Abstract
The use of sp3 C–H bonds—which are ubiquitous in organic molecules—as latent nucleophile equivalents for transition metal–catalyzed cross-coupling reactions has the potential to substantially streamline synthetic efforts in organic chemistry while bypassing substrate activation steps. Through the combination of photoredox-mediated hydrogen atom transfer (HAT) and nickel catalysis, we have developed a highly selective and general C–H arylation protocol that activates a wide array of C–H bonds as native functional handles for cross-coupling. This mild approach takes advantage of a tunable HAT catalyst that exhibits predictable reactivity patterns based on enthalpic and bond polarity considerations to selectively functionalize a-amino and a-oxy sp3 C–H bonds in both cyclic and acyclic systems.
Over the past 50 years, transition metal–catalyzed cross-coupling reactions have transformed the field of synthetic organic chemistry via the evolution of a wide variety of C–C and C–heteroatom bond-forming reactions (1, 2). During this time, the seminal studies of Negishi, Suzuki, Miyaura, Stille, Kumada, and Hiyama have inspired numerous protocols to construct carbon–carbon bonds using palladium, nickel, or iron catalysis. These strategies enable highly efficient and regiospecific fragment couplings with high functional group tolerance, facilitating the application of modular building blocks in early- or late-stage synthetic efforts. Traditionally, cross-coupling methods have relied on the use of organometallic nucleophiles such as aryl or vinyl boronic acids, zinc halides, stannanes, or Grignard reagents that undergo addition to a corresponding metal-activated aryl or vinyl halide.
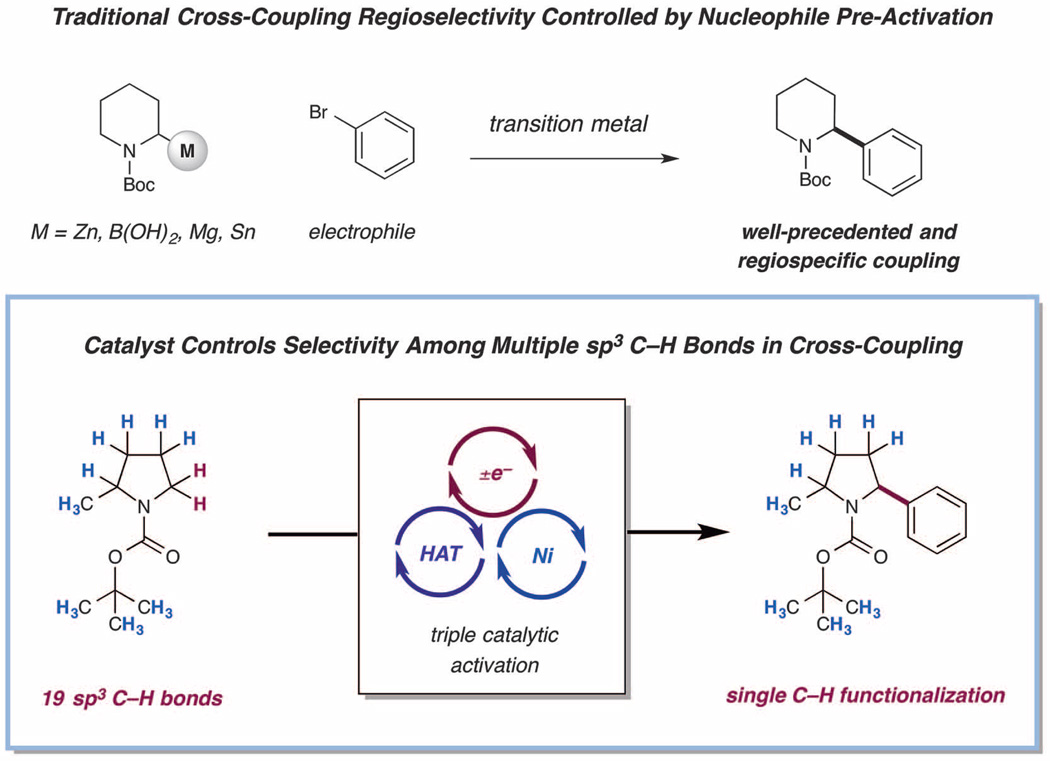
An emerging strategy for C–C bond formation has been the application of native organic functionality as latent nucleophilic handles for transition metal–mediated cross-couplings. In this context, the use of olefin, methoxy, acetoxy, and carboxylic acid moieties as organometallic replacements has enabled a variety of carbon–carbon bond formation protocols using feedstock materials (3–8). However, the most common approach for transition metal–mediated native functionalization has been the use of C–H bonds—the most ubiquitous chemical bonds found in nature—as nucleophilic coupling partners. Among the well-established challenges with sp3 C–H bond functionalization, regioselectivity is perhaps preeminent, given that organic molecules incorporate a diverse combination of methyl, methylene, and/or methine groups. Several elegant methodologies have navigated this question via the use of directing groups to accomplish selective sp3 C–H bond functionalization (9–13), or more recently by focusing on the use of inductive effects to deactivate C–H bonds (14). Enzymes accomplish selective sp3 C–H bond functionalization by taking advantage of the diverse electronic and enthalpic characteristics of carbon–hydrogen bonds found within complex organic molecules (15). Inspired by this biochemical blueprint, we speculated that a small-molecule catalyst platform could be developed that would differentiate between a diverse range of C–H groups using a combination of bond energies and polarization, thereby enabling a unique pathway toward native arylation or vinylation.
A fundamental mechanistic step in organic synthesis is the simultaneous movement of a proton and an electron—a process termed hydrogen atom transfer (HAT) (16, 17). HAT has long served as an effective way to access radical intermediates in organic chemistry; however, the capacity to regioselectively abstract hydrogens among a multitude of diverse C–H locations has been notoriously difficult to control. Recently, driven by developments in small-molecule catalyst design, general methods for C–H bond functionalization via HAT have begun to achieve levels of selectivity that were previously restricted to enzymatic systems (18, 19). In this context, our laboratory has demonstrated that photoredox-mediated HAT catalysis can exploit native sp3 C–H bonds for a range of C–C bond constructions, such as Minisci reactions, conjugate additions, and radical–radical couplings (20–23). Nevertheless, a general strategy for functionalization of C–H bonds via HAT–transition metal cross-coupling has yet to be achieved (24, 25).
We recently questioned whether it would be possible to use a tertiary amine radical cation—generated via a photoredox-mediated single-electron transfer (SET) event (23, 26–28)—to accomplish H-atom abstraction from a diverse range of substrates (Fig. 1). Given the electrophilic nature of amine radical cations, we proposed that such a catalytic strategy might allow the selective abstraction of hydridic, electron-rich C–H bonds in the presence of electron-deficient and neutral C–H bonds, which are abundant throughout organic molecules. We envisioned that the exploitation of polarity effects in the abstraction event would impart a high degree of kinetic selectivity into an otherwise unselective HAT process (29). Thereafter, we assumed the resulting radical intermediate might readily intersect with a Ni-catalyzed coupling cycle, there-by enabling C–C bond formation with a range of aryl electrophiles.
Fig. 1. Photoredox-mediated hydrogen atom transfer and nickel catalysis enables highly selective cross-coupling with sp3 C–H bonds as latent nucleophiles.
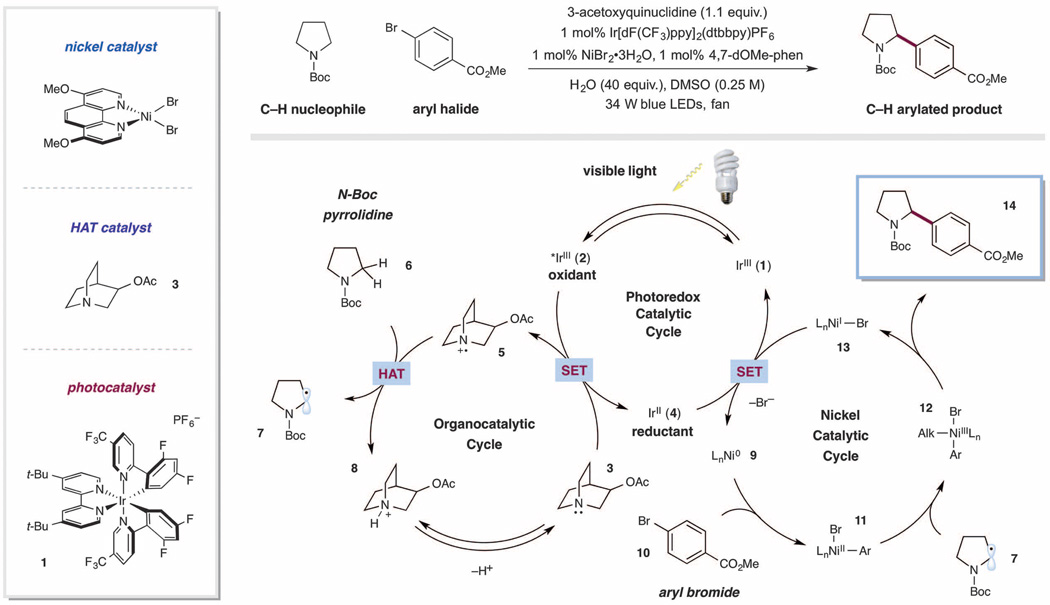
A detailed description of our proposed mechanistic cycle for the sp3 C–H cross-coupling via photoredox HAT-nickel catalysis is outlined in Fig. 2. Initial excitation of the iridium(III) photo-catalyst Ir[dF(CF3)ppy]2(dtbbpy)PF6 [dF(CF3)ppy = 2-(2,4-difluorophenyl)-5-(trifluoromethyl)pyridine; dtbbpy = 4,4'-di-tert-butyl-2,2'-bipyridine] (1) would produce the long-lived photoexcited state 2 (τ= 2.3 µs) (30). The *Ir(III) catalyst 2 is sufficiently oxidizing to undergo SET with a tertiary amine HAT catalyst (such as 3), to generate Ir(II) 4 and amine radical cation 5 [E1/2red (*IrIII/IrII) = +1.21V versus saturated calomel electrode (SCE) in CH3CN; Ep (3-acetoxyquinuclidine) = +1.22 V versus SCE in CH3CN] (30). As a central design element, we postulated that amine radical cation 5 would be sufficiently electron-deficient to engender a kinetically selective HAT event at the most electronrich site of C–H nucleophile substrate 6, thereby exclusively delivering radical intermediate 7. At the same time, we hypothesized that this abstraction event should also be thermodynamically favorable considering the difference in the bond dissociation enthalpies (BDEs) of hydridic α-amino C–H bonds (α-amino C–H = 89 to 94 kcal/mol) and the resultant N–H bond of quinuclidinium cation [H–N+ BDE (quinuclidine) = 100 kcal/mol] (31, 32). Concurrent with this photoredox cycle, we assumed that our active Ni(0) species 9—generated in situ via two SET reductions of (4, 7-dOMe-phen)Ni(II)Br2 (4,7-dOMe-phen = 4,7-dimethoxy-1,10-phenanthroline) by the iridium photocatalyst [E1/2red (IrIII/IrII) = −1.37 V versus SCE in CH3CN; E1/2red (NiII/Ni0) = −1.2 V versus SCE in N,N-dimethylformamide] (30, 33)—would undergo oxidative addition into the aryl halide electrophile 10, forming the electrophilic Ni(II)-aryl intermediate 11. This Ni(II) species would rapidly intercept radical 7 to generate a Ni(III)-aryl-alkyl complex 12, which upon reductive elimination would forge the desired C–C bond to form Ni(I) complex 13 and benzylic amine 14. Reduction of 13 by 4, the Ir(II) state of the photocatalyst, would then reconstitute both Ni(0) catalyst 9 and Ir(III) catalyst 1.
Fig. 2. Photoredox, HAT, and nickel-catalyzed cross-coupling: proposed mechanistic pathway and catalyst combination.
Ac, acetyl; t-Bu, tert-butyl; Boc, tert-butoxycarbonyl; DMSO, dimethyl sulfoxide; LED, light-emitting diode; SET, single-electron transfer; HAT, hydrogen atom transfer.
We began our investigations into the proposed photoredox-mediated HAT nickel cross-coupling by evaluating a broad range of photoredox catalysts, nickel-ligand systems, and quinuclidine analogs. Upon exposing N-Boc pyrrolidine and methyl 4-bromobenzoate to visible light [34 W blue light-emitting diodes (LEDs)] in the presence of iridium photocatalyst Ir[dF(CF3)ppy]2(dtbbpy) PF6, NiBr2•3H2O, 4,7-dimethoxy-1,10-phenanthroline, and 3-acetoxyquinuclidine, we observed 81% yield of the desired α-amino C–C coupled product. Moreover, this product was the only detectable regioisomer formed, indicating that quinuclidine HAT catalyst 3 was selective for themost hydridic C–H bond available. Notably, using quinuclidine in lieu of 3-acetoxyquinuclidine resulted in diminished reactivity, indicating the necessity for an electron-withdrawing substituent. This substantial difference in reaction efficiency illustrates the capacity to tune the reactivity of the HAT catalyst via electronic modification of the substituent at the 3-position. It is important to note that under these reaction conditions, amine 3 serves as both the HAT catalyst and the base (34).
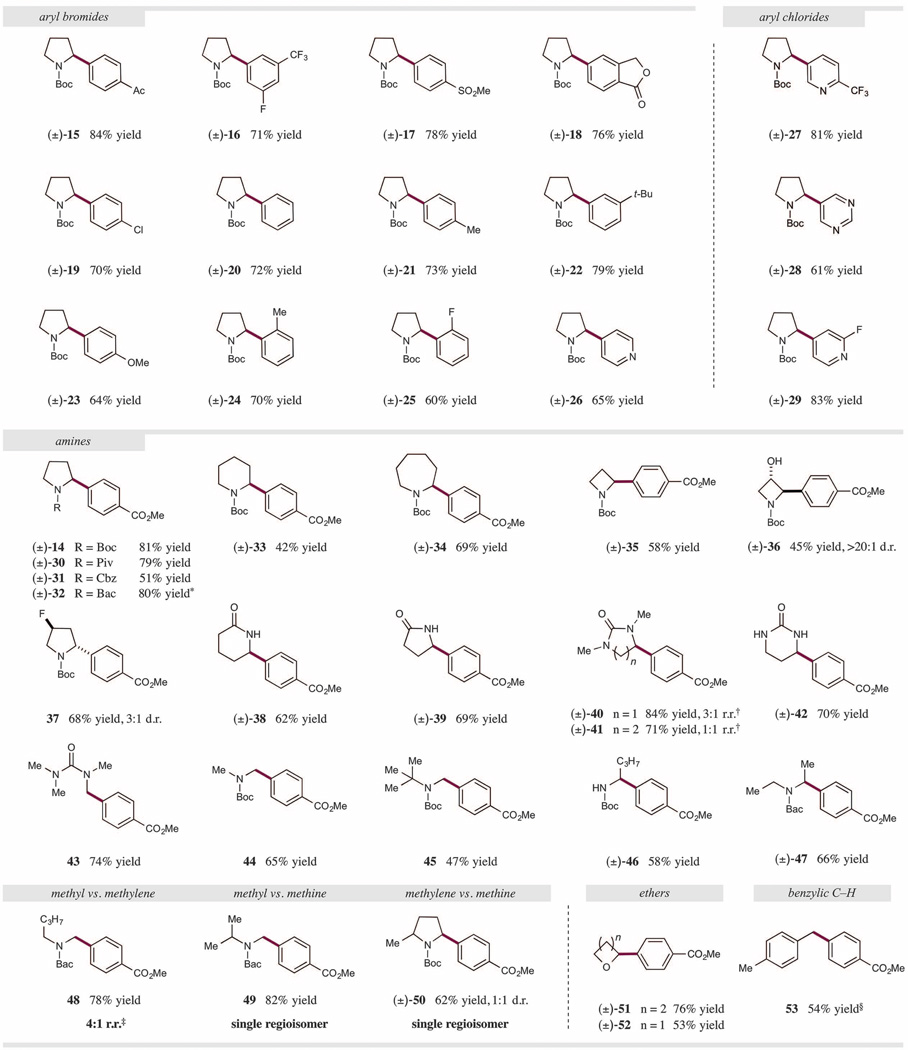
With the optimal conditions in hand, we next sought to examine the generality of this transformation by exploring the scope of the electrophilic aryl halide coupling partner. As outlined in Fig. 3, a wide variety of bromoarenes function efficiently in this HAT cross-coupling protocol. For example, electron-deficient aryl bromides containing ketones, trifluoromethyl groups, fluorines, sulfones, and esters were all effective arylating agents (15 to 18, 71 to 84% yield).Notably, 4-chlorobromobenzene gave chlorophenyl amine product 19 as the only observable arylation product in 70% yield, demonstrating that a high degree of chemoselectivity can be achieved in the oxidative addition step. The HAT arylation strategy is further effective for electron-neutral and electron-rich aryl bromides, as demonstrated by the installation of phenyl, tolyl, t-Bu-phenyl, and anisole groups (20 to 23, 64 to 79% yield). The presence of ortho methyl or fluorine substitution on the aryl halide was not problematic (24 and 25, 70 and 60% yield). With respect to heteroaromatic systems, pyridine rings were incorporated with good efficiency via the use of the corresponding heteroaryl bromide (26, 65% yield). Heteroaryl chlorides were also effective electrophiles in the transformation. For example, electron-deficient pyridines and pyrimidines deliver the benzylic amine products in good efficiency (27 to 29, 61 to 83% yield) (35). The collective one-step synthesis of the aryl pyrrolidine products 14 to 29 from simple N-Boc pyrrolidine clearly demonstrates that synthetic streamlining can be accomplished with this HAT cross-coupling technology (36).
Fig. 3. Photoredox, HAT, and nickel-catalyzed cross-coupling: aryl halide and C–H nucleophile scope.
All yields are isolated yields. Reaction conditions as in Fig. 2; see supplementary materials for experimental details. Ac, acetyl; t-Bu, tert-butyl; Boc, tert-butoxycarbonyl; Piv, pivalate; Cbz, benzyloxycarbonyl; Bac, tert-butylaminocarbonyl. *Reaction performed with 4-bromobenzotrifluoride to deliver N-Bac 2-(4-trifluoromethylphenyl)-pyrrolidine.†Minor regioisomer is arylated on Me position.‡Minor regioisomer is arylated on α-amino methylene position. §Yield determined by 1H–nuclear magnetic resonance.
We next explored the diversity of amino- and oxy-bearing C–H nucleophiles that could be used as substrates in this photoredox-mediated HAT nickel-catalyzed cross-coupling. As demonstrated in Fig. 3, many α-amino methyl- and methylene-containing substrates can be selectively arylated. For example, differentially N-substituted pyrrolidine substrates are effective in the transformation, including those bearing tert-butoxycarbonyl (Boc), benzyloxycarbonyl (Cbz), pivalate (Piv), and tert-butylaminocarbonyl (Bac) groups (14, 30 to 32, 51 to 81% yield). Notably, the arylation of N-Boc pyrrolidine can be achieved on gram scale in a single batch, delivering 1.34 g of the 2-arylpyrrolidine product 14 (78% yield). Cyclic amines of various ring size are readily tolerated, with azetidine, piperidine, and azepane undergoing selective C–H arylation (33 to 35, 42 to 69% yield). Notably, ring systems that incorporate inductively with-drawing alcohols and fluorine substituents at the β-amino position do not unduly retard the C–H abstraction step [36 and 37, 45%yield, >20:1 diastereomeric ratio (d.r.). and 68% yield, 3:1 d.r.]. Moreover, lactams and ureas proved effective latent nucleophiles for this coupling, with both N-Me and N-H substrates providing the corresponding arylated products in good yield (38 to 42, 62 to 84% yield).
The transformation is not restricted to cyclic substrates, as a range of acyclic amines have been efficiently functionalized with this HAT arylation protocol. For example, primary α-amino C–H bonds in both N-Boc alkyl amines and ureas can be arylated in good yield (43 to 45, 47 to 74% yield). N-Boc butylamine, possessing a free N–H bond, undergoes selective α-arylation in 58% yield (46), leaving this latent functional handle available for further derivatization without the need for protection or deprotection steps. For acyclic dialkyl amines containing methyl and methylene C–H bonds,N-Bac–substituted amines delivered the α-arylated products in excellent yield (47 to 49, 66 to 82% yield), whereas the corresponding Boc systems provided diminished yet usable efficiencies (20 to 30% yield). We attribute this interesting reactivity difference to the diminished electron-withdrawing nature of the Bac group in comparison to Boc, resulting in an increased rate of hydrogen atom transfer to the electrophilic amine radical cation 5.
When unsymmetrical amine substrates were exposed to this HAT protocol, some interesting regioselectivity patterns were discovered. For example, methyl C–H bonds undergo preferential coupling over methylene C–H bonds, as shown with N-Bac butylmethylamine [48, 78% yield, 4:1 regioisomeric ratio (r.r.)]. Furthermore, methyl and methylene C–H bonds react exclusively over methine C–H bonds, as demonstrated with N-Bac isopropylmethylamine and N-Boc 2-methylpyrrolidine, respectively (49 and 50, 82 and 62%yield, 1:1 d.r.). This strategy can also be applied to the HAT arylation of α-oxy C–H bonds. Tetrahydrofuran (THF) and oxetane both undergo α-oxy arylation in good efficiency (51 and 52, 76 and 53% yield). Finally, we have demonstrated that this C–H arylation protocol is effective for benzylic systems as para-xylene is arylated in 54% yield (53). Indeed, we expect that application of this strategy to a broad range of α-oxy, α-amino, and benzylic C–H–bearing substrates will demonstrate the general utility of this selective C–H arylation protocol.
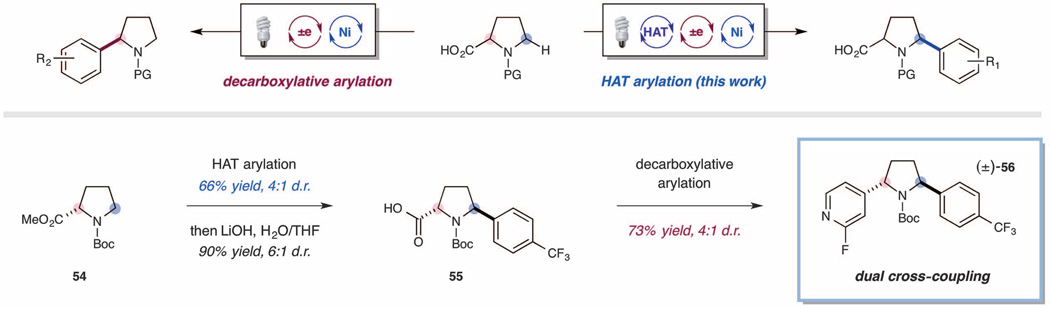
Finally, the capacity to control the regioselectivity of the outlined HAT abstraction along with the opportunity to utilize C–H bonds as latent nucleophiles brings forward the possibility of enabling multiple native functionalizations to be conducted in sequence—a strategy that should allow the rapid construction of molecular complexity from a large variety of readily available organic feedstock chemicals. As one example, we postulated that N-Boc proline methyl ester (54) might be differentially arylated via (i) the photoredox-mediated HAT method presented in this work, followed by (ii) a photoredox-mediated Ni(II) decarboxylative arylation. As shown in Fig. 4, N-Boc proline methyl ester underwent selective arylation at the 5-methylene position using the HAT cross-coupling strategy described herein (66% yield, 4:1 d.r.). The observed regioselectivity is usefully complementary to that which would be expected with established methods for transition metal–catalyzed cross-coupling. Whereas many current strategies use basic conditions to selectively functionalize acidic hydrogens (as in enolate arylations), our developed HAT protocol targets hydridic hydrogen atoms, thereby providing access to fundamentally distinct product classes. Following the successful application of the C–H arylation outlined herein, the corresponding amino acid product 55 underwent decarboxylative coupling with 2-fluoro-4-bromopyridine at the 2-position, delivering the 2,5-diarylated pyrrolidine adduct in excellent yield (56, 73% yield, 4:1 d.r.).We have also demonstrated a HAT arylation followed by a nickel-catalyzed C–O coupling (37). N-Boc 3-hydroxyazetidine can be selectively arylated at the 2-position in 45% yield (36, Fig. 3), leaving the alcohol unreacted. The free alcohol can then be subsequently arylated with 4-bromo-2-methylpyridine to deliver the aryl ether product in 77% yield (see supplementary materials).
Fig. 4. Regioselective arylation: Using C–H arylation and decarboxylative arylation delivers differentially arylated pyrrolidine products.
All yields are isolated yields. See supplementary materials for experimental details.
This HAT strategy represents a powerful demonstration of the versatility of using sp3 C–H bonds as organometallic nucleophile equivalents and will likely find application in the realm of late-stage functionalization. We believe that this protocol will gain widespread use within the synthetic community as a complement to existing cross-coupling technologies.
Supplementary Material
Acknowledgments
We are grateful for financial support provided by the NIH National Institute of General Medical Sciences (R01 GM078201-05) and gifts from Merck and AbbVie. V.W.S. and J.A.T. thank Bristol-Myers Squibb for graduate fellowships. J.D.C. thanks Marie Curie Actions for an International Outgoing Fellowship.
Footnotes
http://www.sciencemag.org/content/352/6291/1304/suppl/DC1
Tables S1 and S2
NMR Spectra
References (38–50)
REFERENCES AND NOTES
- 1.Beller M, Bolm C. Transition Metals for Organic Synthesis. Vol. 1. Weinheim: Wiley-VCH; 2004. [Google Scholar]
- 2.De Meijere A, Diederich F. Metal-Catalyzed Cross-Coupling Reactions. Vol. 1. Weinheim: Wiley-VCH; 2004. [Google Scholar]
- 3.de Meijere A, Meyer FE. Angew. Chem. Int. Ed. Engl. 1995;33:2379–2411. [Google Scholar]
- 4.Gooßen LJ, Deng G, Levy LM. Science. 2006;313:662–664. doi: 10.1126/science.1128684. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez N, Goossen LJ. Chem. Soc. Rev. 2011;40:5030–5048. doi: 10.1039/c1cs15093f. [DOI] [PubMed] [Google Scholar]
- 6.Anka-Lufford LL, Prinsell MR, Weix DJ, Org J. Chem. 2012;77:9989–10000. doi: 10.1021/jo302086g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo JC, Gui J, Yabe Y, Pan C-M, Baran PS. Nature. 2014;516:343–348. doi: 10.1038/nature14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuo Z, et al. Science. 2014;345:437–440. doi: 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaitsev VG, Shabashov D, Daugulis O. J. Am. Chem. Soc. 2005;127:13154–13155. doi: 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]
- 10.Pastine SJ, Gribkov DV, Sames D. J. Am. Chem. Soc. 2006;128:14220–14221. doi: 10.1021/ja064481j. [DOI] [PubMed] [Google Scholar]
- 11.He J, et al. Science. 2014;343:1216–1220. doi: 10.1126/science.1249198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang F-L, Hong K, Li T-J, Park H, Yu J-Q. Science. 2016;351:252–256. doi: 10.1126/science.aad7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topczewski JJ, Cabrera PJ, Saper NI, Sanford MS. Nature. 2016;531:220–224. doi: 10.1038/nature16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee M, Sanford MS. J. Am. Chem. Soc. 2015;137:12796–12799. doi: 10.1021/jacs.5b09099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis JC, Coelho PS, Arnold FH. Chem. Soc. Rev. 2011;40:2003–2021. doi: 10.1039/c0cs00067a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warren JJ, Tronic TA, Mayer JM. Chem. Rev. 2010;110:6961–7001. doi: 10.1021/cr100085k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer JM. Acc. Chem. Res. 2011;44:36–46. doi: 10.1021/ar100093z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen MS, White MC. Science. 2010;327:566–571. doi: 10.1126/science.1183602. [DOI] [PubMed] [Google Scholar]
- 19.Newhouse T, Baran PS. Angew. Chem. Int. Ed. 2011;50:3362–3374. doi: 10.1002/anie.201006368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qvortrup K, Rankic DA, MacMillan DWC. J. Am. Chem. Soc. 2014;136:626–629. doi: 10.1021/ja411596q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuthbertson JD, MacMillan DWC. Nature. 2015;519:74–77. doi: 10.1038/nature14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin J, MacMillan DWC. Nature. 2015;525:87–90. doi: 10.1038/nature14885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeffrey JL, Terrett JA, MacMillan DWC. Science. 2015;349:1532–1536. doi: 10.1126/science.aac8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu D, Liu C, Li H, Lei A. Angew. Chem. Int. Ed. 2013;52:4453–4456. doi: 10.1002/anie.201300459. [DOI] [PubMed] [Google Scholar]
- 25.Liu D, et al. Org. Lett. 2015;17:998–1001. doi: 10.1021/acs.orglett.5b00104. [DOI] [PubMed] [Google Scholar]
- 26.Narayanam JMR, Stephenson CRJ. Chem. Soc. Rev. 2011;40:102–113. doi: 10.1039/b913880n. [DOI] [PubMed] [Google Scholar]
- 27.Prier CK, Rankic DA, MacMillan DWC. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schultz DM, Yoon TP. Science. 2014;343:1239176. doi: 10.1126/science.1239176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts BP. Chem. Soc. Rev. 1999;28:25–35. [Google Scholar]
- 30.Lowry MS, et al. Chem. Mater. 2005;17:5712–5719. [Google Scholar]
- 31.Luo Y-R. Handbook of Bond Dissociation Energies in Organic Compounds. Boca Raton, FL: CRC Press; 2003. [Google Scholar]
- 32.Liu W-Z, Bordwell FG. J. Org. Chem. 1996;61:4778–4783. doi: 10.1021/jo950933r. [DOI] [PubMed] [Google Scholar]
- 33.Durandetti M, Devaud M, Perichon J. New J. Chem. 1996;20:659. [Google Scholar]
- 34.In related studies, we have demonstrated that HAT processes can be achieved with catalytic quantities of 3-acetoxyquinuclidine in the presence of base.
- 35.For heteroaryl halides, no product was observed in the absence of nickel catalyst, supporting the cross-coupling mechanism outlined in Fig. 2. See supplementary materials for control reactions
- 36.Campos KR, Klapars A, Waldman JH, Dormer PG, Chen CY. J. Am. Chem. Soc. 2006;128:3538–3539. doi: 10.1021/ja0605265. [DOI] [PubMed] [Google Scholar]
- 37.Terrett JA, Cuthbertson JD, Shurtleff VW, MacMillan DWC. Nature. 2015;524:330–334. doi: 10.1038/nature14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.