Abstract
Background
Previous studies that included limited numbers of affected dogs have suggested basal cortisol concentrations ≤55 nmol/L (2 μg/dL) are sensitive, but nonspecific, for a diagnosis of hypoadrenocorticism. A detailed assessment of the diagnostic utility of basal cortisol concentrations is warranted.
Hypothesis/Objectives
To evaluate the utility of basal cortisol concentrations for the diagnosis of hypoadrenocorticism in a large number of dogs, including those with and without serum electrolyte abnormalities.
Animals
Five hundred and twenty‐two dogs, including 163 dogs with hypoadrenocorticism, 351 dogs with nonadrenal gland illness, and 8 dogs with equivocal results.
Methods
Retrospective study. Basal and post‐ACTH cortisol concentrations and sodium and potassium concentrations were collected from medical records. A receiver operating characteristic (ROC) curve was constructed for basal cortisol concentrations by standard methodologies. Sensitivity, specificity, and predictive values were determined for various cut‐points.
Results
The area under the ROC curve was 0.988 and was similarly excellent regardless of serum electrolyte concentrations. At the most discriminatory cut‐point of 22 nmol/L (0.8 μg/dL), sensitivity and specificity were 96.9 and 95.7%, respectively. A basal cortisol concentration of ≤55 nmol/L (2 μg/dL) resulted in a sensitivity of 99.4%. Conversely, a basal cortisol concentration of ≤5.5 nmol/L (0.19 μg/dL) resulted in a specificity of 99.1%.
Conclusions and Clinical Importance
Similar to findings in previous studies, basal cortisol concentrations >55 nmol/L (2 μg/dL) are useful in excluding a diagnosis of hypoadrenocorticism. Interestingly, excellent specificities and positive predictive values were observed at lower cut‐point cortisol concentrations.
Keywords: ACTH, Addison's disease, Adrenal gland, Endocrinology
Abbreviations
- Na:K
sodium to potassium
- ROC
receiver operating characteristic
- MSU‐VMC
Michigan State University Veterinary Medical Center
- MSU‐DCPAH
Michigan State University Diagnostic Center for Population and Animal Health
- AUC
area under the curve
- IQR
interquartile range
- HA
hypoadrenocorticism
- NAI
nonadrenal illness
Hypoadrenocorticism is a well‐described endocrinopathy characterized by glucocorticoid deficiency, with or without evidence of concurrent mineralocorticoid deficiency.1 In dogs, most cases result from primary adrenal gland failure, which is thought to be a result of immune‐mediated destruction of the adrenal cortices.1, 2 Although many dogs with naturally occurring disease have classic serum electrolyte abnormalities (hyponatremia and hyperkalemia) suggestive of mineralocorticoid deficiency, a subset of affected animals has normal serum electrolyte concentrations.1, 3, 4, 5 The often vague and nonspecific clinicopathologic features of hypoadrenocorticism are easily confused with primary gastrointestinal, renal, or cardiovascular disease. Affected dogs can deteriorate rapidly, and without appropriate treatment, the disease is life‐threatening. As such, a timely and accurate diagnosis is critical.
The current standard for diagnosis of hypoadrenocorticism is the ACTH stimulation test, which involves measuring plasma or serum cortisol concentrations immediately before and 1 hour after IV administration of synthetic ACTH.1 Cosyntropin, the synthetic ACTH routinely used in stimulation testing, is expensive and not readily available at all veterinary practices. Given these limitations, coupled with the requirement for repeated venipuncture, alternative diagnostic testing is desirable. Recent studies have evaluated the cortisol‐to‐ACTH ratio, the aldosterone‐to‐renin ratio, and various hematologic and biochemical variables as potential means for diagnosing hypoadrenocorticism.6, 7, 8, 9 Because of a variety of limitations, these tests are not used routinely in clinical practice to diagnose hypoadrenocorticism.
Utilization of a single basal cortisol concentration for the diagnosis of hypoadrenocorticism would be desirable given that it is inexpensive, requires only 1 sample collection, avoids the need for synthetic ACTH, and is widely available. Two previous studies have evaluated basal cortisol concentrations for this purpose, both concluding that a cut‐off of ≤55 nmol/L (2 μg/dL) yielded excellent sensitivity but clinically unacceptable specificity.10, 11 The clinical conclusion was that basal cortisol concentrations >55 nmol/L (2 μg/dL) could be used to exclude a diagnosis of hypoadrenocorticism. However, both reports included very low numbers of affected dogs (13 and 14 dogs). Cortisol concentrations <28 nmol/L (1 μg/dL) were not quantitated, and only 2 cut‐points were investigated. Furthermore, test performance was not evaluated independently in 2 important populations of dogs, those with normal and abnormal serum electrolyte concentrations, which could represent various stages or forms of disease.
The primary aim of this study was to investigate the utility of basal cortisol concentrations for the diagnosis of hypoadrenocorticism in dogs using a higher number of affected animals and more evaluated cut‐points as compared to previous reports. Test performance was further evaluated in 2 subpopulations of dogs, those with normal and abnormal serum electrolyte concentrations.
Materials and Methods
Case Selection and Review
Medical records of all dogs undergoing ACTH stimulation testing at the Michigan State University Veterinary Medical Center (MSU‐VMC) between January 2005 and September 2015 were reviewed retrospectively. Dogs were excluded from the study if any of the following were known: ACTH stimulation testing was performed for a suspicion of hyperadrenocorticism, the dog previously had been diagnosed or treated for hyperadrenocorticism, polyuria, and polydipsia were the only clinical signs, the dog had received azole antifungal treatment in the 4 weeks preceding testing, or the dog had received exogenous glucocorticoids in the 4 weeks preceding testing. Dogs were included in the study if the ACTH stimulation test was performed for clinical suspicion of hypoadrenocorticism and no exclusion criteria were met. Information collected from the medical record included basal and post‐ACTH‐stimulated cortisol concentrations and sodium (Na) and potassium (K) concentrations. The reported electrolyte concentrations were obtained on admission to the MSU‐VMC and within 7 days of ACTH stimulation testing.
ACTH Stimulation Testing
The ACTH stimulation testing was performed as described elsewhere.1 Briefly, plasma or serum cortisol concentrations were measured immediately before and 1 hour after IV administration of synthetic ACTH.1 From the years 2005–2008, a standard 250 μg dose was used, whereas in the years 2008–2015, a 5 μg/kg dose (maximum dose of 250 μg) was used. All samples were evaluated at the Michigan State University Diagnostic Center for Population and Animal Health (MSU‐DCPAH). Dogs were included in the hypoadrenocorticism group if the post‐ACTH cortisol concentration was ≤55 nmol/L (2 μg/dL). Dogs were included in the nonadrenal illness group if the post‐ACTH cortisol concentration was >138 nmol/L (5 μg/dL). Dogs with post‐ACTH cortisol concentrations between 55 nmol/L (2 μg/dL) and 138 nmol/L (5 μg/dL) were considered to have equivocal results.10
Cortisol Assays
From January 2005 through November 24, 2014, cortisol was measured in serum or plasma samples with a commercially available radioimmunoassay kit.2 This assay has been commonly used in veterinary studies, and validation data for canine samples have appeared in several publications.12, 13 The radioimmunoassay was performed with reagent and sample volumes described in the manufacturer's protocol but with a 2‐hour incubation at 37°C. Specificity data provided by the manufacturer showed significant cross‐reactivity with prednisolone (76%), methylprednisolone (12%), and 11‐deoxycortisol (11.4%) but negligible (<2.5%) cross‐reactivity with other steroids tested. The assay manufacturer reported an assay sensitivity of 5.5 nmol/L but did not specify the method of determination. In our laboratory, the concentration of cortisol at the point of 2 standard deviations (SD) below the mean total binding of the 0 standard was 5.4 nmol/L (n = 25 assays). The mean ± SD concentration of cortisol at the point of 90% specific binding in these assays was 9.6 ± 0.7 nmol/L. When cortisol was added at amounts of 133, 266, 532, or 798 nmol/L to aliquots of a canine serum pool (97 nmol/L), 114, 117, 118, and 119% were recovered with the assay, respectively. Aliquots of a canine serum pool of 782 nmol/L were diluted at rates of 1:1, 1:3, and 1:7 in distilled water, “0” standard, and protein (0.25% gelatin)‐phosphate buffer to assess dilutional parallelism in different solutions. Recovery of cortisol in diluted samples was not affected by the diluent. The average recovery rates at those dilutions were 92, 94, and 82%, respectively. Assay repeatability was assessed with 3 pools of canine serum with mean concentrations of 8, 97, and 855 nmol/L. The respective intra‐assay % coefficients of variation (CV) for 10 assays of these pools were 12.3, 7.3, and 6.4%. In 10 assay runs, the respective interassay % CV for these pools were 12.7, 5.2, and 5.9%.
When the radioimmunoassay reagents were no longer available after November 2014, cortisol assays were performed on serum samples with a commercially available competitive chemiluminescent immunoassay3 that has been previously used for canine samples.14, 15, 16 The manufacturer reports substantial cross‐reactivity with prednisolone (62%), methylprednisolone (22%), and prednisone (6.1%) but negligible (<1.7%) cross‐reactivity with other steroids tested. The Immulite 2000 analyzer3 is Food and Drug Administration (FDA) approved for diagnostic cortisol testing in human medical laboratories with the default software specification that results below 1 μg/dL (27.6 nmol/L) are reported as <1 μg/dL. However, the manufacturer reports a lower analytical sensitivity of 5.5 nmol/L, similar to the radioimmunoassay. For veterinary medical laboratory use, the manufacturer allows modification of the data reduction program to report numerical results below 27.6 nmol/L. In 6 replicates of a cortisol‐free human serum calibrator provided by the manufacturer, the calculated concentration of cortisol at 2 SD below the mean total binding was 1.1 nmol/L. Another assessment was to look at replicate results of 2 canine serum samples used in assay repeatability studies. One sample had a mean cortisol concentration of 3.4 nmol/L with a range of 2.4–5.2 nmol/L in 15 replicates. Another sample had a mean cortisol concentration of 5.3 nmol/L with a range of 3.9–6.3 nmol/L in 15 replicates. A third sample with a reported cortisol concentration of 12 nmol/had a mean concentration of 13.7 nmol/L with a range of 10.2–15.5 nmol/L in 15 replicate assays, demonstrating the ability of the assay to reliably distinguish cortisol concentrations of <5.5 nmol/L from those in the range of 10–15 nmol/L. Two canine serum samples with cortisol concentrations of <5 nmol/L and 1037 nmol/L were mixed in respective volume combinations of 1:1, 1:2, 2:1, and 4:1 to assess parallelism. Cortisol assays were performed on the mixtures with % observed/expected recovery rates of 86, 86.1, 92.3, and 85.2%. Assay repeatability was assessed with 3 pools of canine serum with mean concentrations of 5, 39, and 632 nmol/L. The respective intra‐assay % CV for 5 assays of these pools were 15.0, 7.0, and 4.0%. Five replicates of each pool were run on 3 consecutive days, and the respective interassay % CV for the 3 daily means of each pool were 13.0, 6.0, and 1.3%.
In preparation for changing cortisol assays, 50 canine samples selected for a wide range of cortisol concentrations (<5–1352 nmol/L) on the radioimmunoassay were run using the chemiluminescence assay, with a 0.981 correlation of results. Five of the samples had radioimmunoassay results of <5 nmol/L, with corresponding results in the chemiluminescent assay. The 7 samples with radioimmunoassay results of 8–15 nmol/L had corresponding results of 9–18 nmol/L with the chemiluminescent assay. As such, cortisol concentrations from both assays, especially at low concentrations, were considered interchangeable in our laboratory. The in‐house assessment of both assays also supported the manufacturer reported sensitivities of 5.5 nmol/L, and all results below this were reported as ≤5.5 nmol/L.
Data Analysis
Using post‐ACTH‐stimulated cortisol concentrations as the diagnostic gold standard, an ROC curve for basal cortisol concentrations was constructed, and the area under the curve (AUC) was calculated by commercially available software.4 The same methodologies were applied to 2 subsets of dogs, those with normal and abnormal serum electrolyte concentrations. Sensitivity and specificity of various basal cortisol concentration cut‐off values for the diagnosis of hypoadrenocorticism were calculated. Positive and negative predictive values were calculated using an estimated disease prevalence of 21% at the MSU‐VMC (see Discussion), as well as 2 previously reported prevalence rates of 3 and 15%.10, 11
Dogs were considered to have abnormal electrolyte profiles if any of the following criteria were met: Na:K ratio < 28, or Na < 143 mmol/L (reference interval [RI], 143–149 mmol/L) in the presence of K > 4.3 mmol/L (RI, 3.4–5.2 mmol/L), or K > 5.2 mmol/L in the presence of Na < 146 mmol/L. All other dogs were considered to have normal electrolyte profiles. This comprehensive approach employing both Na:K ratio and absolute serum electrolyte concentrations was utilized to capture dogs with electrolyte abnormalities or trends consistent with mineralocorticoid deficiency.17, 18, 19
Dogs with equivocal ACTH stimulation test results initially were excluded from analysis similar to a previous report.11 However, these dogs have a subnormal response to exogenous ACTH and could represent an early stage of hypoadrenocorticism. The authors deemed it prudent to consider this alternate possibility. Therefore, sensitivities and specificities of basal cortisol concentrations at various cut‐points also were calculated when cases with equivocal ACTH stimulation test results were included in the hypoadrenocorticism group.
Statistical Analysis
Cortisol concentrations were reported as median and interquartile range (IQR). Comparisons of cortisol concentrations were made using the Mann‐Whitney U‐test. Areas under the ROC curves were compared by standard methodologies.20 , 4 Statistical analyses were performed by commercially available software.5 Cortisol concentrations below the assay sensitivity (5.5 nmol/L) were treated as 3 nmol/L for statistical comparisons. For all analyses, P ≤ .05 was considered significant.
Results
One thousand twenty‐three dogs underwent ACTH stimulation testing between January 2005 and September 2015, and 522 dogs met inclusion criteria after review of medical records. These included 163 dogs with hypoadrenocorticism, 351 dogs with nonadrenal gland illness, and 8 dogs with equivocal results. Twenty‐eight of 163 dogs (17%) with hypoadrenocorticism had normal electrolyte profiles, whereas 135 of 163 (83%) had abnormal electrolyte profiles. Of the dogs with nonadrenal gland illness, 275 of 351 (78%) had normal electrolyte profiles, whereas 76 of 351 (22%) had abnormal electrolyte profiles. The 8 dogs with equivocal results had normal electrolyte profiles.
The median basal cortisol concentrations in dogs with hypoadrenocorticism and nonadrenal gland illness were ≤5.5 nmol/L (IQR, ≤5.5–≤5.5 nmol/L) and 82 nmol/L (IQR, 46–156 nmol/L), respectively (Fig 1). Median ACTH‐stimulated cortisol concentrations in dogs with hypoadrenocorticism and nonadrenal gland illness were ≤5.5 nmol/L (IQR, ≤5.5–≤5.5 nmol/L) and 415 nmol/L (IQR, 342–545 nmol/L), respectively. Baseline and ACTH‐stimulated cortisol concentrations were significantly lower in dogs with hypoadrenocorticism than in dogs with nonadrenal gland illness (P < .001 and P < .001, respectively). In dogs with hypoadrenocorticism, no difference was observed in basal cortisol concentrations of dogs with normal electrolyte profiles as compared to those with abnormal electrolyte profiles (P = .254; Fig 2).
Figure 1.
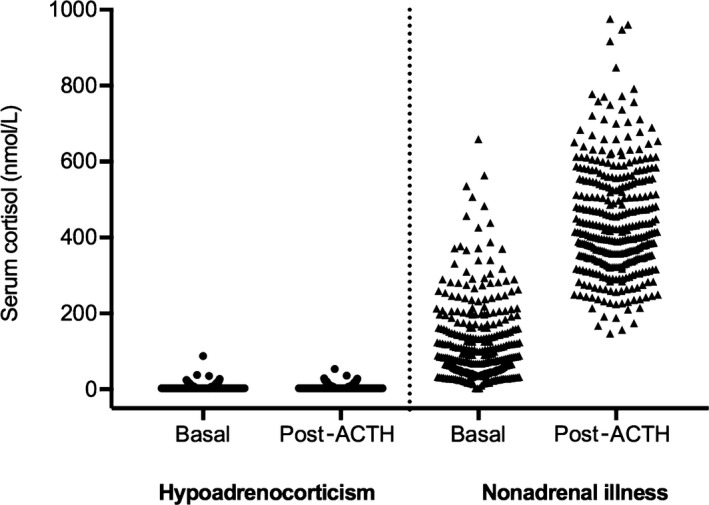
Scatter plot comparing basal and post‐ACTH‐stimulated serum or plasma cortisol concentrations (nmol/L) in dogs with hypoadrenocorticism (n = 163) and dogs with nonadrenal illness (n = 351).
Figure 2.
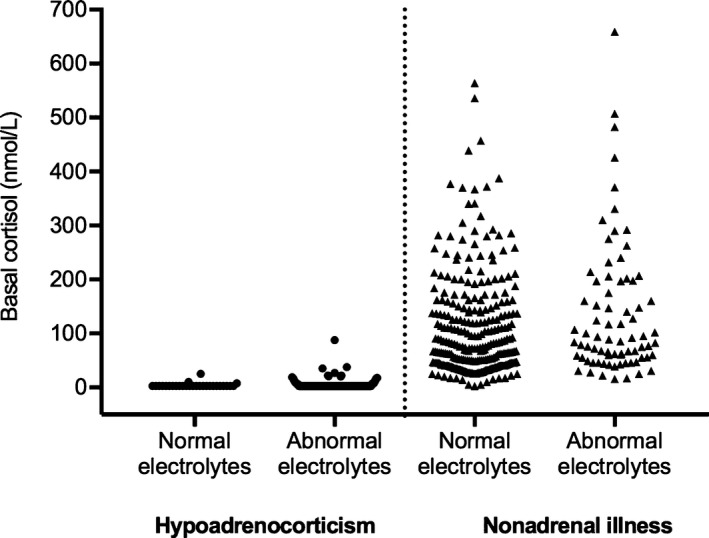
Scatter plot of basal serum or plasma cortisol concentrations (nmol/L) in dogs with hypoadrenocorticism or nonadrenal illness. Results are stratified into dogs with normal and abnormal electrolyte profiles. One hundred thirty‐five of 163 dogs with hypoadrenocorticism and 76 of 351 dogs with nonadrenal illness had abnormal electrolyte profiles.
Commercially available software4 was used to construct an ROC curve for basal cortisol concentrations (Fig 3). The ROC AUC was 0.988 (95% confidence interval [CI], 0.979–0.997). An optimal cut‐point identified from the ROC curve was a basal cortisol concentration of 22 nmol/L, with corresponding sensitivity and specificity of 96.9 and 95.7%, respectively. At a cut‐point of ≤5.5 nmol/L, sensitivity and specificity were 81.6 and 99.1%, respectively. At a cut‐point of ≤55 nmol/L, sensitivity and specificity were 99.4 and 67.0%, respectively. The sensitivity and specificity of various basal cortisol concentrations were summarized (Table 1). At the MSU‐VMC prevalence rate of 21%, the negative predictive value at a cut‐point of ≤55 nmol/L was 99.8%. At the lower cut‐point of ≤5.5 nmol/L, the positive predictive value was 96.0%. The negative and positive predictive values of additional cut‐points at the MSU‐VMC prevalence rate as well as the 2 previously reported prevalence rates10, 11 were summarized (Table 2).
Figure 3.
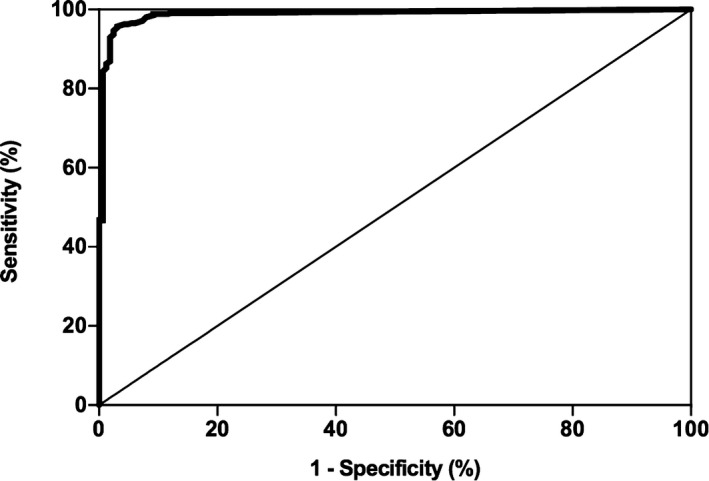
Receiver operating characteristic (ROC) curve (dogs with hypoadrenocorticism versus dogs with nonadrenal illness) for serum or plasma basal cortisol concentrations for the diagnosis of hypoadrenocorticism. The thin diagonal line represents a completely uninformative test, wherein the area under the curve (AUC) is 50%. The area under the ROC curve represented by the thicker line is 98.8% (95% CI, 0.979–0.997). The overall most accurate cut‐point identified on the curve was 22 nmol/L, which had a sensitivity and specificity of 96.9 and 95.7%, respectively.
Table 1.
Calculated sensitivities and specificities of various serum or plasma basal cortisol concentrations for the diagnosis of hypoadrenocorticism in dogs
| Basal Cortisol (nmol/L) | Dogs with HA (total n = 163) | Dogs with NAI (total n = 351) | % Sensitivity (95% CI) | % Specificity (95% CI) |
|---|---|---|---|---|
| ≤5.5 | 133 | 3 | 81.6 (74.8–87.2) | 99.1 (97.5–99.8) |
| ≤10 | 144 | 4 | 88.3 (82.4–92.8) | 98.9 (97.1–99.7) |
| ≤22 | 158 | 15 | 96.9 (93.0–99.0) | 95.7 (93.0–97.6) |
| ≤28 | 160 | 30 | 98.2 (94.7–99.6) | 91.5 (88.0–94.2) |
| ≤40 | 162 | 61 | 99.4 (96.6–100) | 82.6 (78.2–86.4) |
| ≤55 | 162 | 116 | 99.4 (96.6–100) | 67.0 (61.8–71.8) |
The number of dogs with hypoadrenocorticism and nonadrenal illness with basal cortisol concentrations ≤ the listed cut‐point are presented in the first 2 columns. The calculated sensitivity and specificity (and their respective 95% confidence intervals) are presented in the latter 2 columns. HA, hypoadrenocorticism; NAI, nonadrenal illness; CI, confidence interval.
Table 2.
Positive and negative predictive values of various basal cortisol concentrations for the diagnosis of hypoadrenocorticism in dogs
| Basal Cortisol (nmol/L) | Positive Predictive Value (%) | Negative Predictive Value (%) | ||||
|---|---|---|---|---|---|---|
| 3% Prevalence | 15% Prevalence | 21% Prevalence | 3% Prevalence | 15% Prevalence | 21% Prevalence | |
| ≤5.5 | 73.7 | 94.1 | 96.0 | 99.4 | 96.8 | 95.3 |
| ≤22 | 41.1 | 79.9 | 85.7 | 99.9 | 99.4 | 99.1 |
| ≤55 | 8.5 | 34.7 | 44.5 | 100 | 99.8 | 99.8 |
When ROC curves were constructed for dogs with normal and abnormal electrolyte profiles, the ROC AUC were 0.997 (95% CI, 0.993–1.001) and 0.993 (95% CI, 0.985–1.001), respectively. This was not different between groups (P = .41). Sensitivities and specificities at various cut‐points were similar, and at the cut‐point of ≤22 nmol/L, sensitivities of 96.4% (normal electrolyte profile) and 97.0% (abnormal electrolyte profile) and specificities of 95.6% (normal electrolyte profile) and 96.0% (abnormal electrolyte profile) were nearly identical (Table 3).
Table 3.
Sensitivities and specificities of various cut‐points in 2 subpopulations
| Basal Cortisol (nmol/L) | Normal Electrolyte Profile | Abnormal Electrolyte Profile | ||
|---|---|---|---|---|
| % Sens (95% CI) | % Spec (95% CI) | % Sens (95% CI) | % Spec (95% CI) | |
| ≤5.5 | 89.3 (71.8–97.7) | 98.9 (96.9–99.8) | 80.0 (72.3–86.4) | 100 (95.3–100) |
| ≤22 | 96.4 (81.7–99.9) | 95.6 (92.5–97.7) | 97.0 (92.6–99.2) | 96.0 (88.9–99.2) |
| ≤55 | 100 (87.7–100) | 64.7 (58.8–70.3) | 99.3 (95.9–100) | 75.0 (63.7–84.2) |
The sensitivities and specificities (and their respective 95% confidence intervals) of basal cortisol concentrations for the diagnosis of hypoadrenocorticism in dogs with normal and abnormal electrolyte profiles. Sens, sensitivity; Spec, specificity; CI, confidence interval.
Eight of the 523 dogs in this study had equivocal results on the basis of their post‐ACTH‐stimulated cortisol concentrations. Four of these 8 dogs had basal cortisol concentrations ≤55 nmol/L (median, 13 nmol/L; range, ≤5.5–89 nmol/L). Median post‐ACTH cortisol concentration was 109 nmol/L (range, 61–133 nmol/L). Inclusion of these 8 dogs in the hypoadrenocorticism group had minimal impact on test performance. At a cut‐point ≤5.5 nmol/L, sensitivity and specificity were 79.5 and 99.1%, respectively. At a cut‐point of ≤55 nmol/L, sensitivity and specificity were 98.8 and 67.0%, respectively.
Discussion
In recent years, basal cortisol concentrations have been used as a screening test for hypoadrenocorticism in dogs in lieu of performing complete ACTH stimulation testing. Two previous studies have documented that basal cortisol concentrations ≤55 nmol/L are 100% sensitive for a diagnosis of hypoadrenocorticism; therefore, obtaining a value >55 nmol/L reliably excludes the diagnosis. This finding has considerable clinical applicability as many hypoadrenocorticism suspects could avoid the need for provocative testing with synthetic ACTH.10, 11 However, the inclusion of only 13 and 14 affected dogs in prior reports could markedly affect the calculated sensitivities and specificities if their populations were not an accurate representation of all dogs with hypoadrenocorticism, a limitation acknowledged by the authors of both studies. Cut‐points other than 28 nmol/L and 55 nmol/L were not investigated, and any result <28 nmol/L was not quantitated further. Despite this, both reports concluded that basal cortisol concentrations lacked acceptable specificity and positive predictive values. The present study was performed in part to address these limitations. The inclusion of 163 affected dogs represents a 10‐fold increase in the number of affected dogs as compared to previous reports. This large number facilitated an in‐depth analysis of basal cortisol concentrations. An encouraging finding was the similarly excellent sensitivity at a cut‐off value of ≤55 nmol/L, which was maintained at a cut‐off value of ≤40 nmol. The negative predictive value of 99.6% at this cut‐point confirms the previous findings that baseline cortisol concentrations >55 nmol/L reliably exclude a diagnosis of hypoadrenocorticism. The ability to accurately quantify cortisol concentrations <28 nmol/L also allowed a more detailed investigation of alternate cut‐points. Particularly interesting findings were the excellent specificities and positive predictive values observed at some of the low cut‐points.
The consideration of Na:K ratio and absolute electrolyte concentrations was a unique aspect of the present study. The diagnostic utility of basal cortisol concentrations was similar in dogs with and without electrolyte profile abnormalities. Comparable to the cases reported herein, previous reports have documented that approximately 20% of dogs with hypoadrenocorticism have normal serum electrolyte concentrations at the time of diagnosis.3, 4, 5, 8 This subset of dogs has been described as having “atypical” or “glucocorticoid deficient” hypoadrenocorticism by some authors.4, 5 Several etiologies have been proposed, including isolated destruction of the zonas fasciculata and reticularis with sparing of the zona glomerulosa, secondary hypoadrenocorticism (i.e, abnormal pituitary secretion of ACTH), or early stages of primary hypoadrenocorticism.4, 5 Also, previous fluid therapy or other endogenous mechanisms could have maintained normal serum electrolyte concentrations in some of these dogs.21 Regardless of etiology, this subset of dogs could represent an early stage or different form of hypoadrenocorticism. Furthermore, this subset of dogs may be more likely to undergo screening with measurement of basal cortisol concentration as opposed to complete ACTH stimulation testing given the lower index of suspicion for disease. As such, it was important to consider the diagnostic performance of basal cortisol concentrations in both subsets of dogs.
Also, interesting were dogs with equivocal ACTH stimulation test results. Overall, this finding was uncommon, accounting for approximately 1.5% of test results. These cases may be a result of nonadrenal gland disease processes that inhibited normal ACTH responsiveness, or may represent early cases of hypoadrenocorticism. Two of these dogs were diagnosed with nonendocrine disease, but follow‐up information was unavailable. One dog, a middle‐aged Portuguese water dog with intermittent gastrointestinal signs, had equivocal results upon repeat stimulation testing performed 1 month later. In terms of adrenal function, the ultimate status of these dogs is unknown. Even when these 8 dogs were included in the hypoadrenocorticism group, minimal impact on test performance was observed. It is important to note, however, that 1 of these dogs and 1 dog in the original hypoadrenocorticism group had basal cortisol concentrations >55 nmol/L. Although negative predictive values still approach 100% at this cut‐point, complete ACTH stimulation testing should be considered in rare cases in which a high index of suspicion remains despite a basal cortisol concentration >55 nmol/L. Additional investigations are needed to better characterize this uncommon but potentially important subset of dogs with equivocal ACTH stimulation test results.
Several reports have concluded that basal cortisol concentrations cannot be used as a confirmatory test for hypoadrenocorticism.10, 11 Previous studies evaluating serum cortisol concentrations in healthy dogs determined that there is episodic fluctuations in cortisol secretion throughout the day, and occasionally, cortisol concentrations can decrease below reference intervals.21, 22 In 2 separate reports, it was concluded that basal cortisol concentrations lacked acceptable specificities and positive predictive values and that concentrations ≤55 nmol/L provide little information about adrenal function.10, 11 In the aggregate, these reports have led to the conventional belief that basal cortisol concentrations have no confirmatory value for a diagnosis of hypoadrenocorticism. However, reports documenting episodic cortisol secretion and occasionally low basal cortisol concentrations were performed in healthy, acclimatized research dogs.22, 23 This population is very different from client‐owned dogs undergoing testing for hypoadrenocorticism, which are usually sick, or at the minimum, not healthy. A more recent report suggested that the finding of a basal cortisol concentration <6 nmol/L, even in healthy dogs, is extremely uncommon.24 As such, the presence of a markedly decreased basal cortisol concentration in a sick dog is abnormal. In the authors’ experience, it is exceedingly rare for dogs tested for hypoadrenocorticism to have basal cortisol concentrations ≤5.5 nmol/L unless hypoadrenocorticism is actually present. In 2 separate studies of pituitary‐adrenal function in dogs with critical illness, no dogs had basal cortisol concentrations below reference intervals.25, 26 In another study of critically ill dogs with diarrhea caused by parvovirus, the lowest basal cortisol concentration at hospital admission was 19 nmol/L, and the lowest measured basal cortisol concentration at any time point remained >10 nmol/L.27 Unfortunately, the 2 previous reports of basal cortisol concentrations for the diagnosis of hypoadrenocorticism did not evaluate cortisol concentrations <28 nmol/L, perhaps because of assay or laboratory limitations.10, 11 In the present study, cut‐points ranging from ≤5.5–10 nmol/L maintained excellent specificities. Even more clinically applicable, the positive predictive value at ≤5.5 nmol/L was extremely high. However, the translation of these results into clinical practice is not straightforward. The consequences of false positives must be considered, which would include unnecessary lifelong glucocorticoid and possibly mineralocorticoid treatment. In such cases, the cost of treatment would far outweigh the cost of diagnosis. Beyond just cost and the need for frequent veterinary monitoring, unnecessary treatment could have adverse health effects. Additional investigations are needed to substantiate our findings and evaluate the potential benefits and risks of utilizing these low cut‐points in a clinical setting.
Diagnostic sensitivity and specificity are inherent characteristics of a test, which are not influenced by disease prevalence.28, 29 However, prevalence is a major determinant of positive and negative predictive values, which are likely to be of greater clinical utility than sensitivity or specificity. A precise assessment of prevalence for our study period was difficult because several clinicians at MSU‐VMC began using basal cortisol concentrations as a screening test shortly after a previous publication.10 This precluded many dogs from undergoing ACTH stimulation testing, and a prevalence of 31% (163 of 522 dogs) in this study could be a substantial overestimation. We elected to determine the prevalence rate for the years 2005–2007 during which the ACTH stimulation test was the only utilized diagnostic test for hypoadrenocorticism. This resulted in a prevalence of 21%. However, over 750 dogs underwent measurement of basal cortisol concentrations without subsequent ACTH stimulation testing between 2008 and 2015, which could suggest increased screening for hypoadrenocorticism in a low‐risk population of dogs. One hundred and fifty of these dogs had basal cortisol concentrations <55 nmol/L. It is likely that some of these dogs had hypoadrenocorticism, whereas others did not. However, ACTH stimulation testing was not performed at our institution, and definitive classification was not possible. The remaining 600 dogs had basal cortisol concentrations >55 nmol/L, essentially excluding a diagnosis of hypoadrenocorticism. As such, a prevalence of 21% still may be an overestimation. Even if all of the 750 dogs solely undergoing measurement of basal cortisol concentrations were assumed to have nonadrenal illness, the prevalence rate would decrease to 12.5%, a rate similar to that previously reported.10 Considering this, the prevalence at MSU‐VMC is likely somewhere between 12.5 and 21%, but an exact determination is not possible. To offset this limitation, predictive values also were calculated for 2 previously reported prevalence rates.10, 11 Regardless of prevalence rate (3–21%), the positive predictive values at cut‐points ≤5.5 nmol/L and the negative predictive values at cut‐points ≥40 nmol/L remained high.
Given that measurements of basal cortisol concentrations are used extensively to screen for hypoadrenocorticism in dogs, it is critical that clinicians use the test in an appropriate patient population and are aware of potential influencing factors. Widespread testing of an inappropriate or low‐risk patient population could increase the likelihood of erroneous results, and measurements of basal cortisol concentrations should only be performed if a clinical suspicion for hypoadrenocorticism truly exists. In our study, no dogs with nonadrenal illness and abnormal electrolyte profiles had basal cortisol concentrations ≤5.5 nmol/L (100% specificity). However, among dogs with nonadrenal illness and normal electrolyte profiles, 3 dogs (1%) had cortisol concentrations ≤5.5 nmol/L. In 2 of these cases, vague, intermittent gastrointestinal signs were present in otherwise healthy dogs, and there was no biochemical or hematologic evidence of hypoadrenocorticism. This finding highlights the importance of appropriate patient testing and cautious application of results. It is also important to consider the many factors that can influence cortisol concentrations. For instance, azole antifungal agents and both topical and system glucocorticoids can suppress endogenous cortisol concentrations, which could result in a low concentration in a normal dog.30, 31 Conversely, some glucocorticoids could cross‐react with the cortisol assay resulting in an erroneously high basal cortisol concentration in an animal with hypoadrenocorticism.32
One limitation of our study was its retrospective nature. If incorrect information or limited information was present in the medical record, cases may have been inappropriately classified. Medical records were available and thoroughly reviewed for every case, and the overall impact of this limitation was likely minimal. Another potential limitation of our study relates to measurement of cortisol concentrations. The methodology at MSU‐DCPAH changed from radioimmunoassay to a chemiluminescent assay in 2014. Despite this, paired comparisons of multiple samples identified excellent correlation between the 2 methods, especially at low concentrations. However, cortisol concentrations obtained from different laboratories are not necessarily interchangeable.15 The exact reasons for this are unknown but could be due to different assays, reagents, or laboratory techniques. Also, some laboratories do not quantify cortisol concentrations <28 nmol/L, and some assays are unable to quantify the lower cut‐points evaluated in our study.33 For example, these lower cut‐points are less than the dynamic ranges reported for the commonly used in‐house cortisol ELISA.6 Therefore, the results from this study may not be applicable to all assays and laboratories unless they have undergone similar investigations.
In summary, the inclusion of 163 dogs with hypoadrenocorticism in the current report allowed a more detailed assessment of basal cortisol concentrations than has been reported previously. Confirming previous findings, basal serum or plasma cortisol concentrations ≤55 nmol/L are extremely sensitive for hypoadrenocorticism, and as such, concentrations >55 nmol/L are useful to exclude the diagnosis. Test performance did not appear to be influenced by normal or abnormal serum electrolyte concentrations. A new finding was that basal cortisol concentrations ≤5.5 nmol/L had excellent specificities and positive predictive values in this study population. However, additional investigations are needed to corroborate these findings and determine their clinical utility.
Acknowledgments
This work was supported by the Michigan State University College of Veterinary Medicine Trinket Fund.
Conflict of Interest Declaration: Cortisol assays were performed at the Michigan State University Diagnostic Center for Population and Animal Health. The laboratory offers commercial services. This did not affect the study as it was retrospective in nature. All authors were Michigan State University employees at the time of study completion. The authors declare that they have no additional conflict of interests.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Work: This work was performed at the Michigan State University College of Veterinary Medicine.
Prior presentation: Portions of this work were presented in poster form at the 2016 ACVIM Forum, Denver, CO.
Footnotes
Cortrosyn, Amphastar Pharmaceuticals Inc., Rancho Cucamonga, CA
Coat‐a‐Count Cortisol, Siemens Medical Solutions Diagnostics, Los Angeles, CA
Immulite® 2000 Cortisol, Siemens Healthcare Diagnostics Ltd., Gwynedd, UK
GraphPad Prism 7, GraphPad Software, San Diego, CA
SigmaStat 4.0, Systat Software, Inc., San Jose, CA
IDEXX SNAP® Cortisol Test product insert; available at: www.idexx.com/resource-library/smallanimal/snap-cortisol-insert-en.pdf
References
- 1. Peterson ME, Kintzer PP, Kass PH. Pretreatment clinical and laboratory findings in dogs with hypoadrenocorticism: 225 cases (1979–1993). J Am Vet Med Assoc 1996;208:85–91. [PubMed] [Google Scholar]
- 2. Scott‐Moncrieff JC. Hypoadrenocorticism In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 7th ed volume 2 St. Louis, MO: Saunders; 2010:1847–1857. [Google Scholar]
- 3. Sadek D, Schaer M. Atypical Addison's disease in the dog: A retrospective survey of 14 cases. J Am Anim Hosp Assoc 1996;32:159–163. [DOI] [PubMed] [Google Scholar]
- 4. Lifton SJ, King LG, Zerbe CA. Glucocorticoid deficient hypoadrenocorticism in dogs: 18 cases (1986–1995). J Am Vet Med Assoc 1996;209:2076–2081. [PubMed] [Google Scholar]
- 5. Thompson AL, Scott‐Moncrieff JC, Anderson JD. Comparison of classic hypoadrenocorticism with glucocorticoid‐deficient hypoadrenocorticism in dogs: 46 cases (1985–2005). J Am Vet Med Assoc 2007;230:1190–1194. [DOI] [PubMed] [Google Scholar]
- 6. Javadi S, Galac S, Boer P, et al. Aldosterone‐to‐renin and cortisol‐to‐adrenocorticotropic hormone ratios in healthy dogs and dogs with primary hypoadrenocorticism. J Vet Intern Med 2006;20:556–561. [DOI] [PubMed] [Google Scholar]
- 7. Boretti FS, Meyer F, Burkhardt WA, et al. Evaluation of the cortisol‐to‐ACTH ratio in dogs with hypoadrenocorticism, dogs with diseases mimicking hypoadrenocorticism and in healthy dogs. J Vet Intern Med 2015;29:1335–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lathan P, Scott‐Moncrieff JC, Wills RW. Use of the cortisol‐to‐ACTH ratio for diagnosis of primary hypoadrenocorticism in dogs. J Vet Intern Med 2014;28:1546–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seth M, Drobatz KJ, Church DB, et al. White blood cell count and the sodium to potassium ratio to screen for hypoadrenocorticism in dogs. J Vet Intern Med 2011;25:1351–1356. [DOI] [PubMed] [Google Scholar]
- 10. Lennon EM, Boyle TE, Hutchins RG, et al. Use of basal serum or plasma cortisol concentrations to rule out a diagnosis of hypoadrenocorticism in dogs: 123 cases (2000–2005). J Am Vet Med Assoc 2007;231:413–416. [DOI] [PubMed] [Google Scholar]
- 11. Bovens C, Tennant K, Reeve J, et al. Basal serum cortisol concentration as a screening test for hypoadrenocorticism in dogs. J Vet Intern Med 2014;28:1541–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kemppainen RJ, Thompson FN, Lorenz MD. Use of a low dose synthetic ACTH challenge test in normal and prednisone‐treated dogs. Res Vet Sci 1983;35:240–242. [PubMed] [Google Scholar]
- 13. Watson AD, Church DB, Emslie DR. Plasma cortisol concentrations in dogs given cortisone or placebo by mouth. Res Vet Sci 1993;55:379–381. [DOI] [PubMed] [Google Scholar]
- 14. Proverbio D, Groppetti D, Spada E, et al. Comparison of the VIDAS and IMMULITE‐2000 methods for cortisol measurement in canine serum. Vet Clin Pathol 2009;38:332–336. [DOI] [PubMed] [Google Scholar]
- 15. Russell NJ, Foster S, Clark P, et al. Comparison of radioimmunoassay and chemiluminescent assay methods to estimate canine blood cortisol concentrations. Aust Vet J 2007;85:487–494. [DOI] [PubMed] [Google Scholar]
- 16. Midence JN, Drobatz KJ, Hess RS. Cortisol concentrations in well‐regulated dogs with hyperadrenocorticism treated with trilostane. J Vet Int Med 2015;29:1529–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zeugswetter FK, Schwendenwein I. Diagnostic efficacy of the leukogram and the chemiluminometric ACTH measurement to diagnose canine hypoadrenocorticism. Tierarztl Prax Ausg K Kleintiere Heimtiere 2014;42:223–230. [PubMed] [Google Scholar]
- 18. Nielsen L, Bell R, Zoia A, et al. Low ratios of sodium to potassium in the serum of 238 dogs. Vet Rec 2008;162:431–435. [DOI] [PubMed] [Google Scholar]
- 19. Adler JA, Drobatz KJ, Hess RS. Abnormalities of serum electrolyte concentrations in dogs with hypoadrenocorticism. J Vet Intern Med 2007;21:1168–1173. [DOI] [PubMed] [Google Scholar]
- 20. Hanley JA, McNeil BJ. The meaning and use of the area under a Receiver Operating Characteristic (ROC) curve. Radiology 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
- 21. Baumstark ME, Sieber‐Ruckstuhl NS, Müller C, et al. Evaluation of aldosterone concentrations in dogs with hypoadrenocorticism. J Vet Intern Med 2014;28:154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kemppainen RJ, Sartin JL. Evidence for episodic but not circadian activity in plasma concentrations of adrenocorticotrophin, cortisol, and thyroxine in dogs. J Endocrinol 1984;103:219–226. [DOI] [PubMed] [Google Scholar]
- 23. Johnston SD, Mather EC. Canine plasma cortisol (hydrocortisone) measured by radioimmunoassay: Clinical absence of diurnal variation and results of ACTH stimulation and dexamethasone suppression tests. Am J Vet Res 1978;39:1766–1770. [PubMed] [Google Scholar]
- 24. Höglund K, Lequarré AS, Ljungvall I, et al. Effect of breed on plasma endothelin‐1 concentration, plasma renin activity, and serum cortisol concentration in healthy dogs. J Vet Intern Med 2016;30:566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prittie JE, Barton LJ, Peterson ME, et al. Pituitary ACTH and adrenocortical secretion in critically ill dogs. J Am Vet Med Assoc 2002;220:615–619. [DOI] [PubMed] [Google Scholar]
- 26. Martin LG, Groman RP, Fletcher DJ, et al. Pituitary‐adrenal function in dogs with acute critical illness. J Am Vet Med Assoc 2008;233:87–95. [DOI] [PubMed] [Google Scholar]
- 27. Schoeman JP, Goddard A, Herrtage ME. Serum cortisol and thyroxine concentrations as predictors of death in critically ill puppies with parvoviral diarrhea. J Am Vet Med Assoc 2007;231:1534–1539. [DOI] [PubMed] [Google Scholar]
- 28. Martin SW. Estimating disease prevalence and the interpretation of screening test results. Prev Vet Med 1984;2:463–472. [Google Scholar]
- 29. Drobatz KJ. Measures of accuracy and performance of diagnostic tests. J Vet Cardiol 2009;11:33–40. [DOI] [PubMed] [Google Scholar]
- 30. Willard MD, Nachreiner R, McDonald R, et al. Ketoconazole‐induced changes in selected canine hormone concentrations. Am J Vet Res 1986;47:2504–2509. [PubMed] [Google Scholar]
- 31. Abraham G, Gottschalk J, Ungemach FR. Evidence for ototopical glucocorticoid‐induced decrease in hypothalamic‐pituitary‐adrenal axis response and liver function. Endocrinology 2005;146:3163–3171. [DOI] [PubMed] [Google Scholar]
- 32. Krasowski MD, Drees D, Morris CS, et al. Cross‐reactivity of steroid hormone immunoassays: Clinical significance and two‐dimensional molecular similarity prediction. Clinical Pathology 2014;14:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ginel PJ, Perez‐rico A, Moreno P. Validation of a commercially available enzyme‐linked immunosorbent assay (ELISA) for the determination of cortisol in canine plasma samples. Vet Res Comm 1998;22:179–185. [DOI] [PubMed] [Google Scholar]


