Abstract
Background
Apocrine gland adenocarcinoma of the anal sac (AGAAS) is associated with high rates of iliosacral lymph node metastasis, which may influence treatment and prognosis. Magnetic resonance imaging (MRI) recently has been shown to be more sensitive than abdominal ultrasound examination (AUS) in affected patients.
Objective
To compare the rate of detection of iliosacral lymphadenomegaly between AUS and computed tomography (CT) in dogs with AGAAS.
Animals
Cohort A: A total of 30 presumed normal dogs. Cohort B: A total of 20 dogs with AGAAS that underwent AUS and CT.
Methods
Using cohort A, mean normalized lymph node : aorta (LN : AO) ratios were established for medial iliac, internal iliac, and sacral lymph nodes. The CT images in cohort B then were reviewed retrospectively and considered enlarged if their LN : AO ratio measured 2 standard deviations above the mean normalized ratio for that particular node in cohort A. Classification and visibility of lymph nodes identified on AUS were compared to corresponding measurements obtained on CT.
Results
Computed tomography identified lymphadenomegaly in 13 of 20 AGAAS dogs. Of these 13 dogs, AUS correctly identified and detected all enlarged nodes in only 30.8%, and either misidentified or failed to detect additional enlarged nodes in the remaining dogs. Despite limitations in identifying enlargement in all affected lymph nodes, AUS identified at least 1 enlarged node in 100% of affected dogs.
Conclusion and Clinical Importance
Abdominal ultrasound examination is an effective screening test for lymphadenomegaly in dogs with AGAAS, but CT should be considered in any patient in which an additional metastatic site would impact therapeutic planning.
Keywords: Cancer staging, Dog, Lymph node, Metastasis
Abbreviations
- AGAAS
apocrine gland adenocarcinoma of the anal sac
- AUS
abdominal ultrasound
- CT
computed tomography
- HU
hounsfield units
- IILN
internal iliac lymph node
- LN : AO
ratio of the maximum diameter of the lymph node to the aorta
- MILN
medial iliac lymph node
- MRI
magnetic resonance imaging
- SLN
sacral lymph node
- TCSVM
Tufts Cummings School of Veterinary Medicine
Apocrine gland adenocarcinoma of the anal sac (AGAAS) represents 17% of perianal tumors and 2% of all skin tumors in the dog.1, 2, 3 Treatment can be challenging given its local invasiveness, rapid metastasis to regional lymph nodes, and association with paraneoplastic hypercalcemia.4 Reported metastatic rates in dogs with AGAAS are variable, with 36–96% of affected dogs having demonstrable lymph node metastasis at the time of diagnosis.4, 5, 6, 7, 8, 9 Distant metastatic sites, such as the lungs, liver, spleen, bone, and less frequently the heart, adrenal glands, pancreas, kidneys, and mediastinum have been reported to develop later in the course of disease.2, 5, 6, 7
The current standard clinical approach to staging a patient with AGAAS involves CBC, biochemistry profile, urinalysis, 3‐view thoracic radiographs, and abdominal ultrasound examination (AUS).10 These staging tests are important to identify regional or distant metastatic disease, which influences prognosis and helps identify patients in which treatment should include chemotherapy, radiotherapy, or both.6, 9, 11 Recently, magnetic resonance imaging (MRI) has been shown to be more sensitive than AUS in identifying lymphadenopathy in dogs with AGAAS, with AUS failing to detect 67% of lymphadenopathy identified on MRI.1 Conversely, another recent study comparing results of AUS and computed tomography (CT) in assessment found that significantly more nodes within the iliosacral lymphocenter can be identified using CT, but that CT was not superior to AUS in identifying abnormal lymph nodes.12 One major limitation of both of these recent studies is that lymph nodes were subjectively deemed abnormal on the imaging modalities before tissue sampling. Neither study established an objective quantitative means for advanced imaging classification of lymphadenomegaly.
The most common site of metastasis in dogs with AGAAS is the iliosacral lymphocenter.4 This lymphocenter is responsible for draining the pelvic and perineal region and is comprised of the medial iliac, internal iliac (previously termed hypogastric), and sacral lymph nodes. Ultrasonographic and CT features of these lymph nodes have been described in normal dogs.13, 14, 15, 16 In 1 CT study of 19 healthy dogs, these abdominal nodes were described as having an elongated shape (approximately 75% of nodes) with mean dimensions (length × width × thickness) of 22.8 mm × 6.7 mm × 4.6 mm for medial iliac and 10.3 mm × 4.8 mm × 3.7 mm for internal iliac and sacral lymph nodes combined.13 Ultrasonographically, these measurements vary with body size, but normal medial iliac lymph nodes range in width from 4.3 to 8.9 mm.14 In both benign (reactive) and malignant disease, lymph nodes may become larger, more visible, appear hypoechoic, and take on a rounded shape.15, 16
Given lower cost, shorter anesthesia time, and increased availability, as well as being the imaging modality of choice for radiotherapy planning, CT may be a more appropriate imaging modality than MRI for evaluation of the iliosacral lymphocenter in dogs with AGAAS. The purpose of our study was to establish a baseline normalized CT size of nonmetastatic lymph nodes within the iliosacral lymphocenter to assess the rate of detection of iliosacral lymphadenomegaly between AUS and CT in dogs with AGAAS.
Materials and Methods
Cohort Identification
A group of 30 presumed normal dogs was selected to establish a baseline for normal lymph node size of the medial iliac, internal iliac, and sacral lymph nodes. A search of the Tufts Cummings School of Veterinary Medicine (TCSVM) imaging database was performed for abdominal, pelvic, and whole‐body CT studies. Animals in which CT included the region of the lymph nodes of interest and in which underlying disease was determined not to affect the iliosacral lymphocenter were included. This group is referred to as cohort A.
A retrospective search was performed of medical records of dogs with AGAAS evaluated at TCSVM or Tufts Veterinary Emergency Treatment and Specialties between June 2009 and February 2016. Patients were included if they met the following criteria: (1) histologic or cytologic diagnosis of AGAAS, (2) underwent both CT and AUS imaging within 4 weeks of each other, and (3) Digital Imaging and Communications in Medicine (DICOM) images were available for review. Dogs were excluded from the study if they underwent therapy (surgery, chemotherapy, radiotherapy, or some combination) directed at the iliosacral lymph nodes between imaging modalities. Twenty dogs met all of the criteria and were included in the study. This group is referred to as cohort B.
Image Analysis
Computed tomographic images for dogs were acquired with an 8‐ or 16‐slice CT scanner.1 Volume data were obtained, and images were reconstructed in 2.0‐ to 5.0‐mm slice thickness. Technical settings were 110–140 kV, 100–300 mA, 0.5‐ to 1.0‐seconds tube rotation time, 250–500 mm field of view, and a 512 × 512 matrix. Postcontrast images (evaluated only in cohort B) were made 1–10 minutes after manual injection of 2.2 mL/kg body weight of 300 mg I/mL iodinated contrast.2 For cohort A, 26 dogs were scanned in sternal recumbency and 4 dogs in dorsal recumbency. For cohort B, 16 dogs were scanned in lateral recumbency, 2 in dorsal recumbency, and 2 in sternal recumbency. Short‐term apnea was induced with manual hyperventilation in all dogs. All images were reviewed and measurements made in DICOM format, on an American College of Radiology compliant workstation using medical image viewing software.3 All CT images were examined by a single board‐certified veterinary radiologist (RK) using previously reported criteria for lymph node shape, size, and density as guidelines in evaluation.13, 14, 15, 16 Data collected for both cohorts included maximum diameter (largest dimension on a single transverse view) and cross‐sectional area (as calculated by image interpretation softwarec after tracing of lymph node perimeter at the slice of maximum nodal diameter) of each visible lymph node. All lymph node measurements were performed on images in a transverse plane.
For all dogs (both cohorts), lymph node diameter was normalized for body size based on aortic dimension as follows: The diameter of the aorta for each dog was measured on a transverse view of postcontrast images at the level of the L5–L6 intervertebral disk space (Fig 1). The size of each node then was expressed as a ratio of the maximum diameter of the lymph node to the aorta (LN : AO). The mean ratio for each node location (medial iliac, internal iliac, and sacral) was calculated for cohort A.
Figure 1.
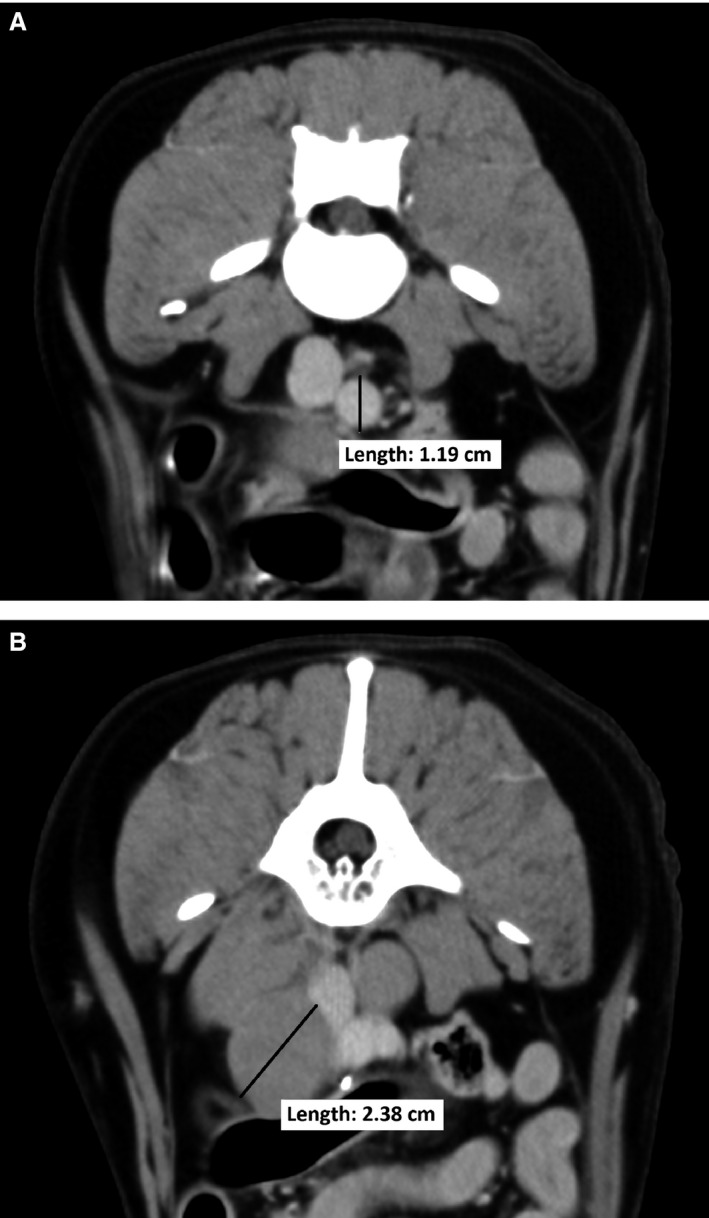
(A) aorta measurement and (B) lymph node measurement. The diameter of the aorta was measured on a transverse view at the level of the L5–L6 intervertebral disk space. The size of each node was then expressed as a ratio of the maximum diameter of the lymph node to the aorta (LN : AO) of the same dog. In this example, the medial iliac LN : AO = 2.4 cm/1.2 cm = 2.0.
Nodes in the AGAAS patients (cohort B) were considered enlarged if their LN : AO ratio measured 2 standard deviations or more above the mean normalized ratio for that particular node in cohort A.
All AUS images were reviewed. When lymph node images for specific lymph nodes were not available, assessment of abnormality, enlargement, or both was obtained from the AUS report. Lymph nodes were considered enlarged based on the opinion of the ultrasonographer performing the scan using previously reported size guidelines.14, 15, 16 Classification for lymph node abnormality and enlargement on AUS was binary (yes/no). For each patient, classification and visibility of nodes identified on AUS were compared to corresponding lymph node measurements obtained on CT.
Statistical Analysis
Statistical analysis was performed using a computerized statistical program.4 Data were checked for normality using the Kolmogorov–Smirnov test using P ≤ .01. All variables were found to be normally distributed except for age of dogs in cohort A and medial iliac lymph node measurements in cohort B overall (n = 20). Lymph node measurements were normally distributed in cohort A and when evaluating only enlarged nodes in cohort B. Differences in mean body weight and age between cohorts A and B were evaluated using a t‐test with P ≤ .05 considered statistically significant. Descriptive statistics (mean ± standard deviation and range for normally distributed variables and median and range for non‐normally distributed variables) were collected for the following variables: age, body weight, maximum nodal diameter, cross‐sectional area of lymph nodes, and LN : AO ratios.
Results
Cohort A
The control group included 10 castrated males, 16 spayed females, 3 intact females, and 1 intact male. The breeds in this group varied and included 5 Labrador Retrievers, 3 Golden Retrievers, 2 Pomeranians, 2 Yorkshire Terriers, and 1 each of the following breeds: Boston Terrier, Old English Sheepdog, Labrador‐Poodle cross, Irish Wolfhound, Miniature Schnauzer, French Bulldog, Great Pyrenees, Newfoundland, Wheaten Terrier, Rottweiler, German Shepherd, Brittany Spaniel, Springer Spaniel, Cocker Spaniel, Pug, Scottish Terrier, Leonberger, and West Highland White Terrier. Reasons for CT imaging in this group included portosystemic shunt, ectopic ureters, hind limb lipoma, adrenal nodule, hemorrhagic gastroenteritis, gallbladder stones, chronic liver disease, microvascular dysplasia, abdominal effusion, insulinoma, cystic calculi, bilateral hip luxation, and total hip replacement planning. The median age was 3.7 years (range, 6 months to 11.3 years), and the mean body weight was 21.2 ± 14.7 kg (range, 2.1–49.9 kg).
Maximum diameters and normalized values for the largest medial iliac, internal iliac, and sacral lymph nodes of each patient in cohort A as measured on CT are listed in Table S1. Computed tomography identified medial iliac lymph nodes in all 30 dogs (mean maximum diameter, 7.4 ± 2.8 mm; mean cross‐sectional area, 34.1 ± 23.5 mm2), internal iliac lymph nodes in 25 dogs (mean maximum diameter, 5.5 ± 2.2 mm; mean cross‐sectional area, 20.5 ± 14.6 mm2), and sacral lymph nodes in 17 dogs (mean maximum diameter, 4.8 ± 2.3 mm; mean cross‐sectional area, 14.6 ± 11.5 mm2). Mean LN : AO ratios were as follows: 0.9 ± 0.2 for medial iliac lymph nodes, 0.6 ± 0.2 for internal iliac lymph nodes, and 0.6 ± 0.2 for sacral lymph nodes. Based on these normal values, we established the following criteria for classification of a lymph node as enlarged in cohort B: a normalized LN : AO ratio ≥1.3 for medial iliac lymph nodes, ≥1.0 for internal iliac lymph nodes, and ≥1.0 for sacral lymph nodes. That is, lymph nodes in cohort B are considered enlarged if maximum diameter was ≥2 standard deviations above the mean normalized ratio in cohort A.
Cohort B
The AGAAS patients included 13 castrated males, 6 spayed females, and 1 intact male. Breeds varied and included 4 German Shepherds, 3 Labrador Retrievers, 2 Golden Retrievers, and 1 each of the following breeds: Belgian Shepherd, hound cross, American Cocker Spaniel, English Springer Spaniel, Bichon Frise, Siberian Husky, Australian Cattle Dog, Shi Tzu, Shiba Inu, Alaskan Klee Kai, and Labrador‐Poodle cross. The mean age was 11.0 ± 2.7 years (range, 6–18.0 years), and mean body weight was 24.4 ± 10.7 kg (range, 6.1–43.2 kg). There was no significant difference in mean weight between the 2 cohorts (21.2 kg versus 24.4 kg, P = .40). Dogs in Cohort B were significantly older than those in Cohort A (11.0 years versus 3.7 years, P < .001). Time between AUS and CT imaging in cohort B ranged from 2 to 28 days.
Overall in cohort B, CT identified medial iliac lymph nodes in 17 dogs (median [range] maximum diameter 12.0 mm [4.8–37.1 mm], median [range] cross‐sectional area 55.3 mm2 [16.4–963.9 mm2], median [range] LN : AO ratio 1.2 [0.5–3.3]), internal iliac nodes in 17 dogs (mean maximum diameter, 21.2 ± 13.8 mm; mean cross‐sectional area, 400.5 ± 202.3 mm2; mean LN : AO ratio, 2.1 ± 1.3), and sacral nodes in 15 dogs (mean maximum diameter, 22.0 ± 15.1 mm; mean cross‐sectional area, 234.7 ± 241.6 mm2; mean LN : AO ratio, 2.1 ± 1.5).
Of the 20 dogs in cohort B, 13 dogs had lymphadenomegaly in the iliosacral lymphocenter identified by CT using the LN : AO classification established in cohort A. Lymphadenomegaly involved a single node of the iliosacral lymphocenter in 3 dogs and multiple nodes of the iliosacral lymphocenter in 10 dogs. In these 13 patients, 8 medial iliac lymph nodes (mean maximum diameter, 22.7 ± 8.5 mm; mean cross‐sectional area, 340.6 ± 318.8 mm2; mean AO : LN ratio, 2.2 ± 0.8), 11 internal iliac lymph nodes (mean maximum diameter, 29.1 ± 10.4 mm; mean cross‐sectional area, 537.8 ± 327.3 mm2; mean AO : LN ratio, 2.8 ± 1.0), and 9 sacral lymph nodes (mean maximum diameter, 31.2 ± 12.4 mm; mean cross‐sectional area, 360.2 ± 222.5 mm2; mean AO : LN ratio, 3.1 ± 1.2) were considered enlarged.
Ultrasound examination correctly identified 7 of 8 (87.5%) enlarged medial iliac lymph nodes, 4 of 11 (36.4%) internal iliac lymph nodes, and 4 of 9 (44.4%) sacral lymph nodes (Table S2). When considering nodal location (medial iliac, internal iliac, or sacral) within the iliosacral lymphocenter, AUS correctly identified all enlarged lymph nodes in 30.8% of patients (n = 4); missed 1 additional enlarged lymph node location in 38.5% of patients (n = 5); and missed 2 additional enlarged lymph node locations in 30.8% of patients (n = 4). A total of 5 of the 20 dogs in cohort B (25%) had cytologic (n = 3) or subsequent histologic (n = 2) evaluation of lymph nodes within the iliosacral lymphocenter. Results identified metastatic adenocarcinoma in all 5 cases. For these 5 dogs (patients 3, 5, 8, 15, and 19 in Table S2), the confirmed metastatic lymph node was identified as enlarged on both AUS and CT imaging. Overall, as compared to CT, AUS is useful for binary classification (yes/no) of the presence of any lymphadenomegaly within the iliosacral lymphocenter (AUS detected at least 1 enlarged node in 100% of cases), but its ability to identify specific nodal lymphadenomegaly is much lower because AUS failed to detect all individually enlarged lymph nodes in 69.2% of cases (n = 9).
Discussion
Identification of lymphadenomegaly in AGAAS patients is an important component of staging and therapeutic planning. Because imaging‐identified lymphadenomegaly does not guarantee metastatic disease and because there are currently no pathognomonic imaging characteristics for lymph node metastasis, any lymph nodes identified as mildly to moderately enlarged should be sampled using ultrasound‐guided fine‐needle aspiration or core biopsy.1, 9 Presence of lymph node metastases has been shown to be a negative prognostic indicator, in that patients with metastatic lymphadenopathy have a significantly shorter median survival time than patients with nodes of normal size.9, 11, 17 Identification and extirpation of metastatic nodes has been correlated with improved survival in dogs without distant metastasis compared to animals in which nodal metastases were not removed.9, 18 Furthermore, incorporation of chemotherapy into a treatment regimen has been suggested to have a survival benefit when administered to patients with lymphadenomegaly >4.5 cm in maximum diameter.9
A recent study has shown MRI to be more sensitive than AUS for detecting abdominal lymphadenopathy in dogs with AGAAS.1 Although informative, this study was relatively small using data from only 6 dogs with AGAAS. It differs from the current study in that normal values for size of nodes in the iliosacral lymphocenter on MRI were not established. Another recent study showed CT to be more sensitive than AUS in detecting absolute numbers of iliosacral lymph nodes, but it failed to detect more abnormal lymph nodes.12 Again, this study had a small sample size of 12 dogs and CT imaging classification before lymph node sampling subjectively deemed nodes to be normal or abnormal based on previously reported variables.12, 13 Our study differs in this regard by objectively defining lymphadenomegaly on CT imaging based on establishment of a normalized LN : AO ratio. This approach may help determine when diagnostic sampling of a lymph node for further evaluation should be considered, whether or not a given lymph node should be included in surgical or radiotherapy planning, or both.
To determine a baseline measurement for each lymph node, we first selected a group of 30 unaffected dogs to serve as presumed normal controls. This control group (cohort A) was weight‐matched to the AGAAS group (cohort B) to account for the potential effect of body size on lymph node size. Based on a prior retrospective study that found the average size of the medial retropharyngeal lymph nodes not affected by age, the cohorts in the current study were not age‐matched.19 We acknowledge these results may not have been appropriately applied to our current population in which different lymph nodes were evaluated.
We also took into consideration potential differences in lymph node size based on patient size by evaluating the LN : AO ratio on CT imaging. A ratio using the aorta has been used previously with ultrasonography to establish reference values for kidney size in healthy dogs,20 and as a landmark for studies identifying left atrial enlargement21 and portal vein diameter in dogs with portosystemic shunts.22 It is uncertain whether this comparison holds true with CT imaging because there are no studies evaluating the technique using CT. Other factors also may affect aortic size, such as body condition score and hydration status, for which we were unable to control.
Given that, for a normalized data set, 95% of the population should fall within 2 standard deviations of the mean, we considered a lymph node enlarged if it fell above this range.23 Using this upper limit, 13 of the 20 dogs in cohort B had enlarged lymph nodes identified by CT. Ultrasound examination identified at least 1 enlarged lymph node in each of these 13 patients, although in most cases (69.2%), it missed 1 or 2 other abnormal lymph nodes identified by CT in the patient, and in other cases, it incorrectly identified which specific node was enlarged. This difference in the detection of lymphadenomegaly may be clinically relevant because surgical planning based on ultrasound imaging alone may result in failure to identify and subsequently resect a lymph node that is potentially metastatic. This concern may be more important for sacral nodes, which are more difficult to visualize or palpate at surgery.
Ultrasound examination was able to identify 87.5% of enlarged medial iliac lymph nodes, but only identified 36.4% internal iliac nodes, and 44.4% sacral nodes found to be enlarged on CT. Sacral nodes are often difficult to image using AUS because of their intrapelvic location directly ventral to the sacral body, and dorsal to the pubis.1 Furthermore, sacral nodes may be inconsistently present in a dog and difficult to differentiate from the internal iliac lymph nodes.13, 24 These factors may have contributed to the low number identified on AUS in our study. Similarly, sacral lymph nodes also may be missed during abdominal exploratory surgery for medial iliac lymph node extirpation, resulting in residual gross disease in the patient. Because of their location directly caudal to the medial iliac lymph nodes, between the internal iliac and median sacral arteries, internal iliac lymph nodes should be more easily visualized on AUS, especially if enlarged.25 Potential causes for the low rate of detection of internal iliac lymph nodes on AUS in our study include close or convergent association with medial iliac lymph nodes (2 lymph nodes measured as 1) or misidentification of internal iliac lymph nodes as either medial iliac or sacral nodes.
A prior study reported a predominance of metastasis to the sacral lymph nodes in patients with AGAAS, given that they are anatomically closest to the anal glands.1 Although our study lacked confirmation of metastasis in the majority of dogs, the incidence of lymphadenomegaly as determined by CT was similar for all 3 nodes: internal iliac lymphadenomegaly was most common (n = 11), followed by sacral (n = 9) and medial iliac (n = 8) lymphadenomegaly.
Because our study design was retrospective, it has inherent limitations. The AUS data collected relied on reports written by the radiologist at the time of the examination, and utilized evaluation of saved rather than real time images. In some cases, the report referred to lymph nodes as “within normal limits” and did not specify if that meant that the operator saw the node and determined that it was of normal size or if he or she failed to visualize the node. Because our primary endpoint was identification of enlarged lymph nodes, we felt that this difference was not relevant because both situations would lead to a classification as negative for lymphadenomegaly and lead to the same clinical outcome. Furthermore, CT slice thickness could not be selected or standardized for CT imaging and in some scans, it may have been larger than ideal for identification of small‐volume structures. An undetected lymph node would only occur if the node was smaller than the given slice thickness and thus would be considered within normal limits for size. A previous report on CT characteristics of presumed normal abdominal lymph nodes in dogs failed to detect a relationship between the number of identified lymph nodes and CT slice thickness.13 However, sacral lymph nodes in particular tend to be the smallest in this lymphocenter, and decreasing slice thickness may improve accuracy of measurement. Another limitation stems from the timing between AUS and CT imaging, leading to the potential that lymph node size could change within 4 weeks. In only 4 dogs was the interval between imaging modalities >14 days. In 3 of these dogs (patients 13, 16, and 18 with a 20‐day, 20‐day, and 27‐day interval, respectively), all lymph nodes were considered within normal limits for size on both AUS and CT. In the dog (patient 3) with the longest interval of 28 days, the medial iliac lymph node was considered enlarged on both AUS and CT, but only CT detected enlarged internal iliac and sacral lymph nodes.
Dogs in cohort A were selected with the intent to avoid including patients with lymphadenopathy, but lack of histopathologic examination to verify absence of pathology of the identified lymph nodes in these presumed normal dogs is a another limitation of the study. The mean (range) maximum diameter of medial iliac lymph nodes of 7.4 mm (2.7–13.0 mm) found in cohort A of our study is similar to the mean CT width (range) of 6.7 mm (2.4–11.2 mm) for presumed normal medial iliac lymph nodes previously reported in dogs.13 This prior CT study in dogs failed to make a clear differentiation between internal iliac and sacral lymph nodes but reported a mean width (range) for these nodes in presumed normal dogs on CT of 4.8 mm (2.0–9.2 mm),13 which again is similar to the mean (range) maximum diameters of 5.5 mm (2.4–10.5 mm) for internal iliac and 4.8 mm (0.9–8.6 mm) for sacral lymph nodes found in cohort A of our study.
Our inclusion criteria included a histopathologic or cytopathologic confirmation of AGAAS from the primary tumor but did not include a lymph node sample. Only 5 of 20 dogs had confirmation of metastatic lymphadenopathy. Interestingly, in these 5 dogs, lymphadenomegaly was detected on both AUS and CT. In the remaining dogs, it is unknown whether the lymph nodes were enlarged because they were reacting to AGAAS or another disease process or whether they were enlarged from metastasis of the cancer. Lack of confirmation of metastasis in enlarged nodes also prevented us from investigating other CT features of the nodes (such as degree and pattern of contrast enhancement) and their association with the detection of metastasis. Although most dogs lacked confirmation of lymph node metastasis, we elected to include them because our study was focused on the identification of lymphadenomegaly between the 2 imaging modalities, not on the utility of CT in confirming lymph node metastasis. In initial staging tests for a patient, if lymphadenomegaly is detected, this finding should prompt the attending clinician to pursue additional diagnostic testing if deemed necessary for confirmation of metastatic disease.
Another potential limitation of our study is bias when assessing lymph nodes because the radiologist was not blinded to the diagnosis of AGAAS before reviewing the studies. To lessen the effect of this bias, we defined lymphadenomegaly on CT based on objective measurements and only classified a lymph node as enlarged when it was above our defined normalized value, thereby eliminating subjectivity.
The findings of our study indicate that AUS is useful for the detection of lymphadenomegaly within the iliosacral lymphocenter. However, CT is a more accurate technique for identification of the full extent of lymphadenomegaly and for identification of which specific nodes are enlarged in dogs with AGAAS. Although AUS identified at least 1 enlarged lymph node in each patient included in the study, CT identified ≥1 additional abnormal lymph nodes in 69.2% of patients with lymphadenomegaly. The clinical relevance of this finding is unclear, although it may be important with regard to surgical and radiotherapy planning. Given these findings, AUS can be considered an effective screening test for iliosacral lymphadenomegaly. However, if lymphadenomegaly is identified in any patient with AGAAS, CT should be considered if the finding of an additional metastatic site would affect therapeutic planning.
Supporting information
Table S1. Maximum Diameters and Normalized Values for the Largest Medial Iliac, Internal Iliac and Sacral Lymph Nodes of Each Patient in Cohort A as Measured on CT.
Table S2. Maximum Diameters and Normalized Values for the Largest Medial Iliac, Internal Iliac and Sacral Lymph Nodes of Each Patient in Cohort B as Measured on CT.
Acknowledgments
Grant support: This study was supported by a NIH Short‐Term Training Grant (OD010963).
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
All work was completed at the Cummings School of Veterinary Medicine, Tufts University.
Footnotes
Aquilion LB 16‐slice CT scanner; Toshiba Medical Systems, Tustin, CA, at TCSVM and CereTom; NeuroLogica Corporation, Danvers, MA, at Tufts Veterinary Emergency Treatment and Specialties.
Iohexol, Omnipaque; GE Healthcare, Princeton, NJ.
Carestream PACS 5.1; Carestream Health, Inc., Rochester, NY.
JMP®, Version 11; SAS Institute Inc., Cary, NC, 1989–2007.
References
- 1. Anderson CL, MacKay CS, Roberts GD, Fidel J. Comparison of abdominal ultrasound and magnetic resonance imaging for detection of abdominal lymphadenopathy in dogs with metastatic apocrine gland adenocarcinoma of the anal sac. Vet Comp Oncol 2015;13:98–105. [DOI] [PubMed] [Google Scholar]
- 2. Berrocal A, Vos JH, van den Ingh TS, et al. Canine perineal tumours. Zentralbl Veterinarmed A 1989;36:739–749. [DOI] [PubMed] [Google Scholar]
- 3. Goldschmidt MH, Shofer FS. Skin Tumors of the Dog and Cat. Oxford, UK: Pergamon Press; 1992. [Google Scholar]
- 4. Williams LE, Gliatto JM, Dodge RK, et al. Carcinoma of the apocrine glands of the anal sac in dogs: 113 cases (1985‐1995). J Am Vet Med Assoc 2003;223:825–831. [DOI] [PubMed] [Google Scholar]
- 5. Goldschmidt MH, Zoltowski C. Anal sac adenocarcinoma in the dog: 14 cases. J Small Anim Pract 1981;22:119–128. [DOI] [PubMed] [Google Scholar]
- 6. Ross JT, Scavelli TD, Matthiesen DT, Patnaik AK. Adenocarcinoma of the apocrine glands of the anal sac in dogs: A review of 32 cases. J Am Anim Hosp Assoc 1991;27:349–355. [Google Scholar]
- 7. Bennett PF, DeNicola DB, Bonney P, et al. Canine anal sac adenocarcinomas: Clinical presentation and response to therapy. J Vet Intern Med 2002;16:100–104. [DOI] [PubMed] [Google Scholar]
- 8. Meuten DJ, Cooper BJ, Capen CC, et al. Hypercalcemia associated with an adenocarcinoma derived from the apocrine glands of the anal sac. Vet Pathol 1981;18:454–471. [DOI] [PubMed] [Google Scholar]
- 9. Polton GA, Brearley MJ. Clinical stage, therapy, and prognosis in canine anal sac gland carcinoma. J Vet Intern Med 2007;21:274–280. [DOI] [PubMed] [Google Scholar]
- 10. Dobson JM, Lascelles BDX. BSAVA Manual of Canine and Feline Oncology, 3rd ed Quedgeley, Gloucester: British Small Animal Veterinary Association; 2011. [Google Scholar]
- 11. Wouda RM, Borrego J, Keuler NS, Stein T. Evaluation of adjuvant carboplatin chemotherapy in the management of surgically excised anal sac apocrine gland adenocarcinoma in dogs. Vet Comp Oncol 2016;14:67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pollard RE, Fuller MC, Steffey MA. Ultrasound and computed tomography of the iliosacral lymphatic centre in dogs with anal sac gland carcinoma. Vet Comp Oncol 2015. Early view online: http://dx.doi.org/10.1111/vco.12160 [DOI] [PubMed] [Google Scholar]
- 13. Beukers M, Gross FV, Voorhout G. Computed tomographic characteristics of presumed normal canine abdominal lymph nodes. Vet Radiol Ultrasound 2013;54:610–617. [DOI] [PubMed] [Google Scholar]
- 14. Mayer MN, Lawson JA, Silver TI. Sonographic characteristics of presumptively normal canine medial iliac and superficial inguinal lymph nodes in the dog. Vet Radiol Ultrasound 2010;51:638–641. [DOI] [PubMed] [Google Scholar]
- 15. De Swarte M, Alexander K, Rannou B, et al. Comparison of sonographic features of benign and neoplastic deep lymph nodes in dogs. Vet Radiol Ultrasound 2011;52:451–456. [DOI] [PubMed] [Google Scholar]
- 16. Llabres‐Diaz FJ. Ultrasonography of the medial iliac lymph nodes in the dog. Vet Radiol Ultrasound 2004;45:156–165. [DOI] [PubMed] [Google Scholar]
- 17. Potanas CP, Padgett S, Gamblin RM. Surgical excision of anal sac apocrine gland adenocarcinomas with and without adjunctive chemotherapy in dogs: 42 cases (2005‐2011). J Am Vet Med Assoc 2015;246:877–884. [DOI] [PubMed] [Google Scholar]
- 18. Hobson HP, Brown MR, Rogers KS. Surgery of metastatic anal sac adenocarcinoma in five dogs. Vet Surg 2006;35:267–270. [DOI] [PubMed] [Google Scholar]
- 19. Burns GO, Scrivani PV, Thompson MS, Erb HN. Relation between age, body weight, and medial retropharyngeal lymph node size in apparently healthy dogs. Vet Radiol Ultrasound 2008;49:277–281. [DOI] [PubMed] [Google Scholar]
- 20. Mareschal A, d'Anjou M, Moreau M, et al. Ultrasonographic measurement of kidney‐to‐aorta ratio as a method of estimating renal size in dogs. Vet Radiol Ultrasound 2007;48:434–438. [DOI] [PubMed] [Google Scholar]
- 21. Hansson K, Haggstrom J, Kvart C, Lord P. Left atrial to aortic root indices using two‐dimensional and M‐mode echocardiography in cavalier King Charles spaniels with and without left atrial enlargement. Vet Radiol Ultrasound 2002;43:568–575. [DOI] [PubMed] [Google Scholar]
- 22. d'Anjou MA, Penninck D, Cornejo L, Pibarot P. Ultrasonographic diagnosis of portosystemic shunting in dogs and cats. Vet Radiol Ultrasound 2004;45:424–437. [DOI] [PubMed] [Google Scholar]
- 23. Witte RS, Witte JS. Statistics, 10th ed Hoboken: John Wiley and Sons, Inc.; 2015. [Google Scholar]
- 24. Evans HE, de Lahunta A. Miller's Anatomy of the Dog, 4th ed St. Louis, MO: Saunders; 2013. [Google Scholar]
- 25. Mattoon JS, Nyland TG. Small Animal Diagnostic Ultrasound, 3rd ed St. Louis, MO: W.B. Saunders Company; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Maximum Diameters and Normalized Values for the Largest Medial Iliac, Internal Iliac and Sacral Lymph Nodes of Each Patient in Cohort A as Measured on CT.
Table S2. Maximum Diameters and Normalized Values for the Largest Medial Iliac, Internal Iliac and Sacral Lymph Nodes of Each Patient in Cohort B as Measured on CT.


