Abstract
A recent survey of pigs in Dong Thap province, Vietnam identified a high frequency of enterovirus species G (EV-G) infection (144/198; 72.7 %). Amongst these was a plethora of EV-G types (EV-G1, EV-G6 and four new types EV-G8–EV-G11). To better characterize the genetic diversity of EV-G and investigate the possible existence of further circulating types, we performed a larger-scale study on 484 pig and 45 farm-bred boar faecal samples collected in 2012 and 2014, respectively. All samples from the previous and current studies were also screened for kobuviruses. The overall EV infection frequency remained extremely high (395/484; 81.6 %), but with comparable detection rates and viral loads between healthy and diarrhoeic pigs; this contrasted with less frequent detection of EV-G in boars (4/45; 8.9 %). EV was most frequently detected in pigs ≤ 14 weeks old (∼95 %) and declined in older pigs. Infections with EV-G1 and EV-G6 were most frequent, whilst less commonly detected types included EV-G3, EV-G4 and EV-G8–EV-G11, and five new types (EV-G12–EV-G16). In contrast, kobuvirus infection frequency was significantly higher in diarrhoeic pigs (40.9 versus 27.6 %; P = 0.01). Kobuviruses also showed contrasting epizootiologies and age associations; a higher prevalence was found in boars (42 %) compared with domestic pigs (29 %), with the highest infection frequency amongst pigs >52 weeks old. Although genetically diverse, all kobuviruses identified belonged to the species Aichivirus C. In summary, this study confirms infection with EV-G was endemic in Vietnamese domestic pigs and exhibits high genetic diversity and extensive inter-type recombination.
Introduction
Porcine enteroviruses (PEVs) are members of the family Picornaviridae and originally consisted of 13 types (PEV-1–PEV-13). Further studies on their genetic relatedness led to the reassignment of these viruses into three separate picornavirus genera: Enterovirus, Sapelovirus and Teschovirus (Kaku et al., 2001; Knowles, 2006; Krumbholz et al., 2002; Zell et al., 2001). Of these, PEV-9 and PEV-10 were classified as EV-G1 and EV-G2 serotypes in enterovirus G, with the subsequently characterized EV-G types EV-G3–EV-G6 reported in single publications from Hungary and South Korea (Boros et al., 2011, 2012a, b; Moon et al., 2012). EV-G7 was subsequently discovered from domestic sheep by A. Boros and N. J. Knowles (unpublished). More recently, our molecular characterization of EVs in a small number of PCR-positive faecal samples from domestic pigs identified a further four types (EV-G8–EV-11) within this species. The presence of such a large amount of genetic diversity within such a modest number of samples suggests that the full range of EV-G types in this virus species has yet to be fully characterized (Van Dung et al., 2014). In contrast to teschoviruses, infections with EV-G have not been linked to any enteric or other disease presentations (Knowles, 2006) except for skin lesions (Knowles, 1988) and flaccid paralysis in an experimental infection of pigs in China (Yang et al., 2013). Viruses in this species have been reported from both healthy and diarrhoeic pigs with no statistically significant difference in infection rates between the two groups (Van Dung et al., 2014).
Porcine kobuvirus (PKV) was first identified in 2008 in Hungary (Reuter et al., 2008). Whilst screening 15 faecal samples from pigs for calicivirus (norovirus and sapovirus) using reverse transcription-PCR, Reuter et al. (2008) detected, in addition to a band specific for sapovirus, a non-specific, strong band of ∼1.1 kb in all tested samples visible on agarose gel. The nucleotide sequence of the non-specific PCR product showed most similarity to the 3C/3D region of bovine and human aichiviruses. Upon analysis of the obtained complete nucleotide sequence, the new PKV was assigned as a member of a new species (Aichivirus C) in the genus Kobuvirus, which also includes the species Aichivirus A and Aichivirus B, originally detected in humans and oxen, respectively. The species Aichivirus C consists of a single type, PKV-1. The existence of PKV-1 in pigs has been well documented in different countries worldwide (Amimo et al., 2014; Barry et al., 2011; Chen et al., 2013; Di Bartolo et al., 2015; Fan et al., 2013; Khamrin et al., 2009, 2010; Okitsu et al., 2012; Park et al., 2010; Reuter et al., 2013; Sisay et al., 2013); however, in Vietnam, PKV-1 has only been reported from river water (Inaba et al., 2014).
We have performed this larger-scale screening study to better understand the prevalence, genetic diversity and disease association of EVs and kobuviruses infecting pigs and farm-bred boars (hereafter referred to as ‘boars’) in Vietnam.
Results
Detection of EVs
EV RNA was detected in all but one (103/104; 99 %) of the farms containing domestic pigs and in three from six (50 %) farms housing boars. In total, 395 of 484 (81.6 %) samples from domestic pigs and four of 55 (8.9 %) boar samples were PCR-positive (Table 1). Pigs ≤ 14 weeks old were the most frequently infected with faecal detection frequencies of nearly 95 %. Lower detection frequencies were observed in older pigs with the lowest rate (50.5 %) in pigs >1 year old. Combining screening data with that of our previous samples, we found no significant difference in the frequency of detection of EV infection between the two groups in all age ranges (Table 1). The lack of any direct causative role of EV-G in enteric disease in pigs was further demonstrated by our observation that pigs with diarrhoea showed slightly lower EV-G RNA viral loads than found in healthy pigs (P = 0.008, Mann–Whitney test; Fig. 1).
Table 1. Detection of EVs and PKVs in pigs.
| Pigs | Age (weeks) | No. of pigs tested* | EV prevalence (%)* | P-value† | PKV prevalence (%)* | P-value† | EV/PKV co-infection (%)* | P-value† |
|---|---|---|---|---|---|---|---|---|
| Suckler | < 7 | 11/78 | 90.9/93.6 | 0.56 | 63.6/59 | 1 | 63.6/53.8 | 0.75 |
| Weaner | 7–14 | 32/147 | 93.8/94.6 | 1 | 18.8/25.9 | 0.4 | 18.8/24.5 | 0.49 |
| Grower | 15–23 | 8/117 | 75/82.9 | 0.63 | 25/16.2 | 0.62 | 25/13.7 | 0.32 |
| Gilts | 24–52 | 0/68 | –/70.6 | – | –/26.5 | – | –/17.6 | – |
| Matured | >52 | 28/106 | 39.3/48.1 | 0.4 | 67.9/25.5 | 2.7 × 10− 5 | 28.6/13.2 | 0.08 |
| Total | – | 79/516‡ | 72.2/79.1 | 0.17 | 43/28.7 | 0.01 | 29.1/23.3 | 0.26 |
Data are presented in pairs of diarrhoeic/non-diarrhoeic samples.
Fisher's exact test or χ2-test.
Only samples from the previous and current studies with available data (age and clinical status) are included.
Fig. 1.
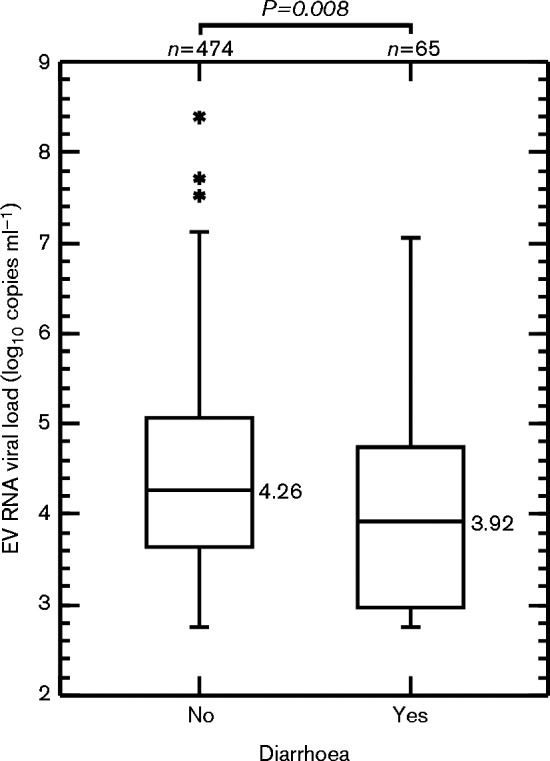
Box plot of EV RNA viral load of EV-G screen-positive sample suspensions from healthy and diarrhoeic pigs. Plots represent median and interquartile range (with outliers). The Mann–Whitney test was applied to compare medians between groups.
Detection of kobuviruses
The overall detection frequencies of kobuviruses in domestic pigs and boars were 29.3 (200/682) and 42.2 % (19/45), respectively. In contrast to EV-G, kobuviruses were significantly associated with diarrhoea in pigs, with an infection frequency of 40.9 % (36/88) in diarrhoeic pigs and an infection frequency of 27.6 % (164/594) in asymptomatic pigs (P = 0.01; χ2-test). This positive association was, however, most marked in pigs >52 weeks old in which the highest prevalence (67.9 %) was recorded. For pigs ≤ 23 weeks old, infection frequencies were highest in sucklers and generally decreased with age.
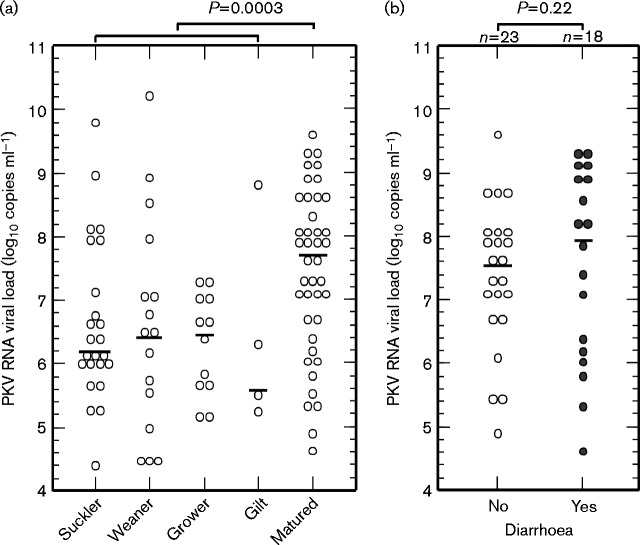
In order to elucidate whether PKV viral loads were associated with the clinical status of pigs, a real-time PCR protocol was developed. The assay had a detection limit of 100 PKV RNA copies per reaction and an amplification efficiency of 89 % (data not shown). It showed good specificity (no false positives in 20 samples negative by nested PCR) and sensitivity (97/100 samples positive by nested PCR were detected by real-time PCR). The median log10 viral load (7.7 copies ml–1) of PKV in 41 matured pig samples was significantly higher than that (6.4 copies ml–1) in the group of 56 younger pig samples (P = 0.0003, Mann–Whitney test; Fig. 2a). However, there was no significant difference in the viral loads (median log10 viral loads of 7.99 and 7.56 copies ml–1, respectively) between matured pigs with and without diarrhoea (P = 0.22 by Mann–Whitney test; Fig. 2b).
Fig. 2.
PKV RNA viral load of pig sample suspensions in (a) different age groups and (b) healthy or diarrhoeic matured pigs. Bars show median values. The Mann–Whitney test was applied to compare medians between groups.
The differences in the co-infection rates were not significant in any age categories between the two groups of diarrhoeic and non-diarrhoeic pigs (P>0.05). Co-infection with EV and PKV was most common in the youngest pigs (Table 1).
Genetic characterization of porcine EVs
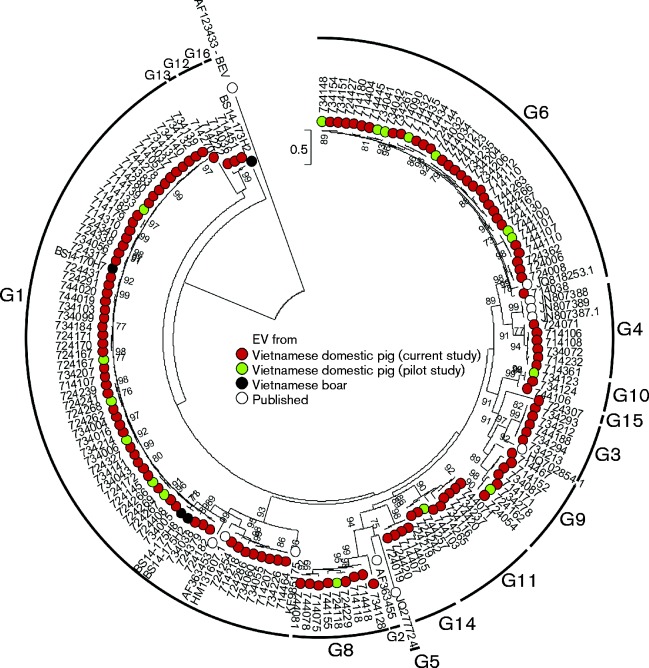
A range of EV variants in a subsample of 177 positive samples representing all sampling districts, Ct value ranges, ages and clinical status of infected pigs, and four boar positive samples were typed by sequencing of the VP1 region. All VP1 sequences obtained from 130 samples (including four from boars) clustered with the species G EVs (Fig. 3). Phylogenetic and sequence distance analysis of this region showed the most commonly identified previously described type was EV-G1 (n = 57 sequences, three boar-derived), followed by EV-G6 (n = 29), EV-G11 (n = 9), EV-G4, EV-G8 and EV-G9 (n = 6 of each type), EV-G3 (n = 5) and EV-G10 (n = 2). The other 10 VP1 nucleotide sequences were substantially divergent (>25 %) from other EV-G types and have been assigned as new types EV-G12–EV-G16 by the International Committee on Taxonomy of Viruses Picornavirus Study Group (Fig. 3).
Fig. 3.
Phylogenetic comparisons of VP1 sequences (nt 2469–3317) from study samples and available sequences of other EV-G variants from GenBank. Maximum-likelihood phylogenetic trees were reconstructed using 1000 bootstrap resamples to demonstrate the robustness of groupings; bootstrap support values of ≥ 70 % are shown. The tree was rooted using bovine enterovirus (BEV; GenBank accession number AF123433) as outgroup. The tree was drawn to scale; bar, estimated number of substitutions per site.
Analysis of data shows that young pigs were more frequently infected with EV-G1 and EV-G6 compared with other EV-G types, with median ages of 2 and 1.75 months, respectively. Meanwhile, other types were generally found in older pigs (median age 4 months) (Table S1, available in the online Supplementary Material). These differences were statistically significant (P < 0.0001 and P = 0.0001 by Mann–Whitney test; Fig. 4).
Fig. 4.
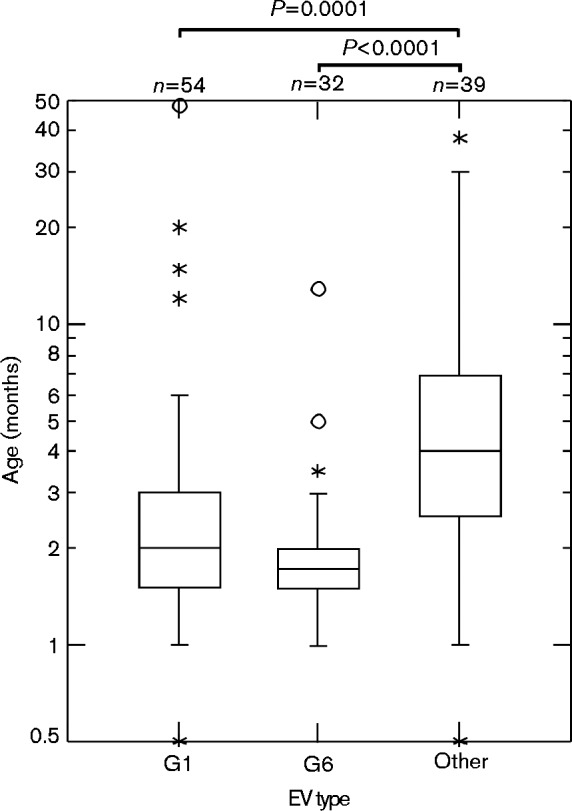
Box plot of EV types by pig ages. Note the y-axis is displayed in a log10 format. Plots represent median and interquartile range (with outliers). The Mann–Whitney test was applied to compare medians between groups.
Sequence and phylogenetic analysis of PKVs
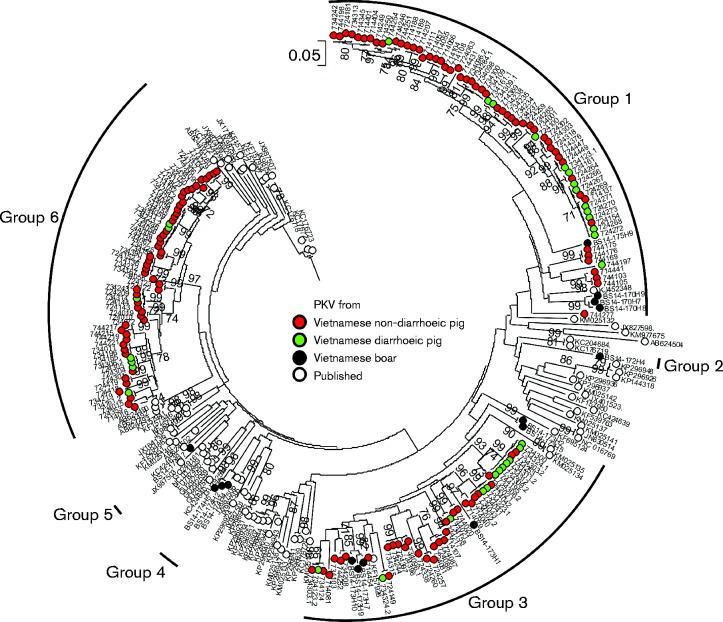
In total, 184 VP1 sequences were obtained from 173 positive samples, including 15 from boars (Fig. 5). Co-infection with two kobuvirus variants was detected in 11 samples by sequencing of VP1. All the VP1 sequences in the current study clustered within aichivirus C with nucleotide and amino acid sequence distances ranging from 0 to 22.3 % (mean 14.3 %) and 0 to 17.6 % (mean 8.5 %), respectively. In comparison with VP1 sequences of aichivirus C available in GenBank, the novel sequences produced here showed 9.1–23.6 and 0.45–17.5 % nucleotide and amino acid distances, respectively. In the resulting phylogeny, sequences from Vietnam were segregated into six groups (groups 1–6) with sequences from boars representing five distinct lineages, whilst those from domestic pigs clustering in three large groups. Groups 1–3 appeared to be most closely related to sequences from China (GenBank accession numbers KJ452348, KF539763 and KF157926), whilst groups 4–6 were most closely related to sequences from the USA (GenBank accession number JX987506), Hungary (GenBank accession number GQ249161.1) and Thailand (GenBank accession number AB624490), respectively. To test whether specific groups were associated with diarrhoea, association index values were calculated using genetic groups 1, 3 and 6 for group assignments and diarrhoea/non-diarrhoea as the associated variable. The calculated association index values of 0.45, 0.85 and 0.66 for the three groups, respectively, provided no evidence for a difference in clinical outcomes between groups.
Fig. 5.
Phylogenetic comparisons of VP1 sequences (nt 3005–3838) from study samples and available sequences of other PKV variants from GenBank. The tree was rooted using human kobuvirus (GenBank accession number NC_001918) and bovine kobuvirus (GenBank accession number GU245693) as outgroups. The tree was drawn to scale; bar, estimated number of substitutions per site.
Recombination of new types EV-G8 and EV-9
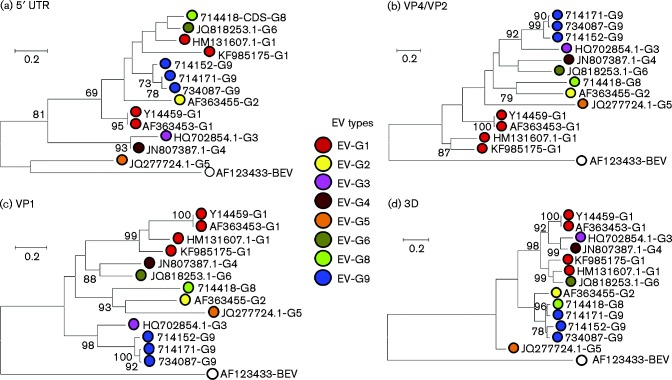
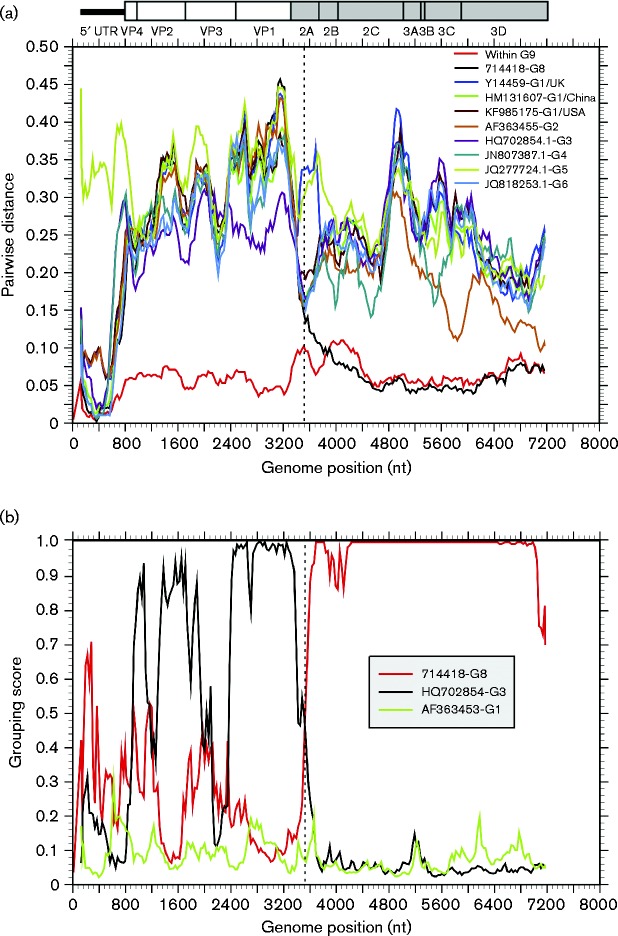
To perform a recombination analysis, complete coding region sequences of two new types were obtained by overlapping PCR amplifications. Phylogenetic relationships between EV-G8 and EV-G9 and other EV-G types for which complete genome sequences were available were inconsistent in several different regions of the genome (5′ UTR, VP4/VP2, VP1 and 3D), indicative of recombination (Fig. 6). Most obviously, whilst sequences from the 5′ UTR and VP4/VP2 and VP1 regions of the two novel EV-G types (EV-G8 and EV-G9) clustered separately from all other known EV-G types, sequences from the 3D regions of these novel viruses clustered together and formed a well-supported monophyletic clade (Fig. 6). To identify sites where recombination was likely to have occurred, a nucleotide distance scan (Fig. 7a) and grouping scan (Fig. 7b) were performed using EV-G9 as the reference sequence. EV-G9 was equally divergent from EV-G8 in P1 as it was from other types, but divergence values between EV-G8 and EV-G9 plunged at the boundary of P1/P2 to values equal to or lower than the mean pairwise distance of sequences within EV-G9. The analysis localized the breakpoint to the middle of the 2A region.
Fig. 6.
Phylogenetic comparisons of sequences from the (a) 5′ UTR (nt 107–809), (b) VP4/VP2 (nt 810–1752), (c) VP1 (nt 2469–3317) and (d) 3D (nt 5934–7316) regions from the study samples and available sequences of other EV-G variants from GenBank. The scale bar shows number of substitutions per site (evolutionary distance).
Fig. 7.
(a) Nucleotide sequence divergence within EV-G9, and between EV-G9 sequences and other EV-G types. Y-axis values show mean pairwise distances between 300-base windows across the genome, incrementing by 30 bases between fragments. (b) Grouping scan analysis of the sequence 734087-G9. Sequence fragments of 300 bases incrementing by 30 bases, 100 bootstrap replicates, were compared with sequences from other types. To show gene boundaries, the P2 and P3 regions are shaded grey and a scale representation of gene positions in the EV polyprotein is shown above the graphs.
Discussion
This is the first large-scale study of the prevalence and genetic diversity of EVs and kobuviruses in pigs and boars, providing substantial new information on their high infection frequencies and co-circulation of multiple types and lineages of both viruses in domestic pigs and boars in Vietnam.
The overall detection frequency of EVs in domestic pigs (81.6 %) was notably higher than those reported in Spain (0 %), Italy (7.5 %), China (8.3 %) and the Czech Republic (50.2 %) (Buitrago et al., 2010; Prodělalová, 2012; Sozzi et al., 2010; Yang et al., 2013). In contrast, detection of EV RNA in boars in this study was much lower than the 50 and 69.4 % prevalence reported in Hungary (Boros et al., 2012a) and the Czech Republic (Prodělalová, 2012), respectively. High rates of EV infection in young pigs (detection rate >90 %) suggested that these viruses maintain endemic transmission in this population. Infection rates declined with the age of the pigs, to 50.5 % in matured pigs, consistent with a limited period of active infection by and faecal excretion of circulating EV-G types, followed by clearance and likely immunity to reinfection. The greater likelihood of encountering the most actively circulating EV-G types (EV-G1 and EV-G6) early in life may account for their increased detection in younger pigs (Fig. 4). As a result of the more limited circulation of other EV-G types, first exposure may be delayed and therefore account for the increased age range of pigs in which these types are detected.
Even though domestic pigs and boars are typically reared separately, we found that EV-G1 infected both animal populations. Furthermore, variants were phylogenetically interspersed, indicating transmission events between the two populations. How this occurs is currently unclear as the boars were sampled in farms without domestic pigs and vice versa.
Our study provided no evidence that infections with EV-G caused diarrhoea, with comparable detection frequencies in pigs with and without symptoms (Table 1), consistent with a previous study (Prodělalová, 2012). To date, only EV-G1 virus has been specifically associated with disease in pigs, based on observations from the experimental infection of pigs in China in which two of 12 specific pathogen-free 2-week-old pigs exhibited flaccid paralysis of the hind limbs with EV RNA detected in plasma, spinal cord and brain. In that experiment, the sample supernatant (not EV isolate) was filtered through 0.22 μm microfilters before inoculation (Yang et al., 2013; Zhang et al., 2012). The sample was negative for a number of pathogens in pigs (e.g. porcine teschoviruses, porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, classical swine fever virus, porcine epidemic diarrhoea virus, porcine rotavirus, hepatitis E virus). However, findings from our study indicate that this must be a rare outcome of infection given the absence of any evident neurological disease in pig farms where EV-G1 (and other EV types) circulate extensively.
Genetic diversity and recombination in EV-G
Findings of four new types (EV-G8–EV-G11) from only 20 positive samples in our previous study suggested that the genetic diversity of EV-G had not been characterized completely (Van Dung et al., 2014). This conclusion was supported by the detection of five more novel types from 130 positive samples in the current study. There is no doubt that more new EV-G types will continue to be detected from pigs from different countries in the future. To further characterize the genetic diversity of EV-G and the potential occurrence of recombination between types, almost complete genome sequences of EV-G8 and EV-G9 were obtained and compared with those of other EV-G types. Collectively, EV-Gs show generic characteristics, such as patterns of divergence, codon usage and non-synonymous to synonymous substitutions that occur in other picornaviruses prone to extensive recombination, including EV-A–EV-C (Van Dung et al., 2014; Oberste et al., 2004; Simmonds, 2006). Consistent with these predictions, recombination in the EV-G genome was detected, with breakpoints located at the border of the 5′ UTR and VP4 of the EV-G5 prototype sequence (Boros et al., 2012b). Our previous study additionally identified other recombination occurring in the HM131607-G1 genome and other EV sequences from domestic pigs in Vietnam (Van Dung et al., 2014). In the current study, sequences of EV-G8 and EV-G9 variants showed significant phylogenetic incongruence between 3D (in which EV-G8 and EV-G9 sequences grouped together) and other regions (5′ UTR, VP4/VP2 and VP1, where EV-G8 clustered separately from EV-G9 sequences). This inconsistency in tree topology is strongly suggestive of recombination. Fragments 3 (nt 2395–3390) and 4 (nt 2679–5419) of the EV-G9 variants, both amplified by a single PCR (Fig. S1), had a long identical overlapping region that was distinct from the corresponding region of EV-G8. This helped rule out the possibility of hybrid viruses formed by assembly error from co-infection of individual pigs with two or more EV variants. When comparing the EV-G8 and EV-G9 variants, a nucleotide sequence divergence scan (Fig. 7a) showed considerable divergence in P1; from the middle of 2B to the end of the coding region, however, divergence declined substantially to levels even lower than that observed within EV-G9 sequences alone. The grouping scan (Fig. 7b), which quantifies degrees of grouping with pre-defined groups (types), similarly identified a recombination breakpoint in the middle of the 2A region.
Infection frequencies, diversity and disease associations of PKV
In accordance with previous studies (Chen et al., 2013; Park et al., 2010), our study showed an association between PKV detection and the presence of diarrhoea in matured pigs (Table 1), indicating its potential aetiological role in gastrointestinal disease, at least in older pigs. In marked contrast to EV infections, however, the highest detection rates were observed in diarrhoeic pigs >52 weeks old (matured pigs). This contrasts with reports from other countries where pigs < 6 months old, particularly sucklers, were most susceptible to PKV infection (Chen et al., 2013; Park et al., 2010). Furthermore, we found viral loads of PKV in matured pigs to be significantly higher than that in the younger pigs.
This observation of increasing detection frequencies with age may simply reflect the possibility that PKV circulates less extensively than other enteric viruses, such as EV-G, and that infections are skewed towards older age groups with greater cumulative exposure. Another hypothesis to explain increasing detection frequencies and greater viral loads in older animals is that PKV infections are naturally persistent, and pigs may sequentially acquire long-term infections with one or more PKV variants over time. Although frequencies of persistence in PKV and other kobuviruses are poorly documented, genomes of all members of this genus possess structured RNA genomes (Davis et al., 2008; Simmonds et al., 2004) – a generic property associated with persistence in several other picornavirus genera and amongst other families of positive-stranded RNA viruses. High rates of detection in older pigs and boars without diarrhoea, and the lack of association between viral load and symptomatic infection in matured pigs, indeed questions a direct aetiological link between PKV and gastrointestinal disease. It is possible, for example, that the greater detection frequency of PKV in cases of diarrhoea was secondary to altered mucosal immunity and inflammatory processes consequent to bacterial or other viral infections causing diarrhoea (Frémont et al., 2013). These processes may serve to reactivate normally relatively quiescent infections with PKV. Consistent with this possible incidental association with gastrointestinal disease, comparison of the phylogeny of VP1 sequences from diarrhoeic and non-diarrhoeic pigs provided no evidence for the existence of specific lineages associated with gastrointestinal disease. Formal testing of the association of this trait with phylogenetic position by calculation of association index values (Wang et al., 2001) confirmed this lack of association.
VP1 sequences of PKV variants clustered in six genetically diverse groups that showed the closest relatedness to sequences from China, the USA, Hungary and Thailand. VP1 contains a number of neutralization domains and the gene encoding this protein has been extensively used for (sero)type identification. The species Aichivirus C is currently classified as a single type, PKV-1, which includes variants with uncorrected VP1 nucleotide sequence distances ranging from 0 to 22.6 %. There is no information on the extent of antigenic, neutralization or protective immunity differences between variants of PKV, or whether members of different genetic groups identified in VP1 correspond to serotypes of other EVs. By analogy with other EVs, EV-A–EV-D (sero)types show >25 % nucleotide sequence distances from each other in VP1 (Oberste et al., 1999), whilst different rhinovirus serotypes in the same genus indeed show divergences of ≥ 12–13 % (McIntyre et al., 2013). As PKV VP1 sequence distances lie between these two thresholds, prediction of their serological properties using this type of comparative approach is problematic. However, the detection of different PKV variants in the same samples is more consistent with them being antigenically distinct and lacking cross-protection, comparable to what is observed with different serotypes of poliovirus and foot-and-mouth disease virus (Alexandersen & Donaldson, 2002; Alexandersen et al., 2002).
Conclusions
This large-scale study showed high prevalence and genetic diversity of EV-G and PKV in pigs and boars in Vietnam. These are clearly widely distributed, with endemic infections circulating in both domestic pigs and boars, and demonstrate the ease with which enteric viruses likely transmitted by faecal/oral routes can become established in and transmit within farmed animal populations. Whilst the pathogenicity of neither virus is clearly established in either this or previous studies, further genetic and epizootiological characterization of these viruses will contribute to our understanding of their transmission patterns and the farming practices that affect their spread. Future studies may also provide new insights into the factors that underlie the transmission and dissemination of more specifically pathogenic viruses, such as rotaviruses, that present a much greater challenge to animal welfare and productivity.
Methods
Sampling
Faecal samples were collected from 104 farms in four districts of Dong Thap province between February and May 2012. At each farm, 10 freshly voided samples from randomly selected healthy pigs and up to four samples from pigs with diarrhoea were collected. From this collection, a subsample of 198 samples, including 70 diarrhoeic samples, were screened in a previous study (Van Dung et al., 2014). In the current study, we extended the screening of EVs to the 18 remaining diarrhoeic samples and 466 samples representing three to five healthy pigs per farm. In addition, 45 faecal samples collected in April 2014 from healthy farm-bred boars that were housed in typical livestock pens on six farms in Dak Lak province were also included. All 727 samples from pigs and boars were also screened for kobuviruses.
Screening and typing of EVs
RNA was extracted from faecal samples using a MagNA Pure 96 Viral NA small volume kit (Roche) and an automated extractor (Roche) as described previously (Van Dung et al., 2014). Reverse transcription using SuperScript III reverse transcriptase (Invitrogen, UK) was performed according to the manufacturer's instruction. EVs were screened by a real-time PCR protocol (Beld et al., 2004). Typing of EVs from 177 positive samples, including 10 from diarrhoeic pigs, representing ranges of geography, Ct values and ages of infected pigs, was performed by amplification and sequencing of the VP1 region as described previously (Van Dung et al., 2014). VP1 products of the nested PCR were sequenced on both strands using BigDye Terminator version 3.1 (Applied Biosystems) and primers for second-round PCR.
Screening and characterization of kobuviruses
Synthesized cDNA was screened for kobuviruses by nested PCR using primers targeting the 3D region of all known kobuviruses (Table S2). The conditions for amplification (using GoTaq DNA polymerase; Promega) were 95 °C for 5 min, and 30 cycles of denaturation (94 °C, 30 s), annealing (54 °C, 30 s) and elongation (72 °C, 45 s). Kobuviruses in positive samples were characterized by amplification and sequencing of the VP1 region with similar amplification conditions for EV VP1 as described previously (Van Dung et al., 2014) but using an annealing temperature of 58 °C in both rounds.
PKV viral load measurement
Using PKV sequences available from GenBank, primers and a probe targeting the 5′ UTR region were designed to develop a real-time PCR protocol for measurement of PKV viral load. The 200 bp target region in the 5′ UTR amplified from a positive sample was cloned into pGEM-T Easy Vector (Promega). The linearized recombinant plasmid was used as template for transcription using a MEGAscript T7 transcription kit (Ambion) as previously described (McLeish et al., 2012). RNA transcripts were used to assess the detection limit and efficiency of the assay before application on a subsample of 100 positive samples. These samples were selected to represent healthy and diarrhoeic pigs of all age groups, with the focus on the group with disease association (matured pigs). The specificity of the assay was checked with 20 randomly selected negative pig samples that were negative for PKV, but positive for EV. Each reaction of 20 μl total volume contained primers (PKV_332s, PKV_533a) and probe (PKV_505a_probe) at final concentrations of 0.4 and 0.2 μM, respectively, cDNA (5 μl), and 10 μl of SensiFAST Probe Hi-ROX kit (Bioline) Master Mix. After initial denaturation at 95 °C (5 min), amplification was performed in 45 cycles of 95 °C for 10 s and 60 °C for 35 s (acquisition step). Primers and probe used in this study for kobuviruses are listed in Table S2.
Complete genome sequencing of EVs
Primers were designed using VP1 sequences available from this study and reference sequences from GenBank to obtain nearly complete genomes of viruses representing EV-G8 and EV-G9 (Fig. S1, Table S3). Nested PCR amplifying regions from ∼700 bp to 2.5 kb in length were performed. For each reaction, 6 μl extracted RNA was used for cDNA synthesis in combination with first-round PCR using a Superscript III One-Step RT-PCR System with Platinum Taq High Fidelity (Invitrogen). Then, 1 μl of the first-round reaction was used as template for the second-round PCR with amplification (using GoTaq DNA polymerase; Promega) conditions as follows: 95 °C for 5 min, and 30 cycles of denaturation (94 °C, 30 s), annealing (50 °C, 30 s) and elongation (72 °C, 60 s) in a thermal cycler. A SequalPrep long PCR kit (Life Technology) was used according to the manufacturer's instruction instead of GoTaq DNA polymerase to amplify fragments >2 kb.
Sequence analysis
Sequences were imported into sse (Simmonds, 2012) for alignment and calculation of sequence divergence values from reference sequences of known EV types. Maximum-likelihood phylogenetic trees were reconstructed as described in our previous study (Van Dung et al., 2014) using the mega 5.2 software package (Tamura et al., 2011) with 1000 bootstrap resamples and best-fitting models (Tamura–Nei, gamma distribution for EV VP4/VP2 and VP1, Tamura three-parameter model, gamma distribution for EV 5′ UTR and 3D, general time-reversible gamma distribution with invariant sites for kobuvirus VP1). Nucleotide distance scans and grouping scans (Simmonds & Midgley, 2005) implemented in sse were applied to identify recombination breakpoints. Analysis of phylogenetic groupings was performed by determining association index values (Wang et al., 2001) using the program in sse with 100 bootstrap replicates.
Statistical analyses
Fisher exact test, χ2-test and Mann–Whitney test incorporated in Minitab 17 were used to compare data between groups of interest when appropriate as specified.
Acknowledgements
We would like to thank the Subdepartment of Animal Health of Dong Thap and all the farmers who participated in the survey for their support. Work has been funded by the Vietnam Initiative on Zoonotic Infections (VIZIONS), part of the Wellcome Trust Major Overseas Programme (UK).
Supplementary Data
Supplementary Data
References
- Alexandersen S., Donaldson A. I. (2002). Further studies to quantify the dose of natural aerosols of foot-and-mouth disease virus for pigs Epidemiol Infect 128313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Zhang Z., Donaldson A. I. (2002). Aspects of the persistence of foot-and-mouth disease virus in animals - the carrier problem Microbes Infect 41099–1110 10.1016/S1286-4579(02)01634-9. [DOI] [PubMed] [Google Scholar]
- Amimo J. O., Okoth E., Junga J. O., Ogara W. O., Njahira M. N., Wang Q., Vlasova A. N., Saif L. J., Djikeng A. (2014). Molecular detection and genetic characterization of kobuviruses and astroviruses in asymptomatic local pigs in East Africa Arch Virol 1591313–1319 10.1007/s00705-013-1942-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. F., Ribeiro J., Alfieri A. F., van der Poel W. H. M., Alfieri A. A. (2011). First detection of kobuvirus in farm animals in Brazil and the Netherlands Infect Genet Evol 111811–1814 10.1016/j.meegid.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Beld M., Minnaar R., Weel J., Sol C., Damen M., van der Avoort H., Wertheim-van Dillen P., van Breda A., Boom R. (2004). Highly sensitive assay for detection of enterovirus in clinical specimens by reverse transcription-PCR with an armored RNA internal control J Clin Microbiol 423059–3064 10.1128/JCM.42.7.3059-3064.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros Á., Pankovics P., Reuter G. (2011). Characterization of a novel porcine enterovirus in domestic pig in Hungary Infect Genet Evol 111096–1102 10.1016/j.meegid.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Boros A., Nemes C., Pankovics P., Bíró H., Kapusinszky B., Delwart E., Reuter G. (2012a). Characterization of a novel porcine enterovirus in wild boars in Hungary Arch Virol 157981–986 10.1007/s00705-012-1255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros A., Pankovics P., Knowles N. J., Reuter G. (2012b). Natural interspecies recombinant bovine/porcine enterovirus in sheep J Gen Virol 931941–1951 10.1099/vir.0.041335-0. [DOI] [PubMed] [Google Scholar]
- Buitrago D., Cano-Gómez C., Agüero M., Fernandez-Pacheco P., Gómez-Tejedor C., Jiménez-Clavero M.Á. (2010). A survey of porcine picornaviruses and adenoviruses in fecal samples in Spain J Vet Diagn Invest 22763–766 10.1177/104063871002200519. [DOI] [PubMed] [Google Scholar]
- Chen L., Zhu L., Zhou Y. C., Xu Z. W., Guo W. Z., Yang W. Y. (2013). Molecular and phylogenetic analysis of the porcine kobuvirus VP1 region using infected pigs from Sichuan Province, China Virol J 10281. 10.1186/1743-422X-10-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M., Sagan S. M., Pezacki J. P., Evans D. J., Simmonds P. (2008). Bioinformatic and physical characterizations of genome-scale ordered RNA structure in mammalian RNA viruses J Virol 8211824–11836 10.1128/JVI.01078-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bartolo I., Angeloni G., Tofani S., Monini M., Ruggeri F. M. (2015). Infection of farmed pigs with porcine kobuviruses in Italy Arch Virol 1601533–1536 10.1007/s00705-015-2397-z. [DOI] [PubMed] [Google Scholar]
- Fan S., Sun H., Ying Y., Gao X., Wang Z., Yu Y., Li Y., Wang T., Yu Z., other authors (2013). Identification and characterization of porcine kobuvirus variant isolated from suckling piglet in Gansu province, China Viruses 52548–2560 10.3390/v5102548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frémont M., Coomans D., Massart S., De Meirleir K. (2013). High-throughput 16S rRNA gene sequencing reveals alterations of intestinal microbiota in myalgic encephalomyelitis/chronic fatigue syndrome patients Anaerobe 2250–56 10.1016/j.anaerobe.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Inaba M., Katayama H., Tran N. V. T., Furuami H. (2014). Detection of genus Kobuvirus for evaluation as virus indicator for fecal contamination source track from Nhue River in Hanoi, Vietnam. In Southeast Asian Water Environment 5, pp. 61–66. Edited by Yamamoto H., Furumai H., Katayama H., Chiemchaisri C., Puetpaiboon U., Visvanathan C., Satoh H.London: IWA Publishing. [Google Scholar]
- Kaku Y., Sarai A., Murakami Y. (2001). Genetic reclassification of porcine enteroviruses J Gen Virol 82417–424 10.1099/0022-1317-82-2-417. [DOI] [PubMed] [Google Scholar]
- Khamrin P., Maneekarn N., Kongkaew A., Kongkaew S., Okitsu S., Ushijima H. (2009). Porcine kobuvirus in piglets, Thailand Emerg Infect Dis 152075–2076 10.3201/eid1512.090724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamrin P., Maneekarn N., Hidaka S., Kishikawa S., Ushijima K., Okitsu S., Ushijima H. (2010). Molecular detection of kobuvirus sequences in stool samples collected from healthy pigs in Japan Infect Genet Evol 10950–954 10.1016/j.meegid.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Knowles N. J. (1988). The association of group III porcine enteroviruses with epithelial tissue Vet Rec 122441–442 10.1136/vr.122.18.441. [DOI] [PubMed] [Google Scholar]
- Knowles N. J. (2006). Porcine enteric picornaviruses. In Diseases of Swine, pp. 337–345. Edited by Straw B. E., Zimmermann J. J., D'Allaine S. D., Taylor D. J.Ames, IA: Blackwell. [Google Scholar]
- Krumbholz A., Dauber M., Henke A., Birch-Hirschfeld E., Knowles N. J., Stelzner A., Zell R. (2002). Sequencing of porcine enterovirus groups II and III reveals unique features of both virus groups J Virol 765813–5821 10.1128/JVI.76.11.5813-5821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre C. L., Knowles N. J., Simmonds P. (2013). Proposals for the classification of human rhinovirus species A, B and C into genotypically assigned types J Gen Virol 941791–1806 10.1099/vir.0.053686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeish N. J., Witteveldt J., Clasper L., McIntyre C., McWilliam Leitch E. C., Hardie A., Bennett S., Gunson R., Carman W. F., other authors (2012). Development and assay of RNA transcripts of enterovirus species A to D, rhinovirus species a to C, and human parechovirus: assessment of assay sensitivity and specificity of real-time screening and typing methods J Clin Microbiol 502910–2917 10.1128/JCM.01172-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. J., Song D., Seon B. H., Kim H. K., Park S. J., An D. J., Kim J. M., Kang B. K., Park B. K. (2012). Complete genome analysis of porcine enterovirus B isolated in Korea J Virol 8610250. 10.1128/JVI.01548-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste M. S., Maher K., Kilpatrick D. R., Pallansch M. A. (1999). Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification J Virol 731941–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste M. S., Peñaranda S., Pallansch M. A. (2004). RNA recombination plays a major role in genomic change during circulation of coxsackie B viruses J Virol 782948–2955 10.1128/JVI.78.6.2948-2955.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okitsu S., Khamrin P., Thongprachum A., Hidaka S., Kongkaew S., Kongkaew A., Maneekarn N., Mizuguchi M., Hayakawa S., Ushijima H. (2012). Sequence analysis of porcine kobuvirus VP1 region detected in pigs in Japan and Thailand Virus Genes 44253–257 10.1007/s11262-011-0692-7. [DOI] [PubMed] [Google Scholar]
- Park S.-J., Kim H.-K., Moon H.-J., Song D.-S., Rho S.-M., Han J.-Y., Nguyen V.-G., Park B.-K. (2010). Molecular detection of porcine kobuviruses in pigs in Korea and their association with diarrhea Arch Virol 1551803–1811 10.1007/s00705-010-0774-1. [DOI] [PubMed] [Google Scholar]
- Prodělalová J. (2012). The survey of porcine teschoviruses, sapeloviruses and enteroviruses B infecting domestic pigs and wild boars in the Czech Republic between 2005 and 2011 Infect Genet Evol 121447–1451 10.1016/j.meegid.2012.04.025. [DOI] [PubMed] [Google Scholar]
- Reuter G., Boldizsár A., Kiss I., Pankovics P. (2008). Candidate new species of Kobuvirus in porcine hosts Emerg Infect Dis 141968–1970 10.3201/eid1412.080797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter G., Nemes C., Boros A., Kapusinszky B., Delwart E., Pankovics P. (2013). Porcine kobuvirus in wild boars (Sus scrofa) Arch Virol 158281–282 10.1007/s00705-012-1456-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P. (2006). Recombination and selection in the evolution of picornaviruses and other mammalian positive-stranded RNA viruses J Virol 8011124–11140 10.1128/JVI.01076-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P. (2012). sse: a nucleotide and amino acid sequence analysis platform BMC Res Notes 550. 10.1186/1756-0500-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Midgley S. (2005). Recombination in the genesis and evolution of hepatitis B virus genotypes J Virol 7915467–15476 10.1128/JVI.79.24.15467-15476.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Tuplin A., Evans D. J. (2004). Detection of genome-scale ordered RNA structure (GORS) in genomes of positive-stranded RNA viruses: Implications for virus evolution and host persistence RNA 101337–1351 10.1261/rna.7640104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisay Z., Wang Q., Oka T., Saif L. (2013). Prevalence and molecular characterization of porcine enteric caliciviruses and first detection of porcine kobuviruses in US swine Arch Virol 1581583–1588 10.1007/s00705-013-1619-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozzi E., Barbieri I., Lavazza A., Lelli D., Moreno A., Canelli E., Bugnetti M., Cordioli P. (2010). Molecular characterization and phylogenetic analysis of VP1 of porcine enteric picornaviruses isolates in Italy Transbound Emerg Dis 57434–442 10.1111/j.1865-1682.2010.01170.x. [DOI] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2011). mega5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods Mol Biol Evol 282731–2739 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dung N., Anh P. H., Van Cuong N., Hoa N. T., Carrique-Mas J., Hien V. B., Campbell J., Baker S., Farrar J., other authors (2014). Prevalence, genetic diversity and recombination of species G enteroviruses infecting pigs in Vietnam J Gen Virol 95549–556 10.1099/vir.0.061978-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. H., Donaldson Y. K., Brettle R. P., Bell J. E., Simmonds P. (2001). Identification of shared populations of human immunodeficiency virus type 1 infecting microglia and tissue macrophages outside the central nervous system J Virol 7511686–11699 10.1128/JVI.75.23.11686-11699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Wang Y., Shen Q., Zhang W., Hua X. (2013). Prevalence of porcine enterovirus 9 in pigs in middle and eastern China Virol J 1099. 10.1186/1743-422X-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zell R., Dauber M., Krumbholz A., Henke A., Birch-Hirschfeld E., Stelzner A., Prager D., Wurm R. (2001). Porcine teschoviruses comprise at least eleven distinct serotypes: molecular and evolutionary aspects J Virol 751620–1631 10.1128/JVI.75.4.1620-1631.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Yang S., Shen Q., Ren L., Shan T., Wei J., Cui L., Hua X. (2012). Complete genome sequence of a novel porcine enterovirus strain in China J Virol 867008–7009 10.1128/JVI.00711-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data