Abstract
Background
Bacterial contamination of whole blood (WB) units can result in transfusion‐transmitted infection, but the extent of the risk has not been established and may be underestimated in veterinary medicine.
Objectives
To detect, quantify, and identify bacterial microorganisms in 49 canine WB units during their shelf life.
Animals
Forty‐nine healthy adult dogs.
Methods
Forty‐nine WB units were included in the study. Immediately after collection, 8 sterile samples from the tube segment line of each unit were aseptically collected and tested for bacterial contamination on days 0, 1, 7, 14, 21, 28, 35, and 42 of storage. A qPCR assay was performed on days 0, 21, and 35 to identify and quantify any bacterial DNA.
Results
On bacterial culture, 47/49 blood units were negative at all time points tested, 1 unit was positive for Enterococcus spp. on days 0 and 1, and 1 was positive for Escherichia coli on day 35. On qPCR assay, 26 of 49 blood units were positive on at least 1 time point and the bacterial loads of the sequences detected (Propionobacterium spp., Corynebacterium spp., Caulobacter spp., Pseudomonas spp., Enterococcus spp., Serratia spp., and Leucobacter spp.) were <80 genome equivalents (GE)/μL.
Conclusions and Clinical Importance
Most of the organisms detected were common bacteria, not usually implicated in septic transfusion reactions. The very low number of GE detected constitutes an acceptable risk of bacterial contamination, indicating that WB units have a good sanitary shelf life during commercial storage.
Keywords: Bacterial contamination, Blood transfusion, Canine, Stored blood units
Abbreviations
- Cq
quantification cycle
- GE
genome equivalent
- NTC
no‐template control
- pRBC
packet red blood cells
- RBC
red blood cells
- WB
whole blood
The World Health Organization reports that over 100 million units of blood are collected globally each year in human medicine.1 However, small animal blood transfusions have only become common in the last 30–60 years.2 Blood transfusions are now a routine, life‐saving therapeutic technique and have become increasingly common in small animal practice,3 but their absolute safety can never be guaranteed. The risks associated with the transfusion of blood products include blood‐borne infectious organisms that can be transmitted by transfusion, and several guidelines suggest the canine donors must be screened for infectious agents.4
As in human medicine, screening programs in veterinary transfusion medicine have decreased the risk of infectious disease transmission from donors. However, the current paramount concern is to improve the safety of transfusion for patients in an attempt to achieve a zero‐risk blood supply.
Some retrospective studies of dogs have suggested that the use of packed red blood cells (pRBCs) and whole blood (WB) is associated with a 3.3–13% incidence of transfusion reaction.5, 6
In human medicine, an association has been demonstrated between transfusion of older stored pRBCs and sepsis,7 but the specific mechanism is not yet known. Red blood cells (RBC) undergo many physical and biochemical changes during storage (eg, increased oxidative stress, formation of microparticles) that can contribute to morbidity and mortality in critical patients,1 but bacterial contamination during the preparation and storage of blood products must also be considered. Severe and even fatal septic transfusion reactions may go unrecognized, and their real prevalence may be higher than reported. For this reason, it is very difficult to determine true morbidity and mortality associated with transfusing bacterially contaminated blood products.
The properties of contaminating microorganisms determine their ability to grow under storage conditions. The mere presence of bacteria in a blood unit is less important, than their replication, which can induce serious septic complications.8
In human medicine, a low but known risk of bacterial contamination has emerged as the greatest risk factor for transfusion‐transmitted disease. In particular, bacterial contamination is considered a long‐standing and persistent risk and has become a matter of increasing concern.9, 10
Although aseptic techniques are practiced in veterinary transfusion medicine, to our knowledge, no bacterial surveillance program is in place and the risk of bacterial contamination of blood units is probably underestimated. Moreover, the types of bacteria that potentially contaminate blood units have not been well described.
A few reports in veterinary medicine have described the spontaneous contamination of feline WB and feline pRBCs units by Serratia spp. and Pseudomonas spp. in hospital blood banks.11, 12, 13
In an effort to understand the real extent of bacterial contamination and the magnitude of the associated risk, we aimed to detect, quantify, and identify the bacterial microorganisms in 49 canine WB bags during their shelf life, by bacteriological culture and biomolecular assays.
Materials and Methods
Blood Donors and Blood collection
The dogs were part of a volunteer blood donor program at the Blood Bank and Veterinary Transfusion Unit (EMOVET‐UNIPG) of the Department of Veterinary Medicine, University of Perugia, Italy.
The inclusion criteria for potential donor animals were as follows: healthy dogs aged 2–8 years, weighing >25 kg; up to date on regular core vaccinations against canine distemper virus, canine adenovirus types 1 and 2, Leptospira interrogans, and canine parvovirus; regular preventative treatment for ecto‐ and endoparasites in the month before screening; no previous history of blood transfusion; and no surgical procedure before blood collection.
Animals were considered healthy based on a screening procedure before blood donation, which included physical examination, CBC, and serum biochemistry panel. The rectal temperatures of the canine blood donors, recorded on the day of donation, were normal. The dogs were tested and shown to be negative for Dirofilaria immitis antigen and antibodies against Anaplasma phagocytophilum, Borrelia burgdorferi,1 Anaplasma platys, Ehrlichia canis, Babesia spp., Leishmania infantum, and Rickettsia rickettsii.2 , 14, 15 The blood bank followed established standard operating procedures in accordance with guidelines set by the Italian Ministry of Health.15, 16
Forty‐nine WB units were included in the study. The blood was collected from 49 healthy dogs in a 1‐month period (August 2014), according to the guidelines cited previously at EMOVET‐UNIPG, Department of Veterinary Medicine, University of Perugia.
To collect each blood sample, the area over 1 jugular vein was carefully shaved and scrubbed with alcohol and povidone iodine. The veterinary medical hospital staff at the transfusion center had been trained to prevent contamination during this procedure. Whole blood was collected from each animal in a standard, sterile, closed 450 mL blood collection system, containing citrate‐adenine‐phosphate‐dextrose solution (CPDA1).3 Within 30 minutes of collection,17 each WB unit was thoroughly mixed, and the tubing was “stripped” to allow the blood in the tube to mix with that in the bag. Eight sterile samples from the tube segment lines, containing 1 mL of blood from each WB unit, were aseptically produced with a mobile tube sealer system4 to allow nondestructive quality control testing of the units (Fig 1). All WB units and their corresponding segments were stored in a blood storage refrigerator at 4 ± 2°C (hypothermic storage, HS) for up to 42 days, 1 week after the expiration date at 35 days.18, 19, 20
Figure 1.
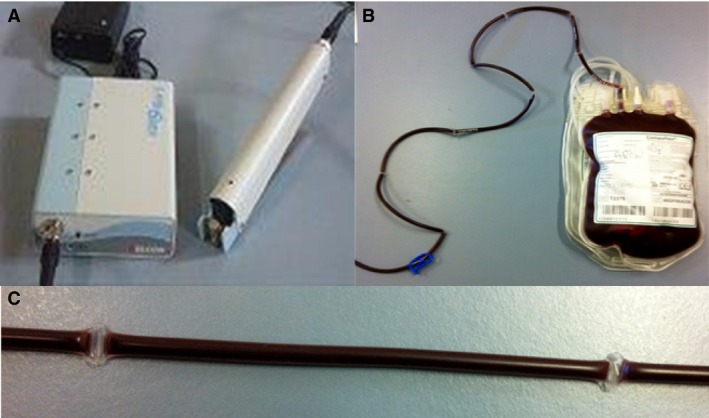
(A) Mobile blood bag tube sealer system that allows nondestructive quality control testing of blood units; (B) whole blood (WB) unit with its 8 sterile corresponding segments; (C) Sample from tube segment line containing 1 mL of blood from a WB unit.
Oscillations in the storage temperature of the refrigerator were monitored with an alarm that was triggered by deviations of 2°C and a recording chart. Each HS WB unit (and its corresponding segments) was assigned a lot number for tracking purposes. During storage, the samples were visually inspected daily for gross color changes, turbidity, and hemolysis, which represent evidence of microbial growth. They were thoroughly mixed every second day, immediately after the check, by the nursing staff. The technicians who handled the blood bags were trained in good laboratory practice and were always the same. The 8 segments contiguous with the bag contents of each unit were sampled aseptically and tested by bacterial culture and microscopic evaluation of a blood smear stained with Romanowsky stain, on days 0 (T0—at room temperature), 1 (T1—24 hours after collection, 4°C), 7 (T7—4°C), 14 (T14—4°C), 21(T21—4°C), 28 (T28—4°C), 35 (T35—4°C), and 42 (T42 —1 week after the expiration date, 4°C). After DNA extraction, a real‐time quantitative PCR (qPCR) assay was performed on the T0, T21, and T35 samples. These time points were chosen because they represent the beginning, middle, and end of the shelf life of the bag.
Thirty of the 49 WB units were transfused into clinical patients at the Veterinary Teaching Hospital, University of Perugia, after different periods of time in storage (between 5 and 27 days in storage), and only the segments were analyzed at all time points. The other 19 WB units and their segments were kept for inspection for the entire 42‐day period of the experiment. All of the microbiological and bimolecular tests were performed under a laminar airflow cabinet.
Bacterial Culture
Tryptic soy broth (10 mL) medium was inoculated with each sample and incubated aerobically and anaerobically at 37°C for 48 hours. After 48 hours, a small amount of each broth culture (100 μL) was subcultured on blood agar, MacConkey agar, and mannitol salt agar and incubated for 48 hours at 37°C. Pure colonies were examined with Gram staining and identified with different biochemical tests, including catalase and coagulase tests for Gram‐positive bacteria and hydrogen sulfide production (H2S), indole test, citrate utilization, gas production, and carbohydrate metabolism for Gram‐negative bacteria.
DNA Extraction
DNA was isolated from the blood units corresponding to T0, T21, and T35, and each sample was processed with the QIAamp DNA Blood Mini Kit,5 according to the manufacturer's instructions. The extracted nucleic acids were eluted in 200 μL of elution buffer. All of the samples were analyzed with a spectrophotometer,6 and electrophoresis on 1% agarose gel to determine the quantity and quality of the DNA.
qPCR Assay
The bacterial DNA loads in the samples were assessed with qPCR,7 with the slightly modified procedure of Nadkarni et al.21 A reference curve relating the quantification cycle (C q) to the bacterial DNA concentration in the samples was constructed with 6 10‐fold serial dilutions of Escherichia coli (ATCC 25922) DNA, starting from 1.97 × 105 bacterial genome equivalents (GE) and diluted to 1.97 × 100. The reference curve was incorporated into each qPCR experiment and used to determine the bacterial loads of the samples. The PCRs were performed in 20 μL containing 10 μL of SsoFast EvaGreen Supermix8 with 100 ng of DNA template. A 466‐bp fragment of the bacterial 16S rDNA was amplified on a CFX96 Touch instrument,8 under the following conditions: 98°C for 3 minutes and 40 cycles of 98°C for 5 seconds, 61°C for 1 minutes. Each reaction was run in triplicate. No‐template controls (NTCs) were included in each run. The data were analyzed with the Bio‐Rad CFX Manager software (ver. 3.2.2), supplied by the manufacturer. The PCR products were all of the expected length, were purified with the Wizard SV Gel and PCR Clean‐up System,9 in accordance with the manufacturer's recommended protocol, and were sequenced directly. The sequences were analyzed with the Basic Local Alignment Search Tool with the GenBank reference database to determine the source genera.
Results
No marked visible color changes, hemolysis, or clots were observed in any of the WB units or segments during the 42 days of storage. A microscopic examination of each blood smear prepared from the segment samples showed no bacteria or parasites. No transfused recipient had an immediate or delayed adverse effect (eg, febrile transfusion reaction, sepsis).
Bacterial Culture
Forty‐seven of the 49 (96%) blood packs were negative at all the time points tested. One pack was positive for Enterococcus sp. at T0 and T1, but negative at all other time points. One pack was positive for E. coli only at T35.
qPCR Assay
The average efficiency of the standard curve was 104.8 ± 4.02%. A cutoff value was determined with which to discriminate contaminated and uncontaminated WB units. Based on the standard curve, the calibration interval, and the observation of the NTC assays, samples were considered positive when the corresponding C q value was ≤30 (corresponding to approximately 20 GE/μL of template). After the analysis, 26 of the 49 blood packs were identified as positive on at least 1 of the time points analyzed (T0, T21, or T35), as shown in Table 1.
Table 1.
Different bacterial genera detected and the bacterial culture and qPCR assay results, with bacterial concentrations at different data point (T0, T21, T35), expressed in GE/μL
| Sample | Bacterial Genus | Bacterial Culture | qPCR | ||
|---|---|---|---|---|---|
| GE/μL | |||||
| T0 | T21 | T35 | |||
| 1 | Propionobacterium spp. | – | 21.58 | 6.46 | 11.40 |
| 2 | Propionobacterium spp. | – | 5.38 | 3.48 | 6.88 |
| 3 | Propionobacterium spp. | – | 9.84 | 23.25 | 26.78 |
| 4 | Propionobacterium spp. | – | 33.01 | 43.52 | 10.66 |
| 5 | Propionobacterium spp. | – | 6.03 | 4.91 | 11.25 |
| 6 | Propionobacterium spp. | – | 14.85 | 35.53 | 14.00 |
| 7 | Propionobacterium spp. | – | 7.06 | 9.15 | 7.26 |
| 8 | Enterococcus spp. | Positive (T0;T1) | 79.93 | 1.22 | 1.38 |
| 9 | Enterococcus spp. | – | 4.17 | 2.02 | 32.69 |
| 10 | Enterococcus spp. | – | 2.56 | 27.14 | 1.06 |
| 11 | Pseudomonas spp. | – | 3.93 | 1.20 | 49.23 |
| 12 | Pseudomonas spp. | – | 23.49 | 1.05 | 1.3 |
| 13 | Pseudomonas spp. | – | 0.95 | 3.89 | 1.99 |
| 14 | Pseudomonas spp. | – | 4.90 | 7.50 | 12.92 |
| 15 | Pseudomonas spp. | – | 4.12 | 48.43 | 3.19 |
| 16 | Pseudomonas spp. | – | 7.94 | 1.79 | 5.19 |
| 17 | Caulobacter spp. | – | 4.02 | 6.07 | 44.19 |
| 18 | Caulobacter spp. | – | 5.85 | 25.67 | 11.08 |
| 19 | Caulobacter spp. | – | 9.27 | 27.48 | 9.59 |
| 20 | Caulobacter spp. | – | 4.32 | 6.78 | 2.14 |
| 21 | Corynebacterium spp. | – | 4.45 | 3.74 | 4.90 |
| 22 | Corynebacterium spp. | – | 2.83 | 21.17 | 4.68 |
| 23 | Serratia spp. | – | 7.83 | 28.23 | 20.88 |
| 24 | Serratia spp. | – | 7.19 | 4.64 | 10.39 |
| 25 | Leucobacter spp. | – | 12.46 | 8.87 | 30.65 |
| 26 | Leucobacter spp. | – | 1.71 | 2.90 | 3.64 |
| 27 | Escherichia coli | Positive (T35) | – | – | – |
Time points considered positive are shown in italics. Samples positive both on bacterial culture and qPCR assay are shown in bolt.
After the sequences were aligned, they were assigned to Propionobacterium spp. (n = 7), Corynebacterium spp. (n = 2), Caulobacter spp. (n = 4), Pseudomonas spp. (n = 6), Enterococcus spp. (n = 3), Serratia spp. (n = 2), and Leucobacter spp. (n = 2). The bacterial loads varied from 4 to 80 GE/μL blood.
Discussion
In our study, with a biomolecular assay, we detected low numbers of GE from widespread bacteria in the WB units, and no transfused recipient had an immediate or delayed adverse transfusion effect. These results suggest that the WB units had good sterile shelf life in terms of their microbial content during their commercial storage.
Testing the quality of blood components commonly involves the removal of samples from WB units by methods that compromise unit sterility, such as inserting a coupler into a unit port and removing a sample with a needle and syringe. Because the aim of our study was to test for possible bacterial contamination, it was essential to maintain a closed blood collection system. Several previous studies22, 23, 24, 25 have assessed the utility of segments as a proxy for assessing some aspects of WB units during storage, without reducing the expiration time of the units. The segments used in our study were designed specifically for bacteriological and biomolecular tests. “Stripping” the tubing during production has been shown to ensure that a more representative sample remains because the blood in the tube is mixed with that in the whole units before the segments are prepared.26, 27
Blood products usually are visually inspected before administration, and bacterial contamination should be suspected when there is any obvious change in color, hemolysis just above the red cell mass, or visible clots. With any of these findings, bacteriological culture must be performed to determine whether or not contamination has occurred and the unit should therefore not be administered.4 In our study, no marked visible color change, hemolysis, or clots were noted in any WB unit (either those administered to recipients or those not transfused) or in any segments during the period of storages. In our previous study,13 we showed that the bacterial load associated with a color change in blood bags during their shelf lives ranged from 1.18 × 107 to 5.22 × 108 GE/mL, suggesting that a gross color change can be observed with this extent of bacterial contamination. However, a color change is not always evident when contamination occurs, and this method is not sufficiently sensitive to detect contaminants present in lower quantities.28
Although the bacterial culture method is considered the gold standard for assessing the presence of contaminants in blood units at most blood centers, cultures of units may be considered negative when low bacterial concentrations are not detected by standard culture methods. The level of contamination at the time of collection is estimated to be relatively low, at approximately 1–10 colony forming units/mL or less.9, 29 However, once the product is contaminated, the inoculated bacterial can proliferate within hours to reach numbers high enough to cause sepsis.12 Moreover, by definition, the process of bacterial culture is slow because the microorganisms require time to develop and reach an appreciable number of cells, and up to 48 hours is required to yield positive results for most microorganisms.14, 30 For this reason, a qPCR assay was performed at T0, T21, and T35, because its analytical sensitivity is higher and the time required for a definitive result much shorter.30
In our study, the percentage of negative bacteriological units was high (47/49, 96%), and among these 22 (22/49, 45%) were negative on both bacteriological and qPCR assays. These results suggest that the blood bags have a good sterile shelf life. The bacteria identified in the 2 culture positive units were Enterococcus spp. (1 unit at T0 and T1) and E. coli (1 unit at T35). We attribute this single example of E. coli growth at T35 (day 35) to a contaminant introduced during laboratory procedures. Supporting this hypothesis is the fact that the same sample was negative on the qPCR assay at all time points.
In the other case, Enterococcus spp. was identified with both the culture and qPCR assays at T0 (room temperature) and in culture at T1 (1 day after collection, 4°C). At all subsequent time points, the unit was negative on both assays. This result can be explained most convincingly by the bactericidal effect of blood, which is attributed to phagocytosis that occurs during the first hours after collection. The antimicrobial activities of blood have been shown to be specifically attributable to leukocytes and serum complement, which explain its phagocytic activity and bacterial removal or killing.8, 31 Different contaminating bacterial species are differentially sensitive to the bactericidal activity of donor blood. The antimicrobial effects of serum and phagocytes play a major role in the clearance of some species of bacteria (eg, Yersinia enterocolitica, Enterococcus faecalis). Conversely, other species (eg Pseudomonas aeruginosa, Serratia spp., Staphylococcus epidermidis) seem less sensitive or completely insensitive to this bactericidal activity.8, 31 Other reports32 have indicated that rapid storage at 4°C is required to avoid these contaminants because this procedure is known to have an excellent antimicrobial effect, and most species of bacteria are unable to grow easily at this temperature. In our study, the time between collection and storage of all blood units was always ≤2 hours and could explain the good results obtained.
The percentage of units that was only positive on qPCR was unexpectedly high (26/49, 53%). The 16S rDNA primer pair used in our study has some limitations, including the detection of laboratory contamination and dead or degraded bacterial DNA, leading to possible false‐positive results (low specificity).12, 33, 34 Alternatively, bacterial DNA can contaminate the PCR reagents. There are several recognized ways to minimize this contamination, including ultraviolet irradiation, enzymatic master mix filtration, and good operating practices that minimize exogenous contaminants. There are also more unconventional techniques, such as decreasing the number of thermal cycles, which obviously also decreases detection sensitivity. The basic issue remains balancing high sensitivity against poor specificity.14 The advantage of a real‐time PCR assay over to bacteriology is its very high sensitivity, rapid detection, quantification of fastidious bacteria with reproducible results, and elimination of postamplification handling. The presence of microbial DNA however does not necessarily mean that the bacterium identified was responsible for an infection.
With qPCR, 3 samples (3/49, 6%) were positive at T0 and negative thereafter; 7 samples (7/49, 14%) were negative at T0 and positive only once thereafter; but 16 WB samples (16/49, 32%) were positive at 2 or 3 of the tested time points. These results are interesting and, if carefully analyzed, may be relevant for the management of blood banks.
The bacteria identified in the units that were positive on qPCR only at T0 (immediately after blood collection, at room temperature) were Pseudomonas spp. (2 samples) and Enterococcus spp. (1 sample). The Enterococcus spp. also was identified on bacterial culture, whereas the other 2 were not identified with that technique, probably because the bacterial load was low (unit # 12 = 23.49 GE/μL; unit # 16 = 7.94 GE/μL). Although it was not possible to determine the actual contamination source of these microorganisms, they were probably skin contaminants from the donor dogs or contaminants introduced during the processing of the units, because they were already present at T0. It is likely that the failure to identify Pseudomonas spp. in the positive units with qPCR at subsequent time points is attributable to residual phagocytic activity of blood leukocytes. One of these 2 units (# 16) was administered on day 23 of storage to a recipient at our veterinary teaching hospital, and no transfusion reaction was detected.
Although most major blood banks for humans are moving toward leukoreduced products, the usefulness of leukocytes in stored units to decrease the bacterial load has not been studied in veterinary medicine. Data from the human literature indicate that the effect of blood leukofiltration in decreasing the bacterial load is controversial. One study35 reported an overall reduction, whereas others31, 36 suggest that the effects vary greatly for different bacterial species, for strains of the specific species, and even according to the physical properties of the filter. Also, filtration may remove opsonins and growth factors useful in the bactericidal activity of the blood.31, 36 The results of our study suggest that leukocytes are useful in the phagocytosis of bacteria in the first hours or days after blood collection and that leukofiltration should not be performed immediately. However, further studies are required on this issue in veterinary medicine.
The bacteria identified by qPCR in the 7 samples (7/49, 14%) negative at T0 and positive only once thereafter were Pseudomonas spp. in 2 units, Enterococcus spp. in 2 units, Leucobacter sp. in 1 unit, and Caulobacter spp. in 2 units. The source of these bacteria was thought to be contamination during laboratory procedure. The clinical relevance of these PCR positive results is likely not important because units were administered after different periods of time in storage to recipients at our veterinary teaching hospital and no transfusion reactions were detected.
The bacteria detected in the 16 WB samples that were only positive by qPCR at 2–3 of the time points tested were identified as Propionobacterium spp. in 7 units, Caulobacter spp. in 2 units, Pseudomonas spp. 2 units, Serratia spp. in 2 units, Corynebacterium spp. 2 units, and Leucobacter sp. in 1 unit.
Most of these microorganisms are saprophytic bacteria found in diverse environments, such as the skin flora, soil, and water. Except for Propionobacterium spp., Pseudomonas spp., and Serratia spp., the bacterial species detected are not commonly involved in transfusion‐associated bacterial contamination. In our study, we did not detect these bacteria on bacteriological culture, perhaps because Propionobacterium spp. have a prolonged lag phase and require 4–6 days to be detected with a bacteriological assay.30 These anaerobic, Gram‐positive microorganisms are of questionable clinical relevance and generally proliferate poorly during storage at 1–6°C.10 Systematic studies of the outcomes of human patients transfused with Propionobacterium spp‐contaminated units indicating that these bacteria are not relevant to transfusion risk.29 These bacteria usually are not virulent and only a few cases have been associated with transfusion‐related sepsis, although the bacterium was not isolated from the patients and the cause‐and‐effect relationship was not confirmed.37 Approximately 80% of transfusion‐associated sepsis involves psychrophilic bacteria capable of growth at refrigeration temperatures, including the Gram‐negative contaminants Pseudomonas spp. and Serratia spp., which have been implicated in blood bag contamination.12, 13, 29, 38, 39, 40 A study has shown that when blood units were inoculated with 1–10 cfu/mL of S. liquefaciens and stored at 4°C, concentrations of 109 cfu/mL were reached after 14 days in storage.41 This study shows the excellent capacity of this microorganism to grow at refrigeration temperature (4°C). In our study, the bacteriological assay was always negative and the numbers of bacterial GE/μL remained constantly low (<48 GE/μL) at T0, T21, and T35 in all 16 WB units. This result probably means that the microorganisms identified were dead or degraded, so that the refrigeration temperature probably killed the bacteria or inhibited their growth.40 Confirming this hypothesis, no transfusion reactions were detected when we administered 7 of these units (2 positive for Propionobacterium spp., 1 positive for Pseudomonas sp., 1 positive for Serratia sp., 1 positive for Caulobacter sp., 1 positive for Corynebacterium sp., and 1 positive for Leucobacter sp.) to recipients after different periods in storage at our veterinary teaching hospital. These concentrations of bacterial GE were probably too low to cause sepsis immediately after transfusion and therefore were not clinically relevant.
The detection of bacteria in blood with real‐time PCR has been described in only a few studies in veterinary medicine.12, 13 Our study was performed with a large number of WB units, collected in a month (August) considered to represent a high risk of contamination of blood units.11 We demonstrated that the qPCR method was sensitive enough to detect the low numbers of bacterial cells present at the beginning of blood product storage. However, this method also has been shown to produce false‐positive results in the presence of dead or degraded bacteria. Very small amounts of contaminants have no clinical relevance when transfused into a recipient, as shown by the transfusion in our hospital of blood units that tested positive.
It will be necessary to identify the cutoff for an acceptable bacterial load for blood products to allow the identification of products that can be transfused.
The outcome of a contaminated transfusion is highly dependent on the amount of bacteria transfused, the type of bacteria and its pathogenicity for dogs, the rate of transfusion, and the clinical status of the recipient.9 Because the risk of bacterial contamination causing serious septic complications increases toward the end of a blood product's shelf life, shorter storage periods will decrease the likelihood that units will contain large numbers of bacteria.42 Despite this, we have demonstrated that these units were tolerably safe from a microbiological point of view for up to 42 days. This is very important for the health of the recipient and for cost‐effectiveness.
In our study, it was not possible to determine the source of contamination with certainty, but all efforts were made to limit contamination. According to another study9, possible mechanisms of bacterial contamination in blood components include donor bacteremia, contamination during the WB collection procedure, contamination of the collection pack, and contamination during the blood processing procedure or storage.
As suggested by the guidelines to decrease the risk of donor bacteremia, we chose clinically healthy dogs that showed no clinical signs of disease indicative of bacteremia, such as diarrhea, hyperthermia, or cough. The donors were routinely screened to detect a possible risk of infection or transient bacteremia (eg, exclusion of animals that had recently undergone surgical procedures). Nevertheless, donor bacteremia can be present even in the absence of clinical signs suggestive of infection. In fact, in human medicine, a source of transfusion reaction seems to be bacteremia in the donor associated with asymptomatic gastroenteritis caused by Yersinia sp.43.
To avoid contamination during the WB collection procedure, the skin was prepared and disinfected. This practice obviously decreases the skin bacterial load, even if a sterile venipuncture cannot be guaranteed because organisms present in sebaceous glands and hair follicles are inaccessible.10, 44 In human medicine, the partial effectiveness of diverting the first 10 mL of blood from the initial collection into a separate receptacle has been shown to remove organisms present in the skin core obtained at venipuncture. Although this was not done in our study, the practice could be used to further decrease or avoid skin contaminants.
In our study, the possibility of unsterile blood bags was decreased by the introduction of aseptic integrally connected plastic containers. However, in Denmark and Sweden, an outbreak of Serratia marcescens contamination of blood seems to have been associated with sterile bag sets that were autoclaved but put into nonsterile outer plastic packages.45 Although there is now greater control on the sterility of blood bags produced by manufacturers, and this source of contamination is unlikely, it is always advisable to check the integrity of the bags and the expiration date before use, as we did in our study.
To avoid contamination from the environment during the blood processing procedure and storage, we closed the blood bags in a sterile manner with a tube sealer system immediately after collection, and the units and their segments were maintained at a constant temperature of 4 ± 2°C within 2 hours after collection in the blood bank refrigerator.46
In conclusion, in our study, the extent of bacterial contamination of the blood bags was low and therefore not clinically relevant. Most of the organisms detected tended to be widespread bacteria, present in soil and water, and except for Enterococcus spp., Propionobacterium spp., Pseudomonas spp., and Serratia spp., are only weakly virulent and not usually implicated in contaminated blood‐associated septic transfusion reactions. They also often were considered dead or degraded bacteria or contaminants related to laboratory procedures. The detection of bacteria in blood units with culture‐based and molecular methods does not necessarily mean that these bacteria propagate in this environment or that they cause sepsis after transfusion. Nevertheless, patients requiring blood transfusion can be seriously debilitated or immunocompromised and critically ill, and the risk of transmitting bacteria that may cause a transfusion reaction cannot be completely excluded.47, 48
The high efficiency of qPCR in screening blood components has been demonstrated, and molecular genetic sterility testing in veterinary blood banking also is recommended as a rapid method, particularly if the blood product has limited durability (eg, fresh WB, platelet concentrate) or is required immediately for patient treatment. Additional studies are needed to improve the applicability of qPCR to routine screening for contamination by transfusion services, especially given the favorable relationship between its cost and benefits. Possible future clinical trials in veterinary medicine should also examine the efficacy of various strategies decreasing the contamination of blood units before transfusion, such as diversion of the initial blood drawn, removal with leukofiltration of leukocytes that could contain phagocytized bacteria8 or the decontamination of units with pathogen inactivation systems, such as irradiation (eg, ultraviolet A or ultraviolet B irradiation) or chemicals.46
Acknowledgments
The authors thank Dr. Elisa Sgariglia and Dr. Tordo Elena for their skillful technical assistance.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The work was done at the Department of Veterinary Medicine (University of Perugia). All the authors participated meaningfully in the study and have seen and approved the final manuscript.
Footnotes
Snap 4DX, IDEXX Laboratories Inc., Westbrook, ME
IDEXX Laboratories Inc.
Baxter Healthcare Corp., Fenwal Division, Deerfield, IL
Hemoweld, Delcon, Italy
Qiagen, Hilden, Germany
NanoDrop 2000 spectrophotometer (Thermo Scientific, Middlesex, MA, USA)
SYBR Green chemistry
Bio‐Rad, Hercules, CA
Promega Corporation, Madison, WI
References
- 1. Wang Y, Giebink A, Spence DM. Microfluidic evaluation of red cells collected and stored in modified processing solutions used in blood banking. Integr Biol (Camb) 2014;6:65–75. [DOI] [PubMed] [Google Scholar]
- 2. Davidow B. Transfusion medicine in small animals. Vet Clin North Am Small Anim Pract 2013;43:735–756. [DOI] [PubMed] [Google Scholar]
- 3. Tocci LJ, Ewing PJ. Increasing patient safety in veterinary transfusion medicine: an overview of pretransfusion testing. J Vet Emerg Crit Care San Antonio 2009;19:66–73. [DOI] [PubMed] [Google Scholar]
- 4. Wardrop KJ, Reine N, Birkenheuer A, et al. Canine and feline blood donor screening for infectious disease. J Vet Int Med 2005;19:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kerl ME, Hohenhaus AE. Packed red blood cell transfusions in dogs: 131 cases (1989). J Am Vet Med Assoc 1993;202:1495–1499. [PubMed] [Google Scholar]
- 6. Callan MB, Oakley DA, Shofer FS, Giger U. Canine red blood cell transfusion practice. J Am Anim Hosp Assoc 1996;32:303–311. [DOI] [PubMed] [Google Scholar]
- 7. Hann L, Brown DC, King LG, Callan MB. Effect of duration of packed red blood cell storage on morbidity and mortality in dogs after transfusion: 3,095 cases (2001–2010). J Vet Intern Med 2014;28:1830–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Högman CF, Engstrand L. Serious bacterial complications from blood components—how do they occur. Transfus Med 1998;8:1–3. [DOI] [PubMed] [Google Scholar]
- 9. Hillyer CD, Josephson CD, Blajchman MA, et al. Bacterial contamination of blood components: risks, strategies, and regulation: joint ASH and AABB educational session in transfusion medicine. Hematology Am Soc Hematol Educ Program 2003;1:575–589. [DOI] [PubMed] [Google Scholar]
- 10. Brecher ME, Hay SN. Bacterial contamination of blood components. Clin Microbiol Rev 2005;18:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hohenhaus AE, Drusin LM, Garvey MS. Serratia marcescens contamination of feline whole blood in a hospital blood bank. J Am Vet Med Assoc 1997;15:794–798. [PubMed] [Google Scholar]
- 12. Kessler RJ, Rankin S, Young S, et al. Pseudomonas fluorescens contamination of a feline packed red blood cell unit and studies of canine units. Vet Clin Pathol 2010;39:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stefanetti V, Miglio A, Cappelli K, et al. Detection of bacterial contamination and DNA quantification in stored blood units in two veterinary hospital blood banks. Vet Clin Pathol 2016; DOI: 10.1111/vcp.12372. 45(3). Article in press (accepted for publication on 11th April 2016). [DOI] [PubMed] [Google Scholar]
- 14. Scherenzel J. Clinical relevance of new diagnostic methods for bloodstream infections. Int J Antimicrob Agents 2007;30S:S2–S6. [DOI] [PubMed] [Google Scholar]
- 15. Antognoni MT, Veronesi F, Morganti G, et al. Natural infection of Anaplasma platys in dogs from Umbria region (Central Italy). Vet Ital 2014;50:49–56. [DOI] [PubMed] [Google Scholar]
- 16. Italian Health Minister . 2007. Guide Line relating to the exercise of the health activity concerning the transfusion medicine in the veterinary field. Available at: http://www.anagrafecaninarer.it/acrer/Portals/0/files/linee%20guida%20trasfusioni.pdf. Accessed 28 April, 2015.
- 17. Ramirez‐Arcos S, Mastronardi C, Perkins H, et al. Evaluating the 4‐hour and 30‐minute rules: effects of room temperature exposure on red blood cell quality and bacterial growth. Transfusion 2013;53:851–859. [DOI] [PubMed] [Google Scholar]
- 18. Kisielewicz C, Self IA. Canine and feline blood transfusions: controversies and recent advances in administration practices. Vet Anaesth Analg 2014;41:233–242. [DOI] [PubMed] [Google Scholar]
- 19. Giger U. Transfusion medicine In: Silverstein DC, Hopper K, eds. Small Animal Critical Care Medicine. Elsevier, St Louis, 2009:281–286. [Google Scholar]
- 20. Lanevschi A, Wardrop KJ. Principles of transfusion medicine in small animals. Can Vet J 2001;42:447–454. [PMC free article] [PubMed] [Google Scholar]
- 21. Nadkarni MA, Martin FE, Jacques NA, Hunter N. Determination of bacterial load by real‐time PCR using a broad‐range (universal) probe and primers set. Microbiology 2002;148:257–266. [DOI] [PubMed] [Google Scholar]
- 22. Farrell SB, Shelat SG, Kim HC, Drew C. Alternative method to determine the hematocrit of red blood cell units: a potential use in the apheresis unit. Transfusion 2009;49:1255–1258. [DOI] [PubMed] [Google Scholar]
- 23. Weiskopf RB, Yau R, Sanchez R, et al. Microarray kit analysis of cytokines in blood product units and segments. Transfusion 2009;49:2269–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Canadian Standards Associations . CAN/CSA‐Z902‐10 Blood and Blood Components, 2010th ed Mississauga, ON: Canadian Standards Association; 2010. [Google Scholar]
- 25. Council of Europe . Guide to the Preparation, Use and Quality Assurance of Blood Components, 11th ed Strasbourg: Council of Europe Publishing; 2005. [Google Scholar]
- 26. Chen J, Garcez RB, Chong C, Carmen R. Red cell concentrate filtration systems: plasma color in high hematocrit segments are a poor predictor of hemolysis in stored red cell concentrates. Transfusion 2000;40:62S. [Google Scholar]
- 27. Garcez RB, Chen J, Chong C, Carmen RA. Whole blood filter systems: low haematocrit segments are a poor predictor of red cell storage hemolysis. Transfusion 2000;40:66S. [Google Scholar]
- 28. Kim DM, Brecher ME, Bland LA, et al. Visual identification of bacterially contaminated red cells. Transfusion 1992;32:221–225. [DOI] [PubMed] [Google Scholar]
- 29. Dreier J, Stormer M, Kleesiek K. Real‐time polymerase chain reaction in transfusion medicine: applications for detection of bacterial contamination in blood products. Transfus Med Rev 2007;21:237–254. [DOI] [PubMed] [Google Scholar]
- 30. Mohammadi T, Pietersz RN, Vandenbroucke‐Grauls CM, et al. Detection of bacteria in platelet concentrates: comparison of broad‐range real‐time 16S rDNA polymerase chain reaction and automated culturing. Transfusion 2005;45:731–736. [DOI] [PubMed] [Google Scholar]
- 31. Siblini L, Lafeuillade B, Ros A, et al. Influence of blood prestorage conditions and white blood cell filtration on the bacterial load of blood deliberately inoculated with Gram‐positive and Gram‐negative pathogens. Vox Sang 2004;87:241–249. [DOI] [PubMed] [Google Scholar]
- 32. Högman CF, Fritz H, Sandberg L. Posttransfusion Serratia marcescens septicemia. Transfusion 1993;33:189–191. [DOI] [PubMed] [Google Scholar]
- 33. Klouche M, Schroder U. Rapid methods for diagnosis of bloodstream infections. Clin Chem Lab Med 2008;46:888–908. [DOI] [PubMed] [Google Scholar]
- 34. Peters RPH, van Agtmael MA, Danner SA, et al. New developments in the diagnosis of bloodstream infections. Lancet Infect Dis 2004;4:751–760. [DOI] [PubMed] [Google Scholar]
- 35. Holden F, Foley M, Devin G, et al. Coagulase negative staphylococcal contamination of whole blood and its components: the effect of WBC reduction. Transfusion 2000;40:1508–1513. [DOI] [PubMed] [Google Scholar]
- 36. Heal JM, Cohen HJ. Do white cells in stored blood components reduce the likelihood of post transfusion bacterial sepsis? Transfusion 1991;31:581–583. [DOI] [PubMed] [Google Scholar]
- 37. Barrett BB, Anderson JW, Anderson KC. Strategies for the avoidance of bacterial contamination of blood components. Transfusion 1993;33:228–233. [DOI] [PubMed] [Google Scholar]
- 38. Boulton FE, Chapman ST, Walsh TH. Fatal reaction to transfusion of red‐cell concentrate contaminated with Serratia liquefaciens . Transfus Med 1998;8:15–18. [DOI] [PubMed] [Google Scholar]
- 39. Zavizion B, SerebryaniK D, Serebryanik I, et al. Prevention of Yersinia enterocolitica, Pseudomonas fluorescens, and Pseudomonas putida outgrowth in deliberately inoculated blood by a novel pathogen‐reduction process. Transfusion 2003;43:135–142. [DOI] [PubMed] [Google Scholar]
- 40. Szewzyk U, Szewzyk R, Stenstrom TA. Growth and survival of Serratia marcescens under aerobic and anaerobic conditions in the presence of materials from blood bags. J Clin Microbiol 1993;7:1826–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roth VR, Arduino MJ, Nobiletti J, et al. Transfusion‐related sepsis due to Serratia liquefaciens in the United States. Transfusion 2000;40:931–935. [DOI] [PubMed] [Google Scholar]
- 42. Wang D, Cortés‐Puch I, Sun J, et al. Transfusion of older stored blood worsens outcomes in canines depending on the presence and severity of pneumonia. Transfusion 2014;54:1712–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McDonald CP, Barbara JA, Hewitt PE, et al. Yersinia enterocolitica transmission from a red cell unit 34 days old. Transfus Med 1996;6:61–63. [DOI] [PubMed] [Google Scholar]
- 44. Wondimu H, Addis Z, Moges F, Shiferaw Y. Bacteriological safety of blood collected for transfusion at university of gondar hospital blood bank, northwest Ethiopia. ISRN Hematol 2013;20:308204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heltberg O, Skov F, Gerner‐Smidt P, et al. Nosocomial epidemic of Serratia marcescens septicemia ascribed to contaminated blood transfusion bags. Transfusion 1993;33:221–227. [DOI] [PubMed] [Google Scholar]
- 46. Kuehnert MJ, Roth VR, Haley NR, et al. Transfusion‐transmitted bacterial infection in the United States, 1998 through 2000. Transfusion 2001;41:1493–1499. [DOI] [PubMed] [Google Scholar]
- 47. Kou Y, Pagotto F, Hannach B, Ramirez‐Arcos S. Fatal false‐negative transfusion infection involving a buffy coat platelet pool contaminated with biofilm‐positive Staphylococcus epidermidis: a case report. Transfusion 2015;55:2384–2389. [DOI] [PubMed] [Google Scholar]
- 48. Rachoin JS, Daher R, Schorr C, et al. Microbiology, time course and clinical characteristics of infection in critically ill patients receiving packed red blood cell transfusion. Vox Sang 2009;97:294–302. [DOI] [PubMed] [Google Scholar]


