Abstract
Background
Pimobendan is effective in treatment of dogs with congestive heart failure (CHF) secondary to myxomatous mitral valve disease (MMVD). Its effect on dogs before the onset of CHF is unknown.
Hypothesis/Objectives
Administration of pimobendan (0.4–0.6 mg/kg/d in divided doses) to dogs with increased heart size secondary to preclinical MMVD, not receiving other cardiovascular medications, will delay the onset of signs of CHF, cardiac‐related death, or euthanasia.
Animals
360 client‐owned dogs with MMVD with left atrial‐to‐aortic ratio ≥1.6, normalized left ventricular internal diameter in diastole ≥1.7, and vertebral heart sum >10.5.
Methods
Prospective, randomized, placebo‐controlled, blinded, multicenter clinical trial. Primary outcome variable was time to a composite of the onset of CHF, cardiac‐related death, or euthanasia.
Results
Median time to primary endpoint was 1228 days (95% CI: 856–NA) in the pimobendan group and 766 days (95% CI: 667–875) in the placebo group (P = .0038). Hazard ratio for the pimobendan group was 0.64 (95% CI: 0.47–0.87) compared with the placebo group. The benefit persisted after adjustment for other variables. Adverse events were not different between treatment groups. Dogs in the pimobendan group lived longer (median survival time was 1059 days (95% CI: 952–NA) in the pimobendan group and 902 days (95% CI: 747–1061) in the placebo group) (P = .012).
Conclusions and Clinical Importance
Administration of pimobendan to dogs with MMVD and echocardiographic and radiographic evidence of cardiomegaly results in prolongation of preclinical period and is safe and well tolerated. Prolongation of preclinical period by approximately 15 months represents substantial clinical benefit.
Keywords: Asymptomatic, Echocardiography, Endocardiosis, Heart failure, Mitral regurgitation, Prevention, Treatment, Vertebral heart sum
Abbreviations
- 2D
two dimensional
- ACEI
angiotensin‐converting enzyme inhibitors
- ALT
alanine aminotransferase
- BCS
body condition score
- BPM
beats per minute
- CHF
congestive heart failure
- CI
confidence intervals
- CKCS
Cavalier King Charles Spaniels
- ECG
electrocardiogram
- EPIC
evaluation of pimobendan in dogs with cardiomegaly caused by preclinical mitral valve disease
- F
female
- FS
female spayed
- FS%
fractional shortening
- GPT
glutamic pyruvate transaminase
- HR
hazard ratio
- IQR
interquartile range
- ITT
intention to treat
- K+
potassium
- LA/Ao
left atrial‐to‐aortic ratio
- LVIDd
left ventricular internal diameter in diastole
- LVIDDN
normalized left ventricular internal diameter in diastole
- LVIDs
left ventricular internal diameter in systole
- LVIDSN
normalized left ventricular internal diameter in systole
- M
male
- MC
male castrated
- MMVD
myxomatous mitral valve disease
- MR
mitral regurgitation
- Na+
sodium
- NA
not able to calculate
- PCV
packed cell volume
- PP
per protocol
- SAP
systolic arterial blood pressure
- TPC
total protein concentration
- UK
United Kingdom
- USA
United States of America
- VHS
vertebral heart sum
Myxomatous mitral valve disease (MMVD) is the most common cardiovascular disease in the dog.1, 2 Progressive degenerative lesions of the valve result in mitral regurgitation (MR) imposing a gradually increasing chronic volume load on the left side of the heart. In some dogs, the volume load will result in clinically detectable enlargement of the left side of the heart and might eventually result in the development of signs of congestive heart failure (CHF), that is, pulmonary venous congestion and edema.
Dogs with MMVD can progress through various stages of the disease. One widely used staging system for this condition divides affected dogs into 4 categories.3 Those at risk for developing the disease are considered to be at stage A; those with evidence of MR and no signs of CHF are at stage B; those with signs of CHF are at stage C; and those with signs of CHF refractory to treatment are considered to be at stage D. Stage B, the preclinical period, is a long period characterized by varying degrees of progression.4 Dogs with early stage B disease and no evidence of cardiac enlargement are categorized as stage B1; dogs in which cardiac enlargement has developed in order to compensate for the volume load, but which have not yet developed signs of CHF, are categorized as stage B2.
A number of therapies are considered effective in dogs with stage C disease3 including pimobendan, which has been shown to improve survival and maintain quality of life.5 Despite the effectiveness of recommended treatments3, median survival time for dogs with stage C disease is less than 1 year.5
Due to the lengthy nature of the preclinical period, any treatment effective in prolonging this period could have a major impact on longevity and quality of life of affected dogs. Currently, there is no consensus about the effectiveness of medical treatment in stage B despite 2 published prospective randomized clinical trials that evaluated the effectiveness of angiotensin‐converting enzyme inhibitors (ACEI) at this stage.6, 7 In these studies, although no clearly beneficial effect of ACEI was found, the median period free of signs of CHF for dogs with stage B2 disease was approximately 27 months (800 days).
The effectiveness of pimobendan in dogs with stage B2 disease has not been previously evaluated. The mechanism of action of pimobendan includes a combination of positive inotropy and balanced vasodilatation caused by calcium sensitization and phosphodiesterase inhibition.8 Four previous studies, 1 evaluating dogs with preclinical dilated cardiomyopathy and 3 concerning dogs with stage C MMVD, demonstrated that pimobendan use is associated with a reduction in heart size.9, 10, 11, 12 A reduction in heart size might also be beneficial in dogs with stage B2 MMVD, provided adequate systemic perfusion is maintained.
On the basis of the known actions of pimobendan, we hypothesized that the chronic oral administration of pimobendan in dogs with evidence of increased heart size secondary to preclinical MMVD, not receiving any other cardiovascular medications, would delay the onset of signs of CHF or cardiac‐related death or euthanasia compared to similar dogs not receiving pimobendan.
Materials and Methods
Trial Design
The “Evaluation of Pimobendan In dogs with Cardiomegaly caused by preclinical mitral valve disease” (EPIC) trial was a prospective multicenter, blinded, randomized, placebo‐controlled study. The trial protocol was prepared by independent cardiologists (AB, JH, and SG) in conjunction with the sponsor and was approved by an ethical review committee at each site where this was required. The contract between the sponsor and lead investigators (AB, JH, and SG) stipulated that the latter would have full access to all results and the right to independently publish, regardless of trial outcome.
Dogs
Enrollment Criteria
Dogs were eligible for participation in the study provided that the owner had given informed consent.
To be eligible for inclusion, a dog had to be 6 years of age or older, have a body weight ≥4.1 and ≤15 kg, have a characteristic systolic heart murmur of moderate to high intensity (≥ grade 3/613) with maximal intensity over the mitral area, have echocardiographic evidence of advanced MMVD defined as characteristic valvular lesions of the mitral valve apparatus, MR on the color Doppler echocardiogram, and have echocardiographic evidence of left atrial and left ventricular dilatation, defined as a left atrial‐to‐aortic root ratio (LA/Ao)14 ≥ 1.6 and body weight normalized left ventricular internal diameter in diastole (LVIDDN)15 ≥ 1.7, in addition to radiographic evidence of cardiomegaly (vertebral heart sum (VHS) > 10.5).16
Exclusion Criteria
Dogs were excluded from the study if they had any of the following: known clinically important systemic or other organ‐related disease that was expected to limit the dog's life expectancy or required chronic cardiovascular medication precluded as part of the trial (Table 1). Dogs with hypothyroidism could be included provided the investigator deemed them clinically stable on treatment. Dogs with current or previous evidence of cardiogenic pulmonary edema, pulmonary venous congestion or both, cardiac disease other than MMVD, clinically significant supraventricular, ventricular tachyarrhythmias or both (i.e, requiring antiarrhythmic treatment), or evidence of pulmonary hypertension considered to be clinically relevant (RV:RA pressure gradient > 65 mmHg) were excluded. Dogs with a history of chronic or recent administration (>14 days of duration or within 30 days of intended enrollment) of any medication listed in Table 1 were excluded. Dogs that were pregnant or lactating were not eligible for enrollment. In the event that before study enrollment, a dog had received short‐term treatment (<14 days) with agents listed in Table 1, but was no longer receiving treatment and had not received it within 30 days of intended enrollment, then the dog was eligible for inclusion.
Table 1.
Prohibited cardiovascular agents. Chronic administration of these agents before study entry was an exclusion criterion. Initiation of any of these agents during the study was considered an event in the time‐to‐first‐event analysis. Initiation of chronic treatment resulted in a dog being censored from the per‐protocol population
| ACE Inhibitors | Enalapril, benazepril, captopril, fosinopril, imidapril, lisinopril, ramipril |
| Angiotensin II receptor blockers | Candesartan, telmisartan |
| Antiarrhythmic drugs | Lidocaine, bretylium, flecainide, mexiletine, procainamide, phenytoin, propafenone, quinidine, tocainide, beta‐blockers [for list see below], amiodarone, sotalol, Ca++channel blockers [diltiazem, verapamil], digoxin, digitoxin |
| Anticholinergics | Atropine, glycopyrrolate, propantheline |
| Beta‐blockers | Atenolol, bisoprolol, carvedilol, esmolol, metoprolol, nadolol, propranolol |
| Diuretics | Furosemide, hydrochlorothiazide, thiazides, chlorothiazide, torasemide (torsemide), spironolactone, eplerenone |
| Inodilators | Pimobendan, levosimendan, milrinone |
| Phosphodiesterase V inhibitors | Sildenafil, tadalafil |
| Positive inotropes | Pimobendan, levosimendan, milrinone, isoproterenol, dobutamine, dopamine, digoxin, digitoxin |
| Pressor agents | Epinephrine, norepinephrine, phenylephrine |
| Vasodilators (including nitric oxide donors) | Amlodipine, hydralazine, prazosin, nitroglycerin (even topical), isosorbide di‐/mononitrate, other nitrates, nitric oxide, sodium nitroprusside, L‐arginine |
| Other | Iloprost, epoprostenol, bosentan, known cardiac toxins, for example, doxorubicin |
Study Sites
Dogs were recruited by investigators specializing in veterinary cardiology at 36 centers: 18 in the United States, 3 in the UK, 3 in Germany, 3 in France, 2 in Japan, 2 in Sweden, 1 in each of Italy, Spain, the Netherlands, Canada, and Australia. (All authors except one (PW) were investigators recruiting cases).
Randomization and Allocation
Block randomization17 was used with a 1:1 allocation ratio in blocks of 20 to maintain similar sample sizes in both treatment groups. The randomization sequence was generated by the manufacturer of the trial medication1 by computer software.2 Each investigator was initially assigned 10 consecutive case numbers from a block of 20, ensuring that each investigator did not know how many cases assigned to each treatment were under their care. When an investigator recruited a new dog, that dog was assigned the next available case number and received the preassigned treatment. During recruitment, some case numbers were reallocated between investigators in order to reach the recruitment target. Case numbers reallocated to an investigator were always from a treatment block different to that from which the investigator's original 10 case numbers came. The maximum allowable number of cases enrolled by any single center was 20.
Blinding
Investigators, owners, study monitors, the statistician3 , and the sponsor were blinded to treatment allocation.
The blinding code for the treatment groups was held by individuals who had no other role in the study. Predefined procedures were available to permit unblinding of individual cases in the event of a medical emergency. Unblinding could be achieved by contacting named individuals who held the randomization list; they could then break the treatment code and inform the investigator of the treatment the animal was receiving. In the event of unblinding, a dog would be censored from the study at the time of unblinding. Neither the investigators, nor the monitor, nor the sponsor of the study had access to the randomization list.
Trial Medication
Pimobendan verum (Vetmedin 2.5 mg chewable tablets) was administered PO at a target dose of 0.4–0.6 mg/kg/d as per registered label instructions in most countries where the product was licensed. The calculated daily dose was divided into 2 administrations and adjusted to a suitable number of tablets. Placebo was administered PO according to the calculated daily dose for pimobendan verum tablets, divided into 2 administrations, and adjusted to a suitable number of placebo tablets. Placebo and verum tablets and packaging containers were visually identical. Dogs were dosed in the morning and evening approximately 12 hours apart. Dose of study medication was not adjusted throughout the study.
Populations Analyzed
Any dog that was randomized and received at least 1 dose of study medication was included in the intention‐to‐treat (ITT) population. Any dog in the ITT population that was confirmed to have met all inclusion criteria (and none of the exclusion criteria) was included in the per‐protocol (PP) population until one of the following occurred: the dog reached the primary endpoint, the dog was censored from the primary endpoint analysis due to the occurrence of an event that precluded continuation in that population, or the end of the study was reached.
Concomitant Treatments
All concomitant medications that dogs were receiving at the time of enrollment or received during the course of the study were recorded.
A dog was censored from the per‐protocol (PP) primary endpoint analysis if an attending veterinarian deemed it necessary to chronically administer open‐label cardiovascular medication(s) for an indication other than having reached the primary endpoint. A list of such cardiovascular medications (Table 1) was prospectively defined and included in the protocol. An exception to this rule was that short‐term use of medications in Table 1 was allowed for a period of <5 days if a dog required anesthesia or sedation, or in the event that a dog enrolled in the study was inadvertently administered one of the medications listed in Table 1 for a period of <3 days, but the reason for administration was not substantiated and chronic medication was unnecessary. Medications, the chronic administration of which did not result in a dog being censored from the PP analysis of the primary endpoint, were predefined in the protocol and are listed in Table 2. Initiation of chronic treatment with any medication in Table 1 or Table 2 was recorded as an event. All commercially available topical treatments for ears and eyes were allowed even if some ingredients are found in Tables 1 and 2, and their chronic administration was not considered an event nor did they preclude enrollment in the study.
Table 2.
Agents that dogs were allowed receive while remaining in the per‐protocol population. Initiation of any of these agents during the study was considered an event in the time‐to‐first‐event analysis
| Bronchodilators | Aminophylline, theophylline, terbutaline |
| Other | Corticosteroids, cough suppressants, mirtazapine, or other appetite stimulants |
Schedule of Events
Before inclusion, a case history was taken for each dog. The dog then underwent a physical examination, measurement of systolic arterial blood pressure (SAP), echocardiography, thoracic radiography, and routine hematology and blood biochemistry (performed at laboratories local to each investigator) with a minimum database consisting of packed cell volume (PCV), ALT (GPT), total protein, creatinine, potassium (K+), and sodium (Na+) concentrations. Re‐examinations were scheduled at day 35, and approximately 4 months after inclusion. Thereafter, the dogs were scheduled for re‐examination every 4 months. Dogs enrolled to the study underwent appropriate clinical monitoring including home monitoring of respiratory rate.
A dog that reached the primary endpoint and survived was treated with open‐label pimobendan and other cardiovascular medications at the discretion of the investigator. All dogs in the ITT population were followed until they died, were euthanized, were lost to follow‐up, or the end of the study was reached. The date of death, if it was known to have occurred, was recorded.
Clinical Evaluation
At inclusion, dog characteristics such as breed, age, sex, and neutering status were documented. The body weight, body condition score (BCS)4, and rectal temperature were measured.
Quality‐of‐Life Observations
After history taking and clinical examination, the following variables were scored (according to the system outlined in Table 3): appetite, demeanor, exercise tolerance, coughing, nocturnal dyspnea/cough, and fainting.
Table 3.
Ordinal scoring system for clinical variables recorded at baseline
| Variable | Score | Clinical Correlate |
|---|---|---|
| Exercise tolerance | 1 (Very good) | Dog moved around with ease and was able to fully exercise |
| 2 (Good) | Dog moved around with ease and was not able to fully exercise; ability to run was reduced | |
| 3 (Moderate) | Dog was less active than normal, moved around a few times per day, avoided long walks | |
| 4 (Poor) | Dog was inactive and would only get up to eat, drink, or urinate | |
| Demeanor | 1 | Alert, responsive |
| 2 | Mildly lethargic | |
| 3 | Moderately lethargic | |
| 4 | Minimally responsive | |
| 5 | Unresponsive | |
| Appetite | 1 | Increased |
| 2 | Normal | |
| 3 | Decreased (2/3 normal) | |
| 4 | Markedly decreased (<2/3 normal) | |
| Respiratory effort | 1 | Normal |
| 2 | Mildly increased rate or effort | |
| 3 | Moderately labored | |
| 4 | Severe respiratory distress | |
| Coughing | 1 | None |
| 2 | Occasional (a few times a week) | |
| 3 | Frequent (a few times a day) | |
| 4 | Persistent (frequently during the day) | |
| Nocturnal dyspnea/cough | 1 | None |
| 2 | Dog coughed from time to time during the night, but no other clinical signs of dyspnea or restlessness were present | |
| 3 | Dog coughed consistently, increased respiratory effort or restlessness during the night | |
| Fainting | 1 | None |
| 2 | Rarely (<once a month) | |
| 3 | Occasionally (a few times a month) | |
| 4 | Frequently (a few times a week or more) |
Circulatory and Respiratory Variables
The respiratory rate was measured. Cardiac auscultation was performed to detect the presence of any arrhythmia, and to measure the heart rate and grade heart murmur intensity on a scale of 1–6.13 Systolic arterial blood pressure was measured before inclusion by 1 of 2 methods: Doppler sphygmomanometry or oscillometry.
Echocardiography
Echocardiography was performed on unsedated dogs. The following measurements were taken from at least 3 cardiac cycles and the mean was recorded: the LA/Ao obtained from the right parasternal short‐axis 2D view as previously described,14 the left ventricular internal diameter at end diastole (LVIDd), and left ventricular internal diameter at end systole (LVIDs) measured on the M‐mode echocardiogram, obtained from the right parasternal short‐axis view.18 M‐mode values were used to derive the fractional shortening (FS%). Normalized dimensions15 were calculated according to the following formulae: normalized LVIDd (LVIDDN) = LVIDd(cm)/(BW (kg))0.294; normalized LVIDs (LVIDSN) = LVIDs(cm)/(BW(kg))0.315.
Thoracic Radiography
Thoracic radiography was performed at inclusion and at the time a dog was considered to have developed signs of CHF. Right lateral and dorsoventral projections were used to evaluate the thorax. Cardiac size was assessed by the VHS method16 and pulmonary edema and congestion were recorded, when considered to be present, by the attending cardiologist.
Primary Endpoint
The primary endpoint was a composite of the development of left‐sided CHF, or euthanasia for a cardiac reason, or death presumed to be cardiac in origin in dogs in the PP population. The primary outcome variable of the study was the time period from inclusion until the primary endpoint was reached.
A dog was considered to have left‐sided CHF when there was radiographic evidence of cardiogenic pulmonary edema as indicated by an interstitial or alveolar lung pattern. In addition to these radiographic findings, the dog must have been showing contemporaneous clinical signs consistent with left‐sided CHF including increased respiratory effort and rate by comparison with previously noted values for this patient.
Two members of an endpoint committee, comprised of the 3 lead investigators (AB, JH, and SG) and 2 additional investigators (RSt and GW), reviewed the radiographs and case records for each dog reaching the CHF component of the primary endpoint in order to verify that the endpoint had been reached. In the event of a disagreement between the 2 members of the endpoint committee, the case was adjudicated by a third endpoint committee member. Endpoint committee members did not evaluate their own cases and were blinded to treatment allocation at the time of adjudication.
If a dog died in the absence of evidence of a noncardiac cause of death before radiographic confirmation of pulmonary edema, it was also considered to have experienced cardiac death and therefore reached the primary endpoint.
Dogs that reached the primary endpoint and in which no relevant protocol deviation or violations occurred during the study were included in the PP analysis. Dogs in which a major protocol deviation occurred during the study (e.g, the owner was not compliant with study procedures, or there were lengthy treatment gaps comprising a single period without trial medication of >30 days or the total duration of period without medication was >10% of the dog's total time on study) were included in the PP analysis until the time when the protocol deviation occurred, at which time they were censored. However, these dogs could still be eligible for inclusion in the ITT analyses.
At any point in the study, an owner or investigator could remove a dog from the study for reasons of animal welfare, suspected adverse drug reaction, and illness or injury that prohibited the dog's continuation.
Secondary Endpoints
A dog was considered to have experienced an event if it reached the primary endpoint, underwent euthanasia or died for a noncardiac reason, had chronic medication initiated (Table 1 or Table 2), had a CHF endpoint that was not verified by the endpoint committee, the owner became noncompliant with study procedures, experienced lengthy treatment gaps, or the dog was withdrawn from the study by the owner or investigator. The time to the first of these events experienced by dogs in the PP population was analyzed as a prospectively defined secondary endpoint. (For further information, see Table S2).
Time to death by any cause was also a prospectively defined secondary endpoint and analyzed in the ITT population.
Appropriate pharmacovigilance was undertaken and adverse events experienced by dogs on the study were noted, classified, and reported in accordance with applicable guidelines.
Data Management
Data management was undertaken by an independent data management company.5 All clinical and dispenser records were collected from all centers. Data were verified and double entered into a database by separate individuals. After data entry, the 2 sets of data were compared to verify accuracy of entry of data and any discrepancies between the 2 databases were explored and resolved. Blinding was maintained during data entry and audit. All decisions on censoring and exclusions from the study were made on the basis of predetermined criteria by the members of the lead committee who remained blinded.
Statistical Methods
Power Calculation
Power calculations for study population size were performed by nQuery platform.6 The planned duration of the accrual period was 2 years and the maximum duration of follow‐up was 5 years. A minimum of 3 years follow‐up was planned after the accrual period. Results of prior studies had shown that the median time to the onset of left‐sided CHF in dogs with stage B2 MMVD is approximately 27 months.6, 7 Based on these assumptions, 150 animals per group were considered necessary to detect a difference in median time to the onset of left‐sided CHF of greater than or equal to 13 months with a power of 80% and an alpha of 5%. To compensate for possible dropouts, 180 animals per group were included in each treatment group.
Populations Analyzed
In both the interim and final analyses, all efficacy analyses with respect to the primary endpoint were planned as analyses comparing treatment groups in the PP population. Time to first event was also compared between treatment groups in the PP population. The safety analyses, all‐cause mortality, and proportion of suspected serious or worse adverse events were evaluated in the ITT population.
Interim Analysis
A preplanned interim analysis was undertaken with predefined stopping criteria for convincing evidence of efficacy and safety, performed on data obtained after 80% of the initial anticipated study period was complete. Unblinding and termination of the study only occurred if deemed necessary by the data interim evaluation committee according to prespecified criteria (Figure S1). The committee consisted of 3 independent (to the study) persons: 1 biostatistician and 2 experts in canine cardiology. The P‐value for stopping on the basis of convincing evidence of efficacy with respect to the primary endpoint was decided by appropriate statistical software6 and set at P < .01477.
Final Analysis
Descriptive statistics for continuous variables are reported as median values with interquartile range (IQR); for categorical and ordinal variables, they are reported as frequency and proportions. For the analysis of the final data, each of the variables obtained at baseline was assessed for homogeneity between treatment groups in the PP populations. All continuous and ordinal baseline variables were compared between groups by a Mann‐Whitney‐Wilcoxon test. For categorical data, a chi‐square or Fisher's exact test was used. No adjustment was made for multiple comparisons. The P‐value for the final analysis was corrected according to the O'Brien‐Fleming alpha spending function.19 The critical two‐sided nominal efficacy alpha level for the final analysis was calculated to be 0.04551 by appropriate statistical software.7 The local alpha levels were calculated for a total type I error level of 5% with a power of 80 % and the assumptions of a hazard ratio of 0.667 and proportional hazards.
In time‐to‐event analyses, a log‐rank test with right censoring was used to determine whether a significant difference existed between the 2 treatment groups in the probability of the event of interest occurring at any time point. The Kaplan‐Meier method was used to estimate the median time to endpoint for each treatment group and plot time to event curves.
Univariate Cox proportional hazards analysis with right censoring was performed for the effect of treatment and each baseline variable, to determine whether there was any association with time to primary endpoint. For the purpose of the Cox proportional hazards analysis, categories of ordinal baseline variables with a small number of observations (<5 observations) at a single level were combined with the adjacent level to create a larger group with the same directional tendency. The robustness of the treatment effect was evaluated by adjusted univariable Cox proportional hazards analysis to determine the influence of each baseline variable individually on the treatment hazard ratio. Multivariable Cox proportional hazards analysis was performed in a backward stepwise manner. Baseline variables were selected for entry to the model if they had a P‐value of <.2 in the univariate analysis. Variables entered into the multivariable analysis were assessed for multicollinearity and to ensure that the proportional hazards assumption was met. The variable with the highest P‐value was eliminated at each subsequent step, with reanalysis between steps, until the final model was obtained with all the remaining variables having a P‐value <.05. A second exploratory multivariable model was created as above but excluding echocardiographic variables in the first iteration, and also run in a backward stepwise manner until all variables in the final model had a P‐value <.05. First‐order interaction terms between each pair of variables remaining in the final model were evaluated and included in each model if found to be significant.
Proportions of dogs that experienced severe or worse adverse events were compared between groups by a chi‐square test.
For all analyses other than those specifically mentioned above, an alpha of 5% was used. All analyses were two‐tailed. Statistical analyses were performed by a statistician3 independent of the sponsor by a commercially available software program.8
In preparation of the manuscript, authors followed the recommendations given in the CONSORT statement for reporting randomized clinical trials.20
Results
Recruitment to the study began in October 2010 and finished in June 2013. Follow‐up was continued until the study was closed on the first of March 2015. The interim analysis was conducted in January 2015 on data collected up to October 1, 2014, in which the criteria for unblinding and stopping the study were met with respect to the time to primary endpoint. The statistician and 1 lead investigator (AB) were therefore unblinded at this time. All other lead investigators (JH and SG) and those involved in data management remained blinded until data entry was complete.
Three hundred and sixty dogs were enrolled in the study and randomized to receive trial medication. The median number of dogs recruited per center was 9 (range 1–20). One dog was allocated a case number but did not complete enrollment and never received trial medication. The outcome for the remaining 359 dogs in the ITT population is summarized in Figure 1. After randomization, 4 dogs randomized to placebo and 1 dog randomized to pimobendan were excluded because they were subsequently found to have failed an inclusion criterion (4 dogs did not meet at least one of the heart size criteria and 1 dog had been chronically pretreated before enrollment to the study). The remaining 354 dogs—178 receiving pimobendan and 176 receiving placebo—comprised the PP population. The PP population consisted of 161 (45.5%) Cavalier King Charles Spaniels (CKCS), 21 (5.9%) Dachshunds, 13 (3.7%) Miniature Schnauzers, 8 (2.3%) each of Poodles and Yorkshire Terriers, 98 (27.7%) other pure breeds, and 45 (12.7%) mixed breeds. The median age of the enrolled population was 9.0 years (IQR 7–11).
Figure 1.
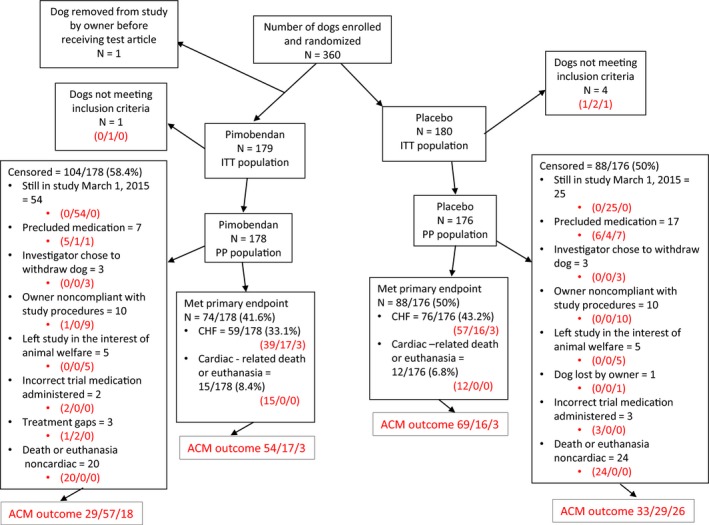
A flow chart indicating the outcome of 360 dogs randomized in the study. Where 3 numbers appear in red in the diagram, they represent the outcome of dogs in each subgroup with respect to the all‐cause mortality analysis. They indicate, in the order in which they appear, dogs known to have died/dogs known to be alive/dogs lost to follow‐up. ACM, all‐cause mortality, ITT; intention to treat; PP, per protocol; CHF, congestive heart failure.
Baseline characteristics of the 2 groups were summarized and compared (Table 4). The groups were balanced for all baseline variables with the exception of breed and BCS. In the PP population of dogs receiving pimobendan, the median dose received was 0.49 mg/kg/d (IQR 0.441–0.528) divided into 2 doses.
Table 4.
Baseline characteristics of the 2 treatment groups in the per‐protocol population and their comparison. Continuous variables are reported as median (interquartile range). Categorical variables are reported as number (%)
| Variable | Treatment groups | P‐value | ||
|---|---|---|---|---|
| Pimobendan n = 178 | Placebo n = 176 | |||
| Dog characteristics | Age (years) | 9.0 (8.0–11.0) | 9.0 (7.0–11.0) | .80 |
| Sex (M/F/MC/FS) (%) | 36/6/75/61 (20/3/38/34) | 35/12/66/63 (20/7/38/36) | .46 | |
| Breed (CKCS/Dachshund/Miniature Schnauzer/Poodle/Yorkshire terrier/mixed breed/other) (%) | 77/12/5/4/0/26/54 (43/7/3/2/0/15/30) | 84/9/8/4/8/19/44 (47/5/5/2/5/11/25) | .02 | |
| Comorbidities | Known comorbidities (yes/no)(%) | 76/102 (43/57) | 71/105 (40/60) | .67 |
| Dose of test medication | Dose pimobendan (mg/kg/day) | 0.49 (0.44–0.53) | NA | NA |
| Quality of life and respiratory variables (See Table S1 for details) | Appetite (decreased/normal/increased) (%) | 4/165/9 (2/93/5) | 3/166/7 (2/94/4) | .89 |
| Demeanor (alert/mildly lethargic/moderately lethargic)(%) | 175/3/0 (98/3/0) | 168/7/1 (95/4/1) | .16 | |
| Exercise tolerance (very good/good/decreased) (%) | 118/53/7 (66/30/4) | 99/70/7 (56/40/4) | .13 | |
| Fainting (none/rarely/occasional) (%) | 175/3/0 (98/2/0) | 170/4/2 (97/2/1) | .35 | |
| Respiratory effort (normal/mildly increased/moderately increased) (%) | 172/5/1 (97/3/1) | 164/10/2 (93/6/1) | .29 | |
| Cough (none/occasional/frequent/persistent) (%) | 108/48/20/2 (61/27/11/1) | 123/35/17/1 (70/20/10/1) | .32 | |
| Nocturnal coughing (none/slight/moderate) (%) | 157/20/0 (89/11/0) | 163/10/2 (93/6/1) | .06 | |
| Physical examination variable | Body weight (kg) | 8.6 (6.9–10.6) | 9.0 (7.1–10.5) | .65 |
| Body condition score (underweight (1–3)/normal (4–6)/overweight (7–9)) (%) | 0/166/12 (0/93/7) | 5/148/23 (3/84/13) | .006 | |
| Rectal temperature (°C) | 38.7 (38.4–39.1) | 38.7 (38.4–39.0) | .69 | |
| Heart rate (BPM) | 124 (110–140) | 122 (112–140) | .96 | |
| Respiratory rate (breaths/min) | 28 (24–36) | 28 (24–36) | .65 | |
| Systolic arterial blood pressure (mmHg) | 140 (130–155) | 140 (130–160) | .87 | |
| Heart murmur intensity (moderate (grade 3–4)/severe (grade 5–6))(%) | 133/45 (75/25) | 133/43 (76/24) | .90 | |
| Diagnostic imaging variables | VHS | 11.3 (10.9–12.0) | 11.5 (11.0–11.9) | .12 |
| LVIDs (cm) | 2.0 (1.75–2.36) | 2.0 (1.74–2.28) | .33 | |
| LVIDSN | 1.03 (0.93–1.14) | 1.02 (0.93–1.10) | .26 | |
| LVIDd (cm) | 3.61 (3.29–4.01) | 3.61 (3.27–3.90) | .76 | |
| LVIDDN | 1.9 (1.8–2.1) | 1.9 (1.8–2.0) | .83 | |
| FS% (%) | 43 (39–48) | 44 (41–49) | .17 | |
| LA/Ao | 1.89 (1.73–2.10) | 1.86 (1.72–2.06) | .60 | |
| Laboratory variables | Na+ (mmol/l) | 148 (145–150) | 148 (146–149) | .54 |
| K+ (mmol/l) | 4.4 (4.1–4.8) | 4.4 (4.1–4.7) | .30 | |
| PCV (%) | 44.0 (41.0–48.0) | 44.0 (40.0–48.1) | .82 | |
| Creatinine (micromol/L) | 70.7 (60.0–88.4) | 70.7 (61.0–82.2) | .72 | |
| TPC (g/L) | 65 (61–69) | 66 (62–70) | .13 | |
| GPT (ALT) (U/L) | 43 (29–68) | 42 (30–66) | .83 | |
ALT, alanine aminotransferase; BCS, body condition score; BPM, beats per minute; CKCS, Cavalier King Charles Spaniels; F, female; FS, female spayed; FS%, fractional shortening; GPT, glutamic pyruvate transaminase; K+, potassium concentration; LA/Ao, left atrial‐to‐aortic root ratio; LVIDd, left ventricular internal diameter in diastole; LVIDDN, normalized left ventricular internal diameter in diastole; LVIDs, left ventricular internal diameter in systole; LVIDSN, normalized left ventricular internal diameter in systole; M, male; MN, male neutered; Na+, sodium concentration; PCV, packed cell volume; TPC, total protein concentration; VHS, vertebral heart sum.
P‐values that appear in bold are < 0.05.
The median time in study for the PP population was 623 days (IQR 292–959). One hundred and sixty‐two of the 354 dogs reached the primary endpoint giving an overall event rate of 45.8%. The median time in study for all dogs that reached the primary endpoint was 433.5 days (IQR 242‐718). One hundred and ninety‐two dogs were censored from the analysis of the primary endpoint, including 104 dogs receiving pimobendan and 88 dogs receiving placebo. Of these, 79 dogs (54 dogs receiving pimobendan and 25 dogs receiving placebo) were still alive, not having reached the primary endpoint, at the end of the study. The median time in study for these dogs was 1056 days (IQR 976‐1238). The cause of censoring for the remaining 113 dogs is shown in Figure 1. The median time in study for these 113 dogs was 502 days (IQR 228–787). The proportion of dogs reaching the primary endpoint was not different between groups (pimobendan, 74/178 (41.6%); placebo, 88/176 (50.0%); P = .14).
The estimated median time to the primary endpoint was 1228 days (95% confidence intervals (CI): 856–NA) in the pimobendan group and 766 days in the placebo group (95% CI: 667–875) (Fig 2). The risk of a dog in the pimobendan group reaching the primary endpoint, at any time point, was lower (P = .0038) and the hazard ratio was 0.64 (95% CI: 0.47–0.87) compared with a dog in the placebo group. The times taken for dogs in the 2 treatment groups to reach the individual components of the composite primary endpoint were compared and are summarized in Table 5. The proportions of dogs in each group experiencing each of the 3 individual components of the primary endpoint, CHF, spontaneous cardiac death, and cardiac euthanasia, were not different between groups (P = .063, P = .134, and P = .218).
Figure 2.
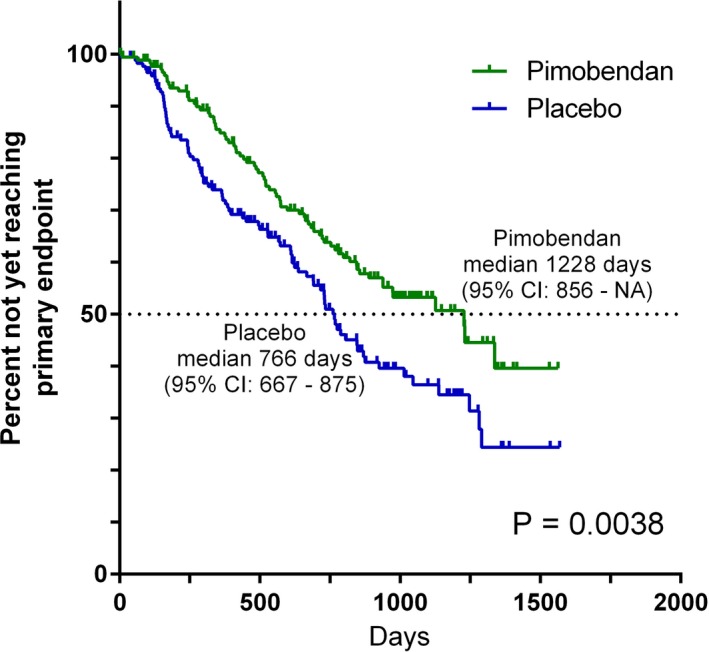
Kaplan‐Meier survival curves plotting the estimated percentage of dogs in each group in the per‐protocol population that have not yet met the primary endpoint (congestive heart failure or cardiac death), against time. There were 178 dogs in the pimobendan group and 176 dogs in the placebo group at the outset. CI, confidence interval; NA, not able to calculate.
Table 5.
Subanalyses of the primary endpoint
| Sub‐endpoint analyzed | Group | Number reaching components of the primary endpoint in the final analysis | Number censored | Median time to reaching sub‐endpoint (95% confidence interval) (days) | Hazard ratio of the pimobendan group compared to the placebo group (95% confidence interval) | P‐value for comparison (log‐rank) | |
|---|---|---|---|---|---|---|---|
| Verified congestive heart failure | Pimobendan | 59/178 (33.1%) | 119/178 (66.9%) | 1337 (1126‐NA) | 0.58 (0.42–0.82) | .0018 | |
| Placebo | 76/176 (43.2%) | 100/176 (56.8%) | 846 (730–1138) | ||||
| Cardiac death or euthanasia | Pimobendan | 15/178 (8.4%) | SCD = 12/178 (6.7%) | 163/178 (91.6%) | Median not reached in 1570 days (NA) | 0.96 (0.45–2.1) | .92 |
| CE = 3/178 (1.7%) | |||||||
| Placebo | 12/176 (6.8%) | SCD = 5/176 (2.8%) | 164/176 (93.2%) | Median not reached in 1570 days (1282‐NA) | |||
| CE = 7/176 (4.0%) |
CE, cardiac euthanasia; NA, Not able to calculate; SCD, spontaneous cardiac death.
Nine dogs initially thought to have developed CHF did not have the presence of radiographic signs of CHF verified by the endpoint committee. Three of these dogs were in the pimobendan group and 6 in the placebo group. Failure to confirm CHF was either due to radiographs being present but failing to demonstrate evidence of an interstitial/alveolar lung pattern, or radiographs not having been obtained at the time of the CHF event. In 8 of these cases, medications listed in Table 1 were administered and these dogs were therefore censored at the time of the suspected heart failure for receiving precluded medication. In 1 case, severe clinical signs resulted in euthanasia of the patient before radiographic confirmation of CHF and this was subsequently (before unblinding) reclassified as a cardiac‐related euthanasia.
In the time‐to‐first‐event analysis, 130 dogs in the pimobendan group and 158 dogs in the placebo group experienced an event. Forty‐eight dogs in the pimobendan group and 18 dogs in the placebo group were censored in this analysis. The median time to the first event was 640 (95% CI: 555–753) days in the pimobendan group versus 406 (95% CI: 316–527) days in the placebo group (P < .0001) (Fig 3). The number of first events attributable to the introduction of medications from Tables 1 and 2 and their reasons for introduction are summarized according to treatment group in Table 6.
Figure 3.
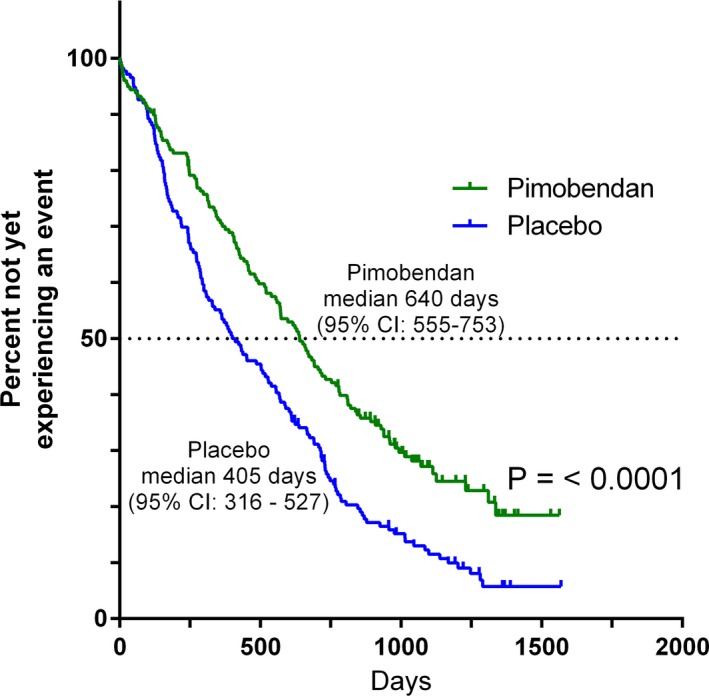
Kaplan‐Meier survival curves plotting the estimated percentage of dogs in each group in the per‐protocol population that have not yet experienced an event (defined as having reached the primary endpoint, undergone euthanasia or died for a noncardiac reason, had chronic medication initiated (Table 1 or Table 2), had a congestive heart failure endpoint that was not verified by the endpoint committee, the owner became noncompliant with study procedures, or the dog was withdrawn from the study by the owner or investigator), against time. There were 178 dogs in the pimobendan group and 176 dogs in the placebo group at the outset. CI, confidence interval.
Table 6.
Numbers of dogs for which administration of medication represented the first event in the “time to first event” including the type of medication administered and the reasons given for initiating medication. Note that more than 1 reason could be given for administration of medication and therefore the numbers in the “reason for administration” may not add up to the total in the adjacent column
| Indication | Type of medication introduced | Pimobendan | Placebo | Total | ||
|---|---|---|---|---|---|---|
| Number (% of those at risk) | Reason for administration | Number (% of those at risk) | Reason for administration | Number (% of those at risk) | ||
| Cardiac indication | Table 1 medication | 8/178 (4.5%) |
Suspected but not verified CHF = 3 Weakness, collapse anorexia = 3 Persistent tachypnoea = 1 Systolic arterial hypotension = 1 Pulmonary hypertension = 2 Coughing = 2 Poor body condition = 1 Syncope = 2 Inappetence = 1 |
13/176 (7.4%) |
Suspected but not verified CHF = 5 Persistent tachypnoea = 1 Arrhythmia = 2 Coughing = 2 Syncope = 1 Right‐sided heart failure = 1 Persistent sinus tachycardia = 1 |
21/354 (5.9%) |
| Table 2 medication | 1/178 (0.6%) | Coughing | 3/176 (1.7%) |
Coughing = 3 Weakness, collapse, anorexia = 1 |
4/354 (1.1%) | |
| Noncardiac indication | Table 1 medication | 3/178 (1.7%) |
Dental procedure = 2 Neurological disease = 1 |
3/176 (1.7%) |
Sedation/anesthesia = 2 Proteinuria = 1 |
6/354 (1.7%) |
| Table 2 medication | 34/178 (19.1%) |
Coughing = 19 Dermatologic disease = 7 Immune‐mediated disease = 4 Neurologic disease = 2 Endocrinopathy = 1 Unknown = 1 |
32/176 (18.2%) |
Coughing = 19 Neurologic disease = 3 Dermatologic disease = 3 Endocrinopathy = 1 Respiratory disease = 3 Immune‐mediated disease = 1 Behavioral problem = 1 Hypertension = 1 |
66/354 (18.6%) | |
| Total | 46/178 (25.8%) | 51/176 (29.0%) | 97/354 (27.4%) | |||
The hazard ratio for the treatment effect, when adjusted for the individual effect of each of the 32 baseline variables, did not change substantially (range 0.575–0.665), and the 95% confidence intervals of the estimated hazard ratio never included the value 1 (Figure S2). In the univariate Cox proportional hazards analysis, in addition to treatment, of the 32 baseline variables tested, the following 16 variables demonstrated an association with the time to primary endpoint at P < .2 and were entered in the first iteration of the multivariable analysis: Miniature Schnauzer (yes/no), appetite, exercise tolerance, fainting, respiratory effort, cough, nocturnal coughing/dyspnea, respiratory rate, heart rate, SAP, VHS, FS%, LVIDDN, LA/Ao, PCV, and creatinine. The final model of the multivariable analysis is summarized in Table 7 (and Figure S3). The final multivariable model without inclusion of echocardiographic measurements is detailed in Table 8.
Table 7.
The final explanatory Cox proportional hazards multivariable model
| Variable | Hazard ratio (HR) | 95.0% confidence interval of the hazard ratio | P‐value |
|---|---|---|---|
| Pimobendan (versus Placebo) | 0.539 | 0.389–0.747 | .0002 |
| Decreased appetite (yes) | 2.558 | 1.029–6.361 | .0433 |
| FS% (HR for 10% increment) | 1.318 | 1.051–1.644 | .0168 |
| LVIDDN (HR for 0.1 unit increment) | 1.214 | 1.116–1.319 | <.0001 |
| LA/Ao (HR for 0.1 unit increment) | 1.112 | 1.061–1.174 | <.0001 |
FS%, fractional shortening; HR, hazard ratio; LA/Ao, left atrial‐to‐aortic root ratio; LVIDDN, normalized left ventricular internal diameter in diastole.
Table 8.
The final exploratory Cox proportional hazards multivariable model without echocardiographic indices
| Variable | Hazard ratio (HR) | 95.0% confidence interval of the hazard ratio | P‐value |
|---|---|---|---|
| Pimobendan (versus Placebo) | 0.623 | 0.454–0.855 | .0033 |
| Heart rate (beats per minute (bpm)) (HR for 10 bpm increment) | 1.116 | 1.030–1.207 | .0044 |
| Systolic arterial blood pressure (mmHg) (HR for 10 mmHg increment) | 0.895 | 0.825–0.970 | .0072 |
| Vertebral heart sum (0.1 unit increment) | 1.027 | 1.008–1.047 | .0047 |
HR, hazard ratio.
With respect to the safety analyses in the ITT population, there were no significant differences between groups in the type and severity of adverse events experienced. The proportion of dogs in each group experiencing adverse events during the study was not different (P = .82) (Table 9).
Table 9.
The nature and severity of adverse events experienced by the dogs in the 2 treatment groups during the study. In the course of the study, dogs could experience more than 1 adverse event or experience more than 1 clinical sign at the time of an adverse event
| Pimobendan N = 179 | Placebo N = 180 | Total N = 359 | ||
|---|---|---|---|---|
| Number of dogs experiencing at least 1 severe or worse adverse event | 19 (10.6%) | 19 (10.6%) | 38 (10.6%) | P = .82 |
| Number of dogs experiencing at least 1 mild or moderate adverse event (but not a severe or worse event) | 61 (34.1%) | 67 (37.2%) | 128 (35.7%) | |
| Number of dogs experiencing no adverse events | 99 (55.3%) | 94 (52.2%) | 193 (53.8%) | |
| Number of recorded adverse events | ||||
| Severe or worse | 23 | 21 | 44 | |
| Mild or moderate | 145 | 153 | 298 | |
| Total | 168 | 174 | 342 | |
| Frequency of specifically recorded adverse events | ||||
| Diarrhea | 21 | 14 | 35 | |
| Vomiting | 27 | 27 | 54 | |
| Anorexia | 7 | 12 | 19 | |
| Lethargy | 13 | 15 | 28 | |
| Tachycardia | 4 | 3 | 7 | |
| Other | 124 | 147 | 271 | |
| Total | 196 | 218 | 414 | |
One hundred and eighty‐six dogs were known to have died by any cause at the date of study closure, 83 of 179 dogs (46.4%) in the pimobendan group and 103 of 180 dogs (57.2%) in the placebo group (P = .0260). The median time to death by all causes was 1059 (95% CI: 952–NA) days in the pimobendan group and 902 (95% CI: 747–1061) days in the placebo group (P = .012) (Figure S4).
Discussion
Myxomatous mitral valve disease is the leading cause of heart disease in dogs, and development of CHF results in substantial morbidity and mortality.2, 21 The EPIC study has shown, for the first time, convincing evidence of benefit of a treatment before the onset of CHF in dogs with cardiac enlargement secondary to MMVD (stage B2). Dogs receiving pimobendan had approximately two‐thirds the risk of reaching the primary endpoint compared with dogs on placebo. This resulted in a 60% prolongation of the preclinical period with dogs receiving pimobendan taking, on average, an additional 462 days, or approximately 15 months, to develop CHF or die as a consequence of MMVD. The subanalyses of the primary endpoint suggest that the majority of this benefit was attributable to delaying the onset of CHF, with relatively few dogs meeting the endpoint by experiencing cardiac death or euthanasia (Table 5). Overall survival was longer in the dogs receiving pimobendan in the ITT population, which included dogs experiencing death or euthanasia due to noncardiac causes. This indicates that chronic administration of pimobendan in this population was safe. Fewer dogs in the pimobendan group were censored before reaching the end of the study. The treatment effect of pimobendan was robust and persisted comparatively unchanged after adjustment for each of the baseline characteristics, either individually, or in combination in the multivariable analyses. In the explanatory multivariable analysis, the treatment effect was strengthened with a lower hazard ratio and narrower confidence intervals after the confounding effect of other dog characteristics measured at baseline was taken into account.
As well as confirming the strength of the treatment effect, the multivariable analysis confirmed the predictive importance of absolute heart size with respect to the onset of CHF as previously reported.22, 23 Two separate echocardiographic indicators of heart size, LA/Ao and LVIDDN, were strongly and independently associated with time to the primary endpoint in the final explanatory multivariable model. Two other nontreatment variables remaining in the model were increased FS% and reduced appetite. Increased FS% might, in this situation, represent a surrogate measure of the severity of MR with a larger total left ventricular stroke volume being associated with worse regurgitation. The significant effect of reduced appetite persisted in the final model despite there being relatively few dogs with this finding. This supports the previously described negative prognostic significance of reduced appetite in dogs with MMVD.24
The exploratory multivariable model, excluding echocardiographic variables, confirmed the prognostic value of widely used clinical and radiographic measurements. This analysis was performed in order to provide information of prognostic value for patients encountered in a setting where accurate echocardiographic measurements might not be available; however, it should be borne in mind when interpreting these results that all dogs included in the analyses also met the echocardiographic inclusion criteria and these results therefore might not be generalizable to all dogs with a VHS > 10.5 in the absence of concurrent echocardiographic measurements and a confirmed diagnosis of MMVD. In our population, increased VHS, lower SAP, and higher heart rate were all independently associated with a worse outcome, although reduced appetite was not found to be significant in this model. The fact that appetite was not included in both final models (explanatory and exploratory) suggests that the effect of this variable is not stable when other variables are considered at the same time. Although a lower (but within reference range) blood pressure has previously been described in dogs with severe MMVD, when compared to dogs with mild to moderate disease,25, 26 this is the first study to demonstrate a worse outcome associated with lower blood pressure, even though no dog enrolled to the study would have been considered clinically hypotensive. Radiographically evident cardiomegaly and increased heart rate have previously been reported to predict a worse outcome.6, 7
The EPIC study design has several notable strengths. It is the largest prospective, randomized clinical trial in small animal cardiovascular medicine. It is comparable in size to average‐sized studies of human patients with cardiovascular disease; with 360 enrolled patients, it would fall within the interquartile range of similar studies of human patients.27 All CHF endpoints were independently verified by an endpoint committee. Events other than reaching the primary endpoint which could have been influenced by the administration of trial medication were also captured in the secondary endpoint of “time to first event”. Analysis of this secondary endpoint demonstrated a significant difference in favor of the group receiving pimobendan, with a P‐value lower than that found in the primary endpoint analysis. This might imply that pimobendan delayed the onset of events other than those incorporated in the primary endpoint, for example, coughing, right‐sided CHF, and syncope. An additional strength of the study is the absence of the confounding effect of any concurrent cardiovascular medications; however, this restriction led to it being necessary to censor a number of the dogs from the primary endpoint analyses when the introduction of these drugs was required. A greater number of dogs in the placebo group needed to be censored from the study for this reason.
This is the first veterinary cardiology study to be terminated after a planned interim analysis with prospectively defined stopping criteria, having demonstrated convincing evidence of efficacy with respect to the primary endpoint. The decision to stop a clinical trial is a difficult one and involves weighing up the validity of the conclusions that can be drawn from a foreshortened study against the risks to animals remaining in a treatment trial where 1 method of treatment appears clearly more efficacious than the other.28 There are risks associated with the premature termination of studies in response to results obtained at interim analyses. Such a study might overestimate the benefit of the treatment effect by basing the results on observation of a smaller number of events than originally anticipated.29 The risk of such an erroneous result is relatively low in the current study. Only 1 interim analysis was planned and only 1 was conducted. This was performed after 4 years when the study was almost 80% complete and all the anticipated patients had been recruited to the study. The final analysis included data acquired during a further 5 months of follow‐up, meaning that the final duration of the study was nearly 90% of the duration originally planned. Finally, to minimize the risk of obtaining a “false‐positive” study result, the O'Brien‐Fleming alpha spending function19 was used to set the P‐value to be used for the interim analysis and the adjustment of the P‐value in the final analysis.
Cardiac‐related death, either spontaneous or due to euthanasia, was incorporated as part of the primary endpoint in the EPIC study, as this was known to be an infrequent but important manifestation of MMVD that might occur in dogs with stage B2 disease. An important reason to include, rather than censor, this outcome was that concerns had previously been raised about possible detrimental effects of the administration pimobendan to dogs with preclinical MMVD.30, 31 If our primary endpoint had focused exclusively on the onset of CHF, with dogs that died being censored, it might have appeared that we were choosing to ignore potentially detrimental effects of the treatment. We found, not unexpectedly, that only a small number of dogs met the primary endpoint in this way. Although a greater number of dogs in the pimobendan group experienced spontaneous cardiac death (12 versus 5), the proportion of dogs in each group experiencing this event was not significantly different. A greater number of dogs in the placebo group underwent cardiac‐related euthanasia (7 versus 3), but again the difference in proportions between groups was not significant. Subanalysis of the time to the separate components of the primary endpoint suggested that the difference in outcome observed between groups is largely attributable to a delay in the onset of signs of CHF. Any previously raised concerns regarding the safety of the medication should be allayed by the longer survival observed in the pimobendan group in the all‐cause mortality analysis.
There was no difference between groups in the rate or type of potential adverse events observed, indicating that pimobendan administration is safe and well tolerated. This is despite the fact that dogs in the pimobendan group spent longer in the study and therefore were at risk of experiencing adverse events for a longer period.
This clinical trial was fully sponsored by a pharmaceutical company (as acknowledged), and industry funding of studies is recognized as a potential source of bias in clinical trials.32 However, it remains the case in studies of human patients that the majority of published clinical trials are sponsored by industry.33 In the veterinary field, there are no large independent funding organizations comparable to the National Institutes of Health (NIH) that are likely to be able to (or wish to) fund a study of the magnitude of the one we describe; therefore, such studies are only likely to be achieved with industry sponsorship. Potential explanations of the increased likelihood of industry‐sponsored research having positive findings include the following: Industrial sponsors might be more likely to support studies that are likely to succeed, industrial sponsors might design studies with an inappropriate comparator group, or there might be publication bias with unsuccessful studies being less likely to be published.34 In the current study, evaluating the use of pimobendan for an indication where there is currently no evidence of a benefit of other treatment, the most appropriate comparator is a placebo, as was used. The risk of publication bias was minimized by the existence of a clause in the lead investigators' contracts guaranteeing the right to publish irrespective of outcome.
Limitations
Diagnosis of CHF and confirmation of cardiac‐related death remain a challenge in clinical trials. In this study, we attempted to minimize the impact of the first of these challenges by use of an independent blinded consensus verification of CHF endpoints.
It is not possible to irrefutably confirm whether any death is cardiac related or not. In the case of dogs that died spontaneously, the investigator classified the death as being cardiac or noncardiac on the basis of the circumstances of the death and their own opinion. Postmortem examinations were not systematically undertaken, and it is therefore possible that in some cases, death was attributed to cardiac disease when in fact it was due to another condition (and vice versa). The study also allowed cardiac‐related euthanasia as part of the primary endpoint. Euthanasia is influenced by owner‐related factors, but it is likely to be precipitated by the animal having a poor quality of life, and therefore, the frequency with which it occurs is likely to be reduced by therapies that delay the onset of clinical signs. The impact of individual owner‐related factors on this outcome should be minimized by randomization of a large population, as was done in this study. Comparatively few dogs met the primary endpoint through experiencing spontaneous cardiac‐related death or euthanasia. The subanalysis of this component of the primary endpoint did not show a significant difference between groups. It appears that most of the observed effect of pimobendan can be attributed to the delay in the onset of CHF; therefore, the impact of any uncertainty in the classification of such cases should be minimal and is likely to be equally distributed between groups.
The recruitment period of the trial was longer than originally expected, but this has had no impact on the outcome of the study, as it was prematurely closed after the findings of the interim analysis.
After the primary endpoint had been reached, all dogs that developed CHF received pimobendan as part of their treatment regime, but other treatment was no longer controlled. Variability of such treatment could not have had an impact on the major conclusions of the study, but introduced a confounding factor in the all‐cause mortality analysis.
Generalizability of the Study
The results of this study are readily generalizable to the large population of dogs at risk of developing MMVD. The population recruited to the study was large, recruited in numerous countries, by many investigators, and is typical of dogs with this disease. The breed, weight, and age distribution of the population are similar to those of other populations described with this condition.4, 35 It is also notable that the outcome for the placebo group, particularly in time to the onset of CHF and event rate, in the current study, was very similar to that reported in previous studies conducted in dogs with preclinical MMVD.6, 7 The restricted weight range of dogs recruited to the study reflected the weight range for which appropriate verum and placebo study medication were available and was not because of any hypothetical limited weight range in which the treatment effect was expected to be seen. It is therefore likely, although not proven, that the conclusions of this study can be extrapolated to dogs of any bodyweight with stage B2 MMVD. The conclusions of this study are only relevant to dogs with cardiac enlargement secondary to preclinical MMVD (stage B2) as all dogs entering the study met or exceeded 3 different heart size criteria (LA/Ao ≥ 1.6, LVIDDN ≥ 1.7, and VHS > 10.5) and no dogs without cardiac enlargement were recruited to the study. Similarly, the conclusions are only relevant to dogs with a murmur of at least a grade 3/6 in intensity. Treatment with pimobendan of all dogs that have a murmur compatible with the presence of MMVD would not be justified on the basis of the findings of this study.
Conclusions
Chronic oral administration of pimobendan to dogs with echocardiographic and radiographic evidence of cardiomegaly secondary to MMVD, in the absence of concurrent cardiovascular medication, results in the prolongation of the preclinical period, and is safe and well tolerated. The median time to the onset of CHF or cardiac‐related death was prolonged by approximately 15 months, and the risk of a dog experiencing this event was reduced by approximately one‐third; the majority of the benefit observed was attributable to delaying the onset of CHF. This substantial degree of prolongation of the preclinical period is of clinical relevance and is of importance to veterinarians and owners of dogs affected by this common disease.
Supporting information
Fig S1. Flow diagram illustrating the pre‐planned decision making process at the time of the interim analysis.
Fig S2. A forest plot showing the hazard ratio and 95% confidence interval estimated from Cox Proportional Hazards analysis for treatment effect from the multivariate analysis (Pimobendan MV), from a univariate analysis (Pimobendan only) and from bivariate Cox Proportional Hazards analyses including treatment analyzed together with each of the 32 baseline variables separately, with time to the primary endpoint (congestive heart failure or cardiac related death) as the dependent variable. The hazard ratio for the treatment effect, when adjusted for the individual effect of each of the baseline variables, did not change substantially and the 95% confidence intervals of the estimated hazard ratio never included the value 1. CKCS, Cavalier King Charles Spaniel; VHS, Vertebral heart sum; LVIDSN, normalized left ventricular internal diameter in systole; LVIDDN, normalized left ventricular internal diameter in diastole; FS, fractional shortening; LA/Ao, left atrial to aortic root ration; Na, Sodium concentration; K, Potassium concentration; PCV, packed cell volume; GPT, Glutamic‐Pyruvate Transaminase. Note the horizontal axis is plotted on a logarithmic scale.
Fig S3. A forest plot showing the hazard ratio and 95% confidence intervals associated with variables remaining in the final explanatory mutivariable Cox Proportional Hazards analysis with time to the primary endpoint (congestive heart failure or cardiac related death) as the dependent variable. Circles represent the hazard ratio and the horizontal bars extend from the lower limit to the upper limit of the 95% confidence interval of the estimate of the hazard ratio. LA/Ao, left atrial to aortic root ratio; LVIDDN, normalized left ventricular internal diameter in diastole; FS%, fractional shortening. (〈0.1 unit) indicates that the hazard ratio illustrated is for a 0.1‐unit increment in the variable of interest; (〈10%) that the hazard ratio illustrated is for a 10% increment in the variable of interest. Note the horizontal axis is plotted on a logarithmic scale.
Fig S4. Kaplan Meier survival curves plotting the estimated percentage of dogs in each group in the intention to treat population that have not yet died, against time. There were 179 dogs in the pimobendan group and 180 dogs in the placebo group at the outset. CI, confidence interval; NA, Not able to calculate.
Table S1 Different events and the consequence of those events in the different time to event analyses undertaken on the per protocol population. Abbreviation: CHF, congestive heart failure.
Acknowledgments
We thank Martin Vanselow for performing statistical analyses; Ray Dillon and Christoph Lombard, members of the interim analysis committee; Olaf Joens, Christoph Schummer, Robert Jones, Auddie Sharp, Kurt Petersen, Lolita Nilsson, Fabrice Thoulon, and Jacques Gossellin for monitoring and administrative support during the study; Kevin Christiansen, Laura Happon, Lisa Cellio, Mel Davis Giorgia Santarelli, Michele Borgarelli, Mari Waterman, Sonya Wesselowski, Michael Aherne, Karen Johnson, Jess Douthat, Linda Slater, Kathy Glaze, Jill VanWhy, Amy Savarino, Matthew Miller, Crystal Hariu, Ryan Fries, Justin Carlson, Randolph Winter, Jordan Vitt, Kay Naden, Véronique Birault, Kristen Antoon, Suzanne Cunningham, Sarah Miller, Peter Holler, Julia Simak, Mary Perricone, April Jackson, Michele Dolson, Regan Rising, Curt Rehling, Geri Lake‐Bakaar, Julie Martin, Herbert W. Maisenbacher, Ashley Jones, Melanie Powell, Brandy Winter, Mary Bohannon, Heidi Chambers, Alice Defarges, Shauna Blois, Anthony Abrams‐Ogg, Nevene Borozan, Kristin A. Jacob, Heather Wink, Dina Berriochoa, Steven Ettinger, and Megan Buckner for assistance in recruitment and management of cases.
Conflict of Interest Declaration: This project was funded by Boehringer Ingelheim Animal Health GmbH. A representative of Boehringer Ingelheim Animal Health GmbH read the final draft before submission. Philip Watson is an employee of Boehringer Ingelheim Animal Health GmbH.
All other authors have received funding from Boehringer Ingelheim Animal Health GmbH within the last 5 years for some or all of the following activities: research, travel, speaking fees, consultancy fees, and preparation of educational materials.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The planning of the EPIC trial was started in 2009. The trial protocol was finalized at investigator meetings in March 2010. There were follow‐up meetings in August 2013, and the submitted manuscript was finalized and approved by the investigators at meetings and by correspondence in April and May 2016. The EPIC Lead committee consisted of Adrian Boswood, Jens Häggström, and Sonya Gordon, and the EPIC endpoint committee consisted of the 3 lead investigators, Gerhard Wess, and Rebecca Stepien. The EPIC interim analysis committee consisted of Christoph Lombard, Ray Dillon, and Martin Vanselow.
Footnotes
Catalent Pharma Solutions, Somerset, NJ
ADLS and Clinicopia
Martin Vanselow
Body condition score chart, Ralston Purina Company, St Louis, Mo
Ondax Scientific, Hondarribia, Spain
nQuery, Statistical Solutions Ltd, Cork, Ireland
nTerim version 1.1, Statistical Solutions Ltd, Cork, Ireland
SAS Version 8.2; SAS Institute Inc., Cary, NC
References
- 1. Buchanan JW. Prevalence of cardiovascular disorders In: Fox PR, Sisson D, Moise NS, eds. Textbook of Canine and Feline Cardiology. Philadelphia: Saunders, W.B.; 1999:457–470. [Google Scholar]
- 2. Egenvall A, Bonnett BN, Hedhammar A, et al. Mortality in over 350,000 insured Swedish dogs from 1995‐2000: II. Breed‐specific age and survival patterns and relative risk for causes of death. Acta Vet Scand 2005;46:121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med 2009;23:1142–1150. [DOI] [PubMed] [Google Scholar]
- 4. Borgarelli M, Savarino P, Crosara S, et al. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med 2008;22:120–128. [DOI] [PubMed] [Google Scholar]
- 5. Haggstrom J, Boswood A, O'Grady M, et al. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: The QUEST study. J Vet Intern Med 2008;22:1124–1135. [DOI] [PubMed] [Google Scholar]
- 6. Atkins CE, Keene BW, Brown WA, et al. Results of the veterinary enalapril trial to prove reduction in onset of heart failure in dogs chronically treated with enalapril alone for compensated, naturally occurring mitral valve insufficiency. J Am Vet Med Assoc 2007;231:1061–1069. [DOI] [PubMed] [Google Scholar]
- 7. Kvart C, Haggstrom J, Pedersen HD, et al. Efficacy of enalapril for prevention of congestive heart failure in dogs with myxomatous valve disease and asymptomatic mitral regurgitation. J Vet Intern Med 2002;16:80–88. [PubMed] [Google Scholar]
- 8. van Meel JC, Diederen W. Hemodynamic profile of the cardiotonic agent pimobendan. J Cardiovasc Pharmacol 1989;14(Suppl 2):S1–S6. [PubMed] [Google Scholar]
- 9. Haggstrom J, Lord PF, Hoglund K, et al. Short‐term hemodynamic and neuroendocrine effects of pimobendan and benazepril in dogs with myxomatous mitral valve disease and congestive heart failure. J Vet Intern Med 2013;27:1452–1462. [DOI] [PubMed] [Google Scholar]
- 10. Haggstrom J, Boswood A, O'Grady M, et al. Longitudinal analysis of quality of life, clinical, radiographic, echocardiographic, and laboratory variables in dogs with myxomatous mitral valve disease receiving pimobendan or benazepril: The QUEST study. J Vet Intern Med 2013;27:1441–1451. [DOI] [PubMed] [Google Scholar]
- 11. Summerfield NJ, Boswood A, O'Grady MR, et al. Efficacy of pimobendan in the prevention of congestive heart failure or sudden death in Doberman Pinschers with preclinical dilated cardiomyopathy (the PROTECT Study). J Vet Intern Med 2012;26:1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woolley R, Smith P, Munro E, et al. Effects of treatment type on vertebral heart size in dogs with myxomatous mitral valve disease. Int J Appl Res Vet Med 2007;5:43–48. [Google Scholar]
- 13. Ettinger SJ, Suter PF. Heart sounds and phonocardiography Canine Cardiology. St Louis: Saunders‐Elsevier; 1970:12–39. [Google Scholar]
- 14. Hansson K, Haggstrom J, Kvart C, et al. Left atrial to aortic root indices using two‐dimensional and M‐mode echocardiography in cavalier King Charles spaniels with and without left atrial enlargement. Vet Radiol Ultrasound 2002;43:568–575. [DOI] [PubMed] [Google Scholar]
- 15. Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Vet Intern Med 2004;18:311–321. [DOI] [PubMed] [Google Scholar]
- 16. Hansson K, Haggstrom J, Kvart C, et al. Interobserver variability of vertebral heart size measurements in dogs with normal and enlarged hearts. Vet Radiol Ultrasound 2005;46:122–130. [DOI] [PubMed] [Google Scholar]
- 17. Altman DG, Bland JM. How to randomise. BMJ (Clinical research ed) 1999;319:703–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. J Vet Intern Med 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 19. O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35:549–556. [PubMed] [Google Scholar]
- 20. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ (Clinical research ed) 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mattin MJ, Boswood A, Church DB, et al. Degenerative mitral valve disease: Survival of dogs attending primary‐care practice in England. Prev Vet Med 2015;122:436–42. [DOI] [PubMed] [Google Scholar]
- 22. Lord P, Hansson K, Kvart C, et al. Rate of change of heart size before congestive heart failure in dogs with mitral regurgitation. J Small Anim Pract 2010;51:210–218. [DOI] [PubMed] [Google Scholar]
- 23. Reynolds CA, Brown DC, Rush JE, et al. Prediction of first onset of congestive heart failure in dogs with degenerative mitral valve disease: The PREDICT cohort study. J Vet Cardiol 2012;14:193–202. [DOI] [PubMed] [Google Scholar]
- 24. Lopez‐Alvarez J, Elliott J, Pfeiffer D, et al. Clinical severity score system in dogs with degenerative mitral valve disease. J Vet Intern Med 2015;29:575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ljungvall I, Hoglund K, Carnabuci C, et al. Assessment of global and regional left ventricular volume and shape by real‐time 3‐dimensional echocardiography in dogs with myxomatous mitral valve disease. J Vet Intern Med 2011;25:1036–1043. [DOI] [PubMed] [Google Scholar]
- 26. Petit AM, Gouni V, Tissier R, et al. Systolic arterial blood pressure in small‐breed dogs with degenerative mitral valve disease: A prospective study of 103 cases (2007–2012). Vet J 2013;197:830–835. [DOI] [PubMed] [Google Scholar]
- 27. Butler J, Tahhan AS, Georgiopoulou VV, et al. Trends in characteristics of cardiovascular clinical trials 2001‐2012. Am Heart J 2015;170:263–272. [DOI] [PubMed] [Google Scholar]
- 28. Pocock SJ. When to stop a clinical trial. BMJ (Clinical research ed) 1992;305:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bassler D, Briel M, Montori VM, et al. Stopping randomized trials early for benefit and estimation of treatment effects: Systematic review and meta‐regression analysis. J Am Med Assoc 2010;303:1180–1187. [DOI] [PubMed] [Google Scholar]
- 30. Chetboul V, Lefebvre HP, Sampedrano CC, et al. Comparative adverse cardiac effects of pimobendan and benazepril monotherapy in dogs with mild degenerative mitral valve disease: A prospective, controlled, blinded, and randomized study. J Vet Intern Med 2007;21:742–753. [DOI] [PubMed] [Google Scholar]
- 31. Tissier R, Chetboul V, Moraillon R, et al. Increased mitral valve regurgitation and myocardial hypertrophy in two dogs with long‐term pimobendan therapy. Cardiovasc Tox 2005;5:43–51. [DOI] [PubMed] [Google Scholar]
- 32. Bhandari M, Busse JW, Jackowski D, et al. Association between industry funding and statistically significant pro‐industry findings in medical and surgical randomized trials. Can Med Assoc J 2004;170:477–480. [PMC free article] [PubMed] [Google Scholar]
- 33. Anderson ML, Chiswell K, Peterson ED, et al. Compliance with results reporting at ClinicalTrials.gov. N Engl J Med 2015;372:1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lexchin J, Bero LA, Djulbegovic B, et al. Pharmaceutical industry sponsorship and research outcome and quality: Systematic review. BMJ (Clinical research ed) 2003;326:1167–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mattin MJ, Boswood A, Church DB, et al. Prevalence of and risk factors for degenerative mitral valve disease in dogs attending primary‐care veterinary practices in England. J Vet Intern Med 2015;29:847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Flow diagram illustrating the pre‐planned decision making process at the time of the interim analysis.
Fig S2. A forest plot showing the hazard ratio and 95% confidence interval estimated from Cox Proportional Hazards analysis for treatment effect from the multivariate analysis (Pimobendan MV), from a univariate analysis (Pimobendan only) and from bivariate Cox Proportional Hazards analyses including treatment analyzed together with each of the 32 baseline variables separately, with time to the primary endpoint (congestive heart failure or cardiac related death) as the dependent variable. The hazard ratio for the treatment effect, when adjusted for the individual effect of each of the baseline variables, did not change substantially and the 95% confidence intervals of the estimated hazard ratio never included the value 1. CKCS, Cavalier King Charles Spaniel; VHS, Vertebral heart sum; LVIDSN, normalized left ventricular internal diameter in systole; LVIDDN, normalized left ventricular internal diameter in diastole; FS, fractional shortening; LA/Ao, left atrial to aortic root ration; Na, Sodium concentration; K, Potassium concentration; PCV, packed cell volume; GPT, Glutamic‐Pyruvate Transaminase. Note the horizontal axis is plotted on a logarithmic scale.
Fig S3. A forest plot showing the hazard ratio and 95% confidence intervals associated with variables remaining in the final explanatory mutivariable Cox Proportional Hazards analysis with time to the primary endpoint (congestive heart failure or cardiac related death) as the dependent variable. Circles represent the hazard ratio and the horizontal bars extend from the lower limit to the upper limit of the 95% confidence interval of the estimate of the hazard ratio. LA/Ao, left atrial to aortic root ratio; LVIDDN, normalized left ventricular internal diameter in diastole; FS%, fractional shortening. (〈0.1 unit) indicates that the hazard ratio illustrated is for a 0.1‐unit increment in the variable of interest; (〈10%) that the hazard ratio illustrated is for a 10% increment in the variable of interest. Note the horizontal axis is plotted on a logarithmic scale.
Fig S4. Kaplan Meier survival curves plotting the estimated percentage of dogs in each group in the intention to treat population that have not yet died, against time. There were 179 dogs in the pimobendan group and 180 dogs in the placebo group at the outset. CI, confidence interval; NA, Not able to calculate.
Table S1 Different events and the consequence of those events in the different time to event analyses undertaken on the per protocol population. Abbreviation: CHF, congestive heart failure.


