of the mean difference in N2 LNN (a) and LNS (b) in the VATS group vs. the open group
Five articles were identified for N2 LNS comparison. They involved 473 patients in the VATS group and 473 patients in the open group. The mean difference in N2 LNS between the two groups was not significant (95% CI −0.46 to 0.23, p = 0.50), with significant heterogeneity across studies (p = 0.002, I 2 = 76%, Fig. 3b).
Comparison of N1 LNN and LNS
Four articles were identified for N1 LNN comparison. They involved 288 patients in the VATS group and 686 patients in the open group. The heterogeneity between these studies was acceptable (p = 0.44, I 2 = 60%). The mean difference in N1 LNN between the two groups was not significant (95% CI −0.71 to 0.08, p = 0.11, Fig. 4).
Fig. 4.
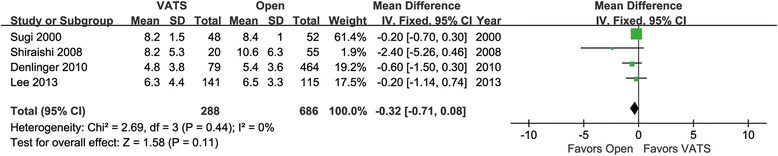
Forest plot of the mean difference in N1 LNN in the VATS group vs. the open group
Only one article was identified for N1 LNS comparison. They involved 141 patients in the VATS group and 115 patients in the open group. The result showed that fewer N1 LN stations were dissected in the VATS group (1.4 ± 0.5 vs. 1.6 ± 0.6, p = 0.04).
Publication bias
The funnel plot for publication bias (standard error by total LNN comparison) demonstrated marked evidence of symmetry (Fig. 5), indicating no publication bias. The combined effect size yielded a Z value of 5.64, with a corresponding p < 0.00001. This result indicates that the fail-safe N value was relevant.
Fig. 5.
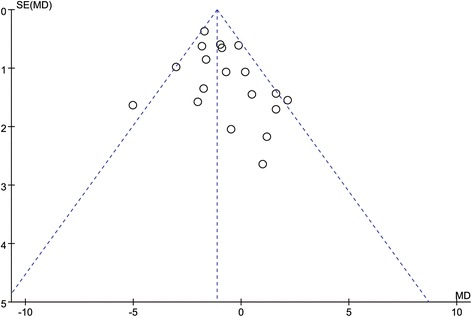
Funnel plot of the mean difference in total LNN in the VATS group vs. the open group
Discussion
The presence or absence of mediastinal LN metastases is a critical component to accurate staging and therefore a key component of the surgical management of NSCLC [10]. Both overall and disease-free survival have been associated with the number of LNs dissected [11, 12]. Lardinois compared LNs dissection versus sampling; the results showed a longer disease-free survival and better local tumor control in dissection groups [13]. Focusing on stage IA lung cancer, Xu reported a similar result and suggested that the number of N2 stations served as a more significant prognostic factor [14]. As a result, current guidelines from the National Comprehensive Cancer Network and European Society of Thoracic Surgeons recommend that all patients with resectable NSCLC should have a complete systematic nodal dissection, with at least three N2 stations dissected [15, 16].
Although VATS is associated with many benefits in comparison to thoracotomy, whether VATS can achieve the same LN dissection efficacy in patients with lung cancer remains controversial [7, 17, 18]. Flores reported that the surgical field in VATS facilitated the dissection of LNs adjacent to the blood vessels and the trachea and found that smaller nodes achieved better LN dissection [19]. Ramos showed that VATS dissected more total LNS (5.1 ± 1.1 vs. 4.5 ± 1.2, p < 0.001) and mediastinal LNS (3.4 ± 0.9 vs. 3.2 ± 0.9, p = 0.022) when compared with thoracotomy [20]. By contrast, Denlinger reported that significantly more overall LNs were dissected in the open group than in the VATS group (8.9 ± 5.2 vs. 7.1 ± 5.2, p = 0.0006) [21]. Similar results were reported by Lee and colleagues in their retrospective study of LN evaluation achieved by VATS lobectomy compared with that by open lobectomy [22]. A secondary analysis including 752 original participants of the American College of Surgeons Oncology Group Z0030 trial compared patients who underwent lobectomy by VATS with patients who underwent thoracotomy. The results showed that there was no significant difference in the overall number of LNs retrieved between the two groups (15 vs. 19, p = 0.147) [23].
The present study included a total of 7568 patients from nine countries, providing the most comprehensive evidence for LN dissection efficacy by VATS to date. The meta-analysis showed that fewer total LNN and similar total LNS were dissected in the VATS group as compared with that in the open group. If we only included studies focusing on clinical stage I lung cancer, the results were similar (total LNN, 95% CI −1.67 to −0.61, p < 0.0001; total LNS, 95% CI −0.62 to 0.02, p = 0.07). It suggests that surgeons may not have the ability to perform systematic lymphadenectomy in VATS or ignore the importance of systematic lymphadenectomy for various reasons (earlier tumor stage, worrying about damage to vital organs, and so on).
In the comparison of N1 and N2 LN dissection, our results showed that similar number of N1 LNN and N2 LNS could be harvested by VATS, while fewer N2 LNN were harvested by VATS as compared with thoracotomy. Only one article reported on N1 LNS comparison between the two groups and showed better efficiency in the open group (1.4 ± 0.5 vs. 1.6 ± 0.6, p = 0.04) [22].
It was controversial that removing more N2 LNs could increase the accuracy of clinical staging of NSCLC. Boffa et al. compared the completeness of surgical LN evaluation during anatomic resection of primary lung cancer by open and VATS approaches in 11,531 patients from The Society of Thoracic Surgeons-General Thoracic Database. The results showed nodal upstaging in 14.3% (1024 patients) of the open group and in 11.6% (508 patients) of the VATS group (p < 0.001). The study suggested that surgeons should be encouraged to apply a systematic approach to hilar and peribronchial LN dissection during VATS lobectomy for lung cancer, particularly as they were adopting this approach [24].
Some surgeons might also worry about the complications caused by the systematic LN dissection. As with open thoracotomy, systematic mediastinal LN dissection under VATS may increase the risk of intraoperative bleeding (bronchial arteries, etc.), tracheobronchial injury, recurrent nerve injury, prolonged air leak, atrial fibrillation, and pulmonary edema [25]. In other papers, Watanabe et al. reported similar mortality and morbidity of mediastinal LN dissection by VATS vs. open lobectomy, indicating that systematic mediastinal LN dissection by VATS is a safe procedure [26]. Zhang et al. compared complications such as chylothorax and nerve injury between VATS and open thoracotomy in a meta-analysis. The results showed that these events were similar in both groups [27].
The possible limitations of our study must be considered when interpreting the findings described herein. First, including only English papers might have resulted in language bias. Second, including 7568 participants from 36 studies with only three RCTs might have weakened the quality of the results. Third, the number of dissected LNs varied significantly between the included studies. Different doctors have different understanding of LN dissection and might be at different stages of the learning curve. Some data did not meet the National Comprehensive Cancer Network guide requirements for lung cancer surgery treatment of systematic LN dissection, which may have affected the reliability of the results. Fourth, there is great potential for LN fragmentation during dissection. Different pathologists and different counting procedures might lead to false LNN counts, which might increase the heterogeneity between studies but would not alter the overall results. Finally, we did not analyze the survival difference between VATS and open thoracotomy. Our analysis compared LN harvest capability between two evaluation procedures only from a surgical point of view and tried to give further proof of satisfied oncologic efficacy by VATS.
Conclusions
Less total and mediastinal LNs were evaluated with VATS than with thoracotomy in the present study. Both approaches harvested a similar number of total LN stations, mediastinal LN stations, and N1 LNs. However, owing to the possible existing bias in the original studies, inter-study heterogeneity, and the inherent limitations of our meta-analysis, the findings require validation in high-quality, large-scale RCTs.
Acknowledgements
We would like to thank all the investigators, surgeons, and patients who participated in this study.
Funding
This study was partially supported by The National Natural Science Foundation of China (grant no. 81160293).
Availability of data and materials
All the data used in the study can be obtained from the original articles.
Authors’ contributions
WXZ and DYL performed the experiment conception and design. WXZ, YPW, and HJ did the literature search. WXZ and JJX performed the data analysis. WXZ and DYL did the paper writing. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All analyses were based on previous published studies; thus, no consent for publication is required.
Ethics approval and consent to participate
All analyses were based on previous published studies; thus, no ethical approval and patient consent are required.
Abbreviations
- CHN
China
- CI
Confidence interval
- CT
Computed tomography
- DNK
Denmark
- GER
Germany
- JPN
Japan
- LN
Lymph node
- LNN
Lymph node number
- LNS
LN station number
- NOS
Newcastle-Ottawa Scale
- NSCLC
Non-small cell lung cancer
- PET
Positron emission tomography
- RCTs
Randomized controlled trials
- RR
Relative risk
- TR
Turkey
- USA
United States of America
- VATS
Video-assisted thoracic surgery
Contributor Information
Wenxiong Zhang, Email: zwx123dr@126.com.
Yiping Wei, Email: weiyiping2015@163.com.
Han Jiang, Email: jhan3939@163.com.
Jianjun Xu, Email: xujianjun3526@163.com.
Dongliang Yu, Phone: 13576133363, Email: yudongliang2015@163.com.
References
- 1.Oda M, Ishikawa N, Tsunezuka Y, et al. Closed three-port anatomic lobectomy with systematic nodal dissection for lung cancer. Surg Endosc. 2007;21(8):1464–5. doi: 10.1007/s00464-006-9074-y. [DOI] [PubMed] [Google Scholar]
- 2.Chen WQ, Zhang SW, Zou XN. Estimation and projection of lung cancer incidence and mortality in China. Chin J Lung Cancer. 2010;13(5):488–93. doi: 10.3779/j.issn.1009-3419.2010.05.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Cunha J, Herndon JE, Herzan DL, et al. Cancer and leukemia group B. Poor correspondence between clinical and pathologic staging in stage 1 non-small cell lung cancer: results from CALGB 9761, a prospective trial. Lung Cancer. 2005;48(2):241–6. doi: 10.1016/j.lungcan.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Stephens N, Rice D, Correa A, et al. Thoracoscopic lobectomy is associated with improved short-term and equivalent oncological outcomes compared with open lobectomy for clinical stage I non-small cell lung cancer: a propensity-matched analysis of 963 cases. Eur J Cardiothorac Surg. 2014;46(4):607–13. doi: 10.1093/ejcts/ezu036. [DOI] [PubMed] [Google Scholar]
- 5.Liu C, Li Z, Bai C, et al. Video-assisted thoracoscopic surgery and thoracotomy during lobectomy for clinical stage I non-small-cell lung cancer have equivalent oncological outcomes: a single-center experience of 212 consecutive resections. Oncol Lett. 2015;9(3):1364–72. doi: 10.3892/ol.2014.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nwogu CE, D’Cunha J, Pang H, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance) Ann Thorac Surg. 2015;99(2):399–405. doi: 10.1016/j.athoracsur.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho S, Do YW, Lee EB. Comparison of costs for video-assisted thoracic surgery lobectomy and open lobectomy for non-small cell lung cancer. Surg Endosc. 2011;25(4):1054–61. doi: 10.1007/s00464-010-1315-4. [DOI] [PubMed] [Google Scholar]
- 8.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. Ottawa: Ottawa Health Research Institute; 2012. [Google Scholar]
- 9.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 10.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 11.Reichert M, Steiner D, Kerber S, et al. A standardized technique of systematic mediastinal lymph node dissection by video-assisted thoracoscopic surgery (VATS) leads to a high rate of nodal upstaging in early-stage non-small cell lung cancer. Surg Endosc. 2016;30(3):1119–25. doi: 10.1007/s00464-015-4312-9. [DOI] [PubMed] [Google Scholar]
- 12.Ou SH, Zell JA. Prognostic significance of the number of lymph nodes removed at lobectomy in stage IA non-small cell lung cancer. J Thorac Oncol. 2008;3(8):880–6. doi: 10.1097/JTO.0b013e31817dfced. [DOI] [PubMed] [Google Scholar]
- 13.Lardinois D, Suter H, Hakki H, et al. Morbidity, survival, and site of recurrence after mediastinal lymph-node dissection versus systematic sampling after complete resection for non-small cell lung cancer. Ann Thorac Surg. 2005;80(1):268–75. doi: 10.1016/j.athoracsur.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Xu F, Qi L, Yue D, Wang C. The effect of the extent of lymph node dissection for stage IA non-small-cell lung cancer on patient disease-free survival. Clin Lung Cancer. 2013;14(2):181–7. doi: 10.1016/j.cllc.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2010;8(7):740–801. doi: 10.6004/jnccn.2010.0056. [DOI] [PubMed] [Google Scholar]
- 16.Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg. 2006;30(5):787–92. doi: 10.1016/j.ejcts.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Sawada S, Komori E, Yamashita M, et al. Comparison in prognosis after VATS lobectomy and open lobectomy for stage I lung cancer: retrospective analysis focused on a histological subgroup. Surg Endosc. 2007;21(9):1607–11. doi: 10.1007/s00464-007-9200-5. [DOI] [PubMed] [Google Scholar]
- 18.Shiraishi T, Shirakusa T, Iwasaki A, et al. Video-assisted thoracoscopic surgery (VATS) segmentectomy for small peripheral lung cancer tumors: intermediate results. Surg Endosc. 2004;18(11):1657–62. doi: 10.1007/s00464-003-9269-4. [DOI] [PubMed] [Google Scholar]
- 19.Flores RM. Video-assisted thoracic surgery (VATS) lobectomy: focus on technique. World J Surg. 2010;34(4):616–20. doi: 10.1007/s00268-009-0340-8. [DOI] [PubMed] [Google Scholar]
- 20.Ramos R, Girard P, Masuet C, Validire P, Gossot D. Mediastinal lymph node dissection in early-stage non-small cell lung cancer: totally thoracoscopic vs thoracotomy. Eur J Cardiothorac Surg. 2012;41(6):1342–8. doi: 10.1093/ejcts/ezr220. [DOI] [PubMed] [Google Scholar]
- 21.Denlinger CE, Fernandez F, Meyers BF, et al. Lymph node evaluation in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Ann Thorac Surg. 2010;89(6):1730–5. doi: 10.1016/j.athoracsur.2010.02.094. [DOI] [PubMed] [Google Scholar]
- 22.Lee PC, Nasar A, Port JL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 2013;96(3):951–61. doi: 10.1016/j.athoracsur.2013.04.104. [DOI] [PubMed] [Google Scholar]
- 23.Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg. 2010;139(4):976–81. doi: 10.1016/j.jtcvs.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 24.Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg. 2012;94(2):347–53. doi: 10.1016/j.athoracsur.2012.04.059. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, D’Amico TA. Efficacy of mediastinal lymph node dissection during thoracoscopic lobectomy. Ann Cardiothorac Surg. 2012;1(1):27–32. doi: 10.3978/j.issn.2225-319X.2012.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe A, Koyanagi T, Ohsawa H, et al. Systematic node dissection by VATS is not inferior to that through an open thoracotomy: a comparative clinicopathologic retrospective study. Surgery. 2005;138:510–7. doi: 10.1016/j.surg.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Feng H, Wang X, et al. Can lymph node evaluation be performed well by video-assisted thoracic surgery? J Cancer Res Clin Oncol. 2015;141(1):143–51. doi: 10.1007/s00432-014-1785-1. [DOI] [PubMed] [Google Scholar]
- 28.Kirby TJ, Mack MJ, Landreneau RJ, et al. Lobectomy video-assisted thoracic surgery versus muscle-sparing thoracotomy. A randomized trial. J Thorac Cardiovasc Surg. 1995;109(5):997–1001. doi: 10.1016/S0022-5223(95)70326-8. [DOI] [PubMed] [Google Scholar]
- 29.Morikawa T, Katoh H, Takeuchi E, et al. Technical feasibility of video-assisted lobectomy with radical lymphadenectomy for primary lung cancer. Surg Laparosc Endosc. 1998;8(6):466–73. doi: 10.1097/00019509-199812000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Luketich JD, Meehan MA, Landreneau RJ, et al. Total videothoracoscopic lobectomy versus open thoracotomy for early-stage non small-cell lung cancer. Clin Lung Cancer. 2000;2(1):56–60. doi: 10.3816/CLC.2000.n.018. [DOI] [PubMed] [Google Scholar]
- 31.Sugi K, Kaneda Y, Esato K. Video-assisted thoracoscopic lobectomy achieves a satisfactory long-term prognosis in patients with clinical stage IA lung cancer. World J Surg. 2000;24(1):27–30. doi: 10.1007/s002689910006. [DOI] [PubMed] [Google Scholar]
- 32.Nomori H, Horio H, Naruke T, et al. What is the advantage of a thoracoscopic lobectomy over a limited thoracotomy procedure for lung cancer surgery? Ann Thorac Surg. 2001;72(3):879–84. doi: 10.1016/S0003-4975(01)02891-0. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe A, Koyanagi T, Obama T, et al. Assessment of node dissection for clinical stage I primary lung cancer by VATS. Eur J Cardiothorac Surg. 2005;27(5):745–52. doi: 10.1016/j.ejcts.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Petersen RP, Pham D, Toloza EM, et al. Thoracoscopic lobectomy: a safe and effective strategy for patients receiving induction therapy for non-small cell lung cancer. Ann Thorac Surg. 2006;82(1):214–8. doi: 10.1016/j.athoracsur.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 35.Shigemura N, Akashi A, Funaki S, et al. Long-term outcomes after a variety of video-assisted thoracoscopic lobectomy approaches for clinical stage IA lung cancer: a multi-institutional study. J Thorac Cardiovasc Surg. 2016;132(3):507–12. doi: 10.1016/j.jtcvs.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 36.Shiraishi T, Hiratsuka M, Yoshinaga Y, et al. Thoracoscopic lobectomy with systemic lymph node dissection for lymph node positive non-small cell lung cancer—is thoracoscopic lymph node dissection feasible? Thorac Cardiovasc Surg. 2008;56(3):162–6. doi: 10.1055/s-2007-989368. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe A, Mishina T, Ohori S, et al. Is video-assisted thoracoscopic surgery a feasible approach for clinical N0 and postoperatively pathological N2 non-small cell lung cancer? Eur J Cardiothorac Surg. 2008;33(5):812–8. doi: 10.1016/j.ejcts.2008.01.064. [DOI] [PubMed] [Google Scholar]
- 38.Whitson BA, D’Cunha J, Andrade RS, et al. Thoracoscopic versus thoracotomy approaches to lobectomy: differential impairment of cellular immunity. Ann Thorac Surg. 2008;86(6):1735–44. doi: 10.1016/j.athoracsur.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Nakanishi R, Oka S, Odate S. Video-assisted thoracic surgery major pulmonary resection requiring control of the main pulmonary artery. Interact Cardiovasc Thorac Surg. 2009;9(4):618–22. doi: 10.1510/icvts.2009.210310. [DOI] [PubMed] [Google Scholar]
- 40.Okur E, Baysungur V, Tezel Ç, et al. Comparison of perioperative results of conventional versus thoracoscopic lobectomy for clinical stage I lung carcinoma. Turkish J Thorac Cardiovasc Surg. 2009;17(3):191–7. [Google Scholar]
- 41.D’Amico TA, Niland J, Mamet R, et al. Efficacy of mediastinal lymph node dissection during lobectomy for lung cancer by thoracoscopy and thoracotomy. Ann Thorac Surg. 2010;92:226–32. doi: 10.1016/j.athoracsur.2011.03.134. [DOI] [PubMed] [Google Scholar]
- 42.Bu L, Li Y, Yang F, et al. Completely video-assisted thoracoscopic lobectomy versus open lobectomy for non-small cell lung cancer greater than 5 cm: a retrospective study. Chin Med J (Engl) 2012;125(3):434–9. [PubMed] [Google Scholar]
- 43.Li Y, Wang J. Comparison of clinical outcomes for patients with clinical N0 and pathologic N2 non-small cell lung cancer after thoracoscopic lobectomy and open lobectomy: a retrospective analysis of 76 patients. J Surg Oncol. 2012;106(4):431–5. doi: 10.1002/jso.23104. [DOI] [PubMed] [Google Scholar]
- 44.Licht PB, Jorgensen OD, Ladegaard L, et al. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg. 2013;96(3):943–9. doi: 10.1016/j.athoracsur.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Fan XL, Liu YX, Tian H. Video-assisted thoracoscopic surgery for treatment of early- stage non-small cell lung cancer. Asian Pac J Cancer Prev. 2013;14(5):2871–7. doi: 10.7314/APJCP.2013.14.5.2871. [DOI] [PubMed] [Google Scholar]
- 46.Palade E, Passlick B, Osei-Agyemang T, et al. Video-assisted vs open mediastinal lymphadenectomy for stage I non-small-cell lung cancer: results of a prospective randomized trial. Eur J Cardiothorac Surg. 2013;44(2):244–9. doi: 10.1093/ejcts/ezs668. [DOI] [PubMed] [Google Scholar]
- 47.Zhong C, Yao F, Zhao H. Clinical outcomes of thoracoscopic lobectomy for patients with clinical N0 and pathologic N2 non-small cell lung cancer. Ann Thorac Surg. 2013;95(3):987–92. doi: 10.1016/j.athoracsur.2012.10.083. [DOI] [PubMed] [Google Scholar]
- 48.Li C, Ma H, He J, et al. Video-assisted thoracoscopic lobectomy with a single utility port is feasible in the treatment of elderly patients with peripheral lung cancer. Thoracic Cancer. 2014;5:219–24. doi: 10.1111/1759-7714.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai HB, Li YX, Li Z. Short term curative effect of video assisted thoracoscopic lobectomy for early-stage lung cancer. Indian J Cancer. 2015;51(2):37–41. doi: 10.4103/0019-509X.151993. [DOI] [PubMed] [Google Scholar]
- 50.Kuritzky AM, Aswad BI, Jones RN, et al. Lobectomy by video-assisted thoracic surgery vs muscle-sparing thoracotomy for stage I lung cancer: a critical evaluation of short- and long-term outcomes. J Am Coll Surg. 2015;220(6):1044–53. doi: 10.1016/j.jamcollsurg.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 51.Murakawa T, Ichinose J, Hino H, et al. Long-term outcomes of open and video-assisted thoracoscopic lung lobectomy for the treatment of early stage non-small cell lung cancer are similar: a propensity-matched study. World J Surg. 2015;39(5):1084–91. doi: 10.1007/s00268-014-2918-z. [DOI] [PubMed] [Google Scholar]
- 52.Zhang S, Pan SB, Lyu QH, et al. Postoperative regulatory T-cells and natural killer cells in stage I nonsmall cell lung cancer underwent video-assisted thoracoscopic lobectomy or thoracotomy. Chin Med J (Engl) 2015;128(11):1502–9. doi: 10.4103/0366-6999.157672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data used in the study can be obtained from the original articles.


