Abstract
In prior studies we identified a 57-kDa protein in the lumen of the endoplasmic reticulum that, in addition to having both protein disulfide isomerase and thyroid hormone-binding protein activities, bound a photoaffinity probe containing the N-glycosylation-site sequence Asn-Xaa-Ser/Thr. It was hypothesized that this multifunctional protein, called glycosylation site-binding protein (GSBP), participated in the process of N-glycosylation of proteins. To test this hypothesis we have employed various conditions to deplete the lumen of GSBP and then assess the level of N-glycosylation catalyzed by oligosaccharyltransferase (OTase). Although most conditions leading to depletion resulted in partial loss of OTase activity, this loss was independent of the extent of GSBP depletion. Indeed, virtually complete loss (greater than 99%) of GSBP with partial retention of OTase activity was frequently observed. Moreover, repletion of the microsomal lumen with GSBP did not restore OTase activity to control levels. Thus, no correlation between GSBP content and OTase activity before or after reconstitution was found. These results suggest that this multifunctional 57-kDa protein is not an essential component of the enzymatic reaction in which oligosaccharide chains are transferred from dolichyl-P-P-GlcNAc2Man9Glc3 to nascent polypeptides or to synthetic tripeptide acceptors.
Full text
PDF

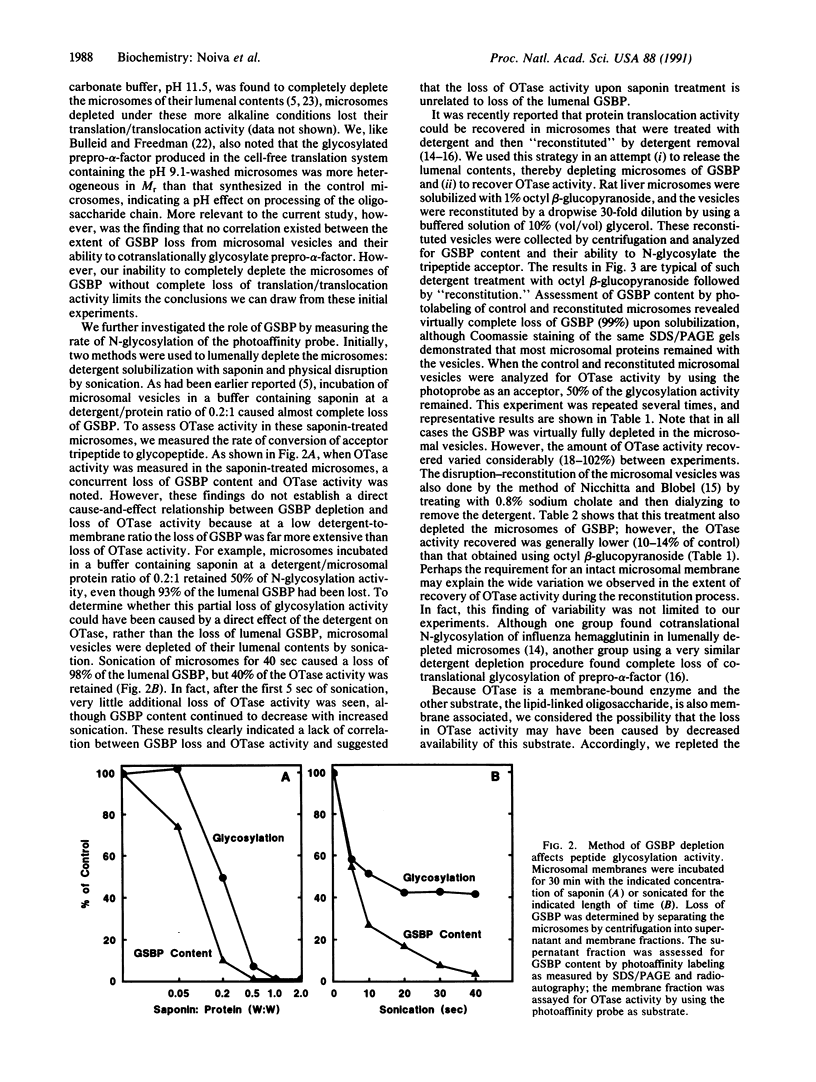

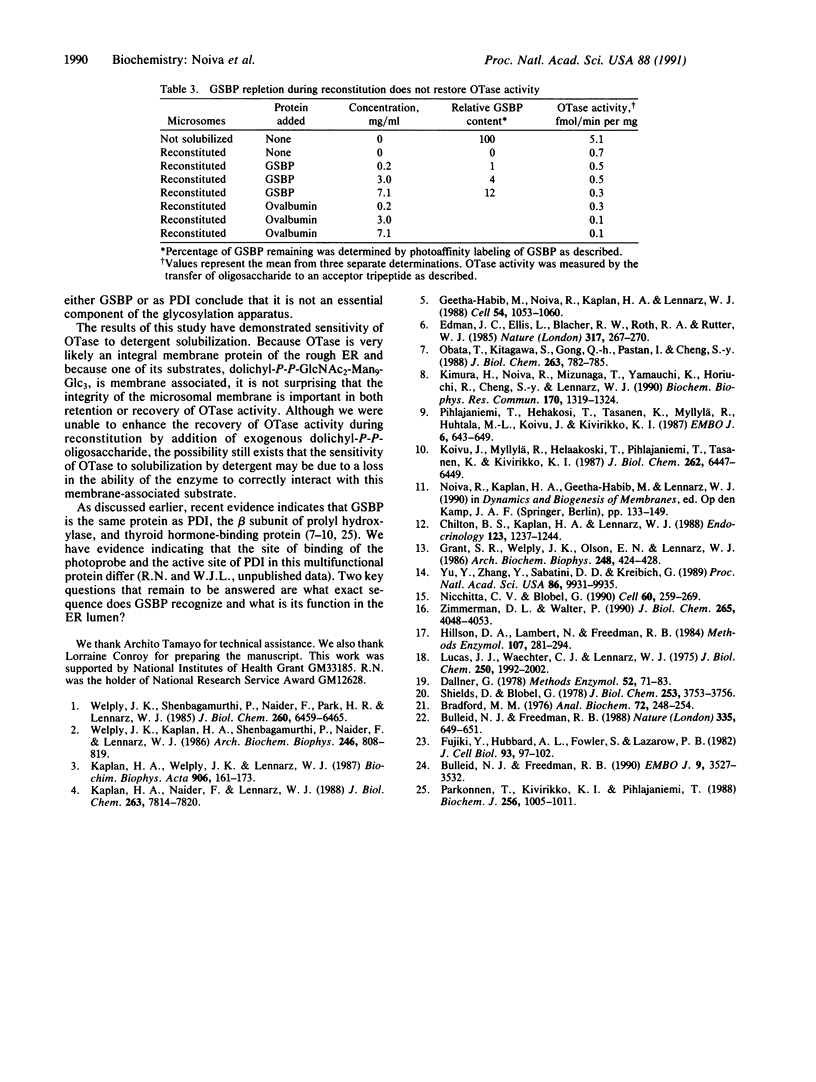
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bulleid N. J., Freedman R. B. Cotranslational glycosylation of proteins in systems depleted of protein disulphide isomerase. EMBO J. 1990 Nov;9(11):3527–3532. doi: 10.1002/j.1460-2075.1990.tb07561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulleid N. J., Freedman R. B. Defective co-translational formation of disulphide bonds in protein disulphide-isomerase-deficient microsomes. Nature. 1988 Oct 13;335(6191):649–651. doi: 10.1038/335649a0. [DOI] [PubMed] [Google Scholar]
- Chilton B. S., Kaplan H. A., Lennarz W. J. Estrogen regulation of the central enzymes involved in O- and N-linked glycoprotein assembly in the developing and the adult rabbit endocervix. Endocrinology. 1988 Sep;123(3):1237–1244. doi: 10.1210/endo-123-3-1237. [DOI] [PubMed] [Google Scholar]
- Dallner G. Isolation of microsomal subfractions by use of density gradients. Methods Enzymol. 1978;52:71–82. doi: 10.1016/s0076-6879(78)52007-7. [DOI] [PubMed] [Google Scholar]
- Edman J. C., Ellis L., Blacher R. W., Roth R. A., Rutter W. J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature. 1985 Sep 19;317(6034):267–270. doi: 10.1038/317267a0. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha-Habib M., Noiva R., Kaplan H. A., Lennarz W. J. Glycosylation site binding protein, a component of oligosaccharyl transferase, is highly similar to three other 57 kd luminal proteins of the ER. Cell. 1988 Sep 23;54(7):1053–1060. doi: 10.1016/0092-8674(88)90120-1. [DOI] [PubMed] [Google Scholar]
- Grant S. R., Welply J. K., Olson E. N., Lennarz W. J. Oligosaccharyltransferase activity is markedly increased during differentiation of a nonfusing myoblast cell line. Arch Biochem Biophys. 1986 Jul;248(1):424–428. doi: 10.1016/0003-9861(86)90439-x. [DOI] [PubMed] [Google Scholar]
- Hillson D. A., Lambert N., Freedman R. B. Formation and isomerization of disulfide bonds in proteins: protein disulfide-isomerase. Methods Enzymol. 1984;107:281–294. doi: 10.1016/0076-6879(84)07018-x. [DOI] [PubMed] [Google Scholar]
- Kaplan H. A., Naider F., Lennarz W. J. Partial characterization and purification of the glycosylation site recognition component of oligosaccharyltransferase. J Biol Chem. 1988 Jun 5;263(16):7814–7820. [PubMed] [Google Scholar]
- Kaplan H. A., Welply J. K., Lennarz W. J. Oligosaccharyl transferase: the central enzyme in the pathway of glycoprotein assembly. Biochim Biophys Acta. 1987 Jun 24;906(2):161–173. doi: 10.1016/0304-4157(87)90010-4. [DOI] [PubMed] [Google Scholar]
- Kimura H., Noiva R., Mizunaga T., Yamauchi K., Horiuchi R., Cheng S. Y., Lennarz W. J. Thyroid hormone binding protein contains glycosylation site binding protein activity. Biochem Biophys Res Commun. 1990 Aug 16;170(3):1319–1324. doi: 10.1016/0006-291x(90)90538-x. [DOI] [PubMed] [Google Scholar]
- Koivu J., Myllylä R., Helaakoski T., Pihlajaniemi T., Tasanen K., Kivirikko K. I. A single polypeptide acts both as the beta subunit of prolyl 4-hydroxylase and as a protein disulfide-isomerase. J Biol Chem. 1987 May 15;262(14):6447–6449. [PubMed] [Google Scholar]
- Lucas J. J., Waechter J., Lennarz W. J. The participation of lipid-linked oligosaccharide in synthesis of membrane glycoproteins. J Biol Chem. 1975 Mar 25;250(6):1992–2002. [PubMed] [Google Scholar]
- Nicchitta C. V., Blobel G. Assembly of translocation-competent proteoliposomes from detergent-solubilized rough microsomes. Cell. 1990 Jan 26;60(2):259–269. doi: 10.1016/0092-8674(90)90741-v. [DOI] [PubMed] [Google Scholar]
- Obata T., Kitagawa S., Gong Q. H., Pastan I., Cheng S. Y. Thyroid hormone down-regulates p55, a thyroid hormone-binding protein that is homologous to protein disulfide isomerase and the beta-subunit of prolyl-4-hydroxylase. J Biol Chem. 1988 Jan 15;263(2):782–785. [PubMed] [Google Scholar]
- Parkkonen T., Kivirikko K. I., Pihlajaniemi T. Molecular cloning of a multifunctional chicken protein acting as the prolyl 4-hydroxylase beta-subunit, protein disulphide-isomerase and a cellular thyroid-hormone-binding protein. Comparison of cDNA-deduced amino acid sequences with those in other species. Biochem J. 1988 Dec 15;256(3):1005–1011. doi: 10.1042/bj2561005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajaniemi T., Helaakoski T., Tasanen K., Myllylä R., Huhtala M. L., Koivu J., Kivirikko K. I. Molecular cloning of the beta-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene. EMBO J. 1987 Mar;6(3):643–649. doi: 10.1002/j.1460-2075.1987.tb04803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields D., Blobel G. Efficient cleavage and segregation of nascent presecretory proteins in a reticulocyte lysate supplemented with microsomal membranes. J Biol Chem. 1978 Jun 10;253(11):3753–3756. [PubMed] [Google Scholar]
- Welply J. K., Kaplan H. A., Shenbagamurthi P., Naider F., Lennarz W. J. Studies on properties of membrane-associated oligosaccharyltransferase using an active site-directed photoaffinity probe. Arch Biochem Biophys. 1986 May 1;246(2):808–819. doi: 10.1016/0003-9861(86)90337-1. [DOI] [PubMed] [Google Scholar]
- Welply J. K., Shenbagamurthi P., Naider F., Park H. R., Lennarz W. J. Active site-directed photoaffinity labeling and partial characterization of oligosaccharyltransferase. J Biol Chem. 1985 May 25;260(10):6459–6465. [PubMed] [Google Scholar]
- Yu Y. H., Zhang Y. Y., Sabatini D. D., Kreibich G. Reconstitution of translocation-competent membrane vesicles from detergent-solubilized dog pancreas rough microsomes. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9931–9935. doi: 10.1073/pnas.86.24.9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman D. L., Walter P. Reconstitution of protein translocation activity from partially solubilized microsomal vesicles. J Biol Chem. 1990 Mar 5;265(7):4048–4053. [PubMed] [Google Scholar]