Abstract
Background
Assessment of blood compatibility, typically by tube agglutination (TUBE) and hemolysis crossmatch or, less commonly, by blood typing and alloantibody screening, often is performed before blood transfusion in horses. In contrast, gel column (GEL) and immunochromatographic strip (STRIP) techniques are preferred for compatibility testing in dogs and cats.
Objective
To determine the accuracy of novel and standard crossmatch and typing methods.
Animals
Thirty‐eight healthy horses, previously blood typed and alloantibody screened.
Methods
TUBE and GEL crossmatches were performed on 146 different recipient‐donor pairs with 56 incompatible TUBE crossmatches. Crossmatches were compared by nonparametric area under the curve of receiver operating characteristic (AUC‐ROC) analyses. Horses also were blood typed by the novel immunochromatographic Ca typing STRIP.
Results
Compared to TUBE crossmatch, GEL had excellent accuracy for agglutination (AUC‐ROC = 0.903), but marginal accuracy for hemolysis (AUC‐ROC = 0.639). Compared to macroscopic TUBE, microscopic TUBE had excellent accuracy for agglutination (AUC‐ROC = 0.912). The predicted crossmatch compatibility based on blood type and alloantibody assay showed excellent accuracy compared to TUBE and GEL (AUC‐ROC = 0.843 and 0.897, respectively). However, there were more recipient‐donor pairs identified as incompatible by both TUBE and GEL than predicted by blood type and antibody screen, suggesting the presence of unidentified alloantibodies. A Ca typing STRIP exhibited 100% sensitivity and specificity for the 35 Ca+ and 3 Ca‐ horses tested.
Conclusions and Clinical Relevance
Gel column crossmatch and Ca typing immunochromatographic strip are simple and accurate methods to evaluate clinical blood compatibility.
Keywords: Agglutination, Blood typing, Crossmatch, Hemolysis
Abbreviations
- ACD
acid‐citrate‐dextrose
- AUC
area under the curve
- EDTA
ethylenediaminetetraacetic acid
- GEL
gel column agglutination test
- MICRO
standard tube agglutination test (microscopic)
- NPV
negative predictive value
- PBS
phosphate‐buffered saline
- PPV
positive predictive value
- PREDICTED
predicted compatibility based on blood type
- QH
Quarter Horse
- RBC
red blood cell
- ROC
receiver operating characteristics
- STBD
Standardbred
- STRIP
Immunochromatographic strip Ca blood typing kit
- TB
Thoroughbred
- TUBE
standard tube agglutination test (macroscopic)
- WB
Warmblood
Blood transfusions are commonly performed to support horses with hemorrhage and anemia. Seven blood group systems (A, C, D, K, P, Q, and U) with >30 red blood cell (RBC) antigens currently are recognized in the horse.1 Because over 400,000 antigenic combinations may occur, horses are never given truly “type‐specific” blood transfusions, and maintaining a herd of blood donors compatible for a variety of recipients can be difficult, particularly when dealing with different breeds.2, 3
Alloantigens on the surface of RBCs may incite alloantibody production when foreign RBC antigens are introduced into an animal. Approximately 90% of horses have no naturally occurring RBC antibodies.3 However, of the approximately 10% that do have alloantibodies, Aa and Ca antibodies are present most often. Horses lacking the Aa antigen frequently have anti‐Aa antibodies, and horses missing the Ca antigen typically have anti‐Ca antibodies.3 One therefore can assume that any horse negative for Aa and Ca antigens may have anti‐Ca and anti‐Aa antibodies. Anti‐Aa antibodies are capable of causing acute hemolytic reactions after incompatible transfusions.4 In addition, alloantibodies can form after pregnancy and transfusion and are most commonly associated with Aa and Qa incompatibilities, whereas incompatibilities associated with Ca are believed to be more rare.5, 6
Ideally, blood typing should be performed to ensure compatibility between donor and recipient horses before any allogeneic blood transfusion. However, equine blood typing to identify suitable blood donors is time‐consuming and limited to only a few laboratories.4, 7 Point‐of‐care canine and feline card, gel column agglutination, and immunochromatographic strip blood typing kits with monoclonal antibodies have been available,8, 9, 10, 11 but no such test has been developed and marketed in horses, mainly because of a lack of monoclonal antibodies.4, 7
In comparison with blood typing, the donor‐recipient crossmatch procedure is more readily available in equine referral clinics as a bench top assay,12 although laborious and requiring considerable expertise. Although the prospect of achieving a perfectly type‐specific donor is unlikely, a crossmatch may be performed to minimize the risk of sensitization and subsequent adverse hemolytic transfusion reactions.2 The majority of equine clinics currently use standard tube agglutination and hemolysis methods for major and minor crossmatch. To date, there have been no studies in horses evaluating the efficacy of the gel column agglutination crossmatch method used in humans and small animals13 compared to tube agglutination methods.
The primary objective of our study was to assess the accuracy of gel column (GEL) crossmatch and standard tube agglutination (TUBE) crossmatch by fresh equine blood from predicted incompatible and compatible blood pairings, based on previous blood type and alloantibody screen. We hypothesized that the GEL would be an accurate and easier method of evaluating crossmatch compatibility as compared to the TUBE methodology. A second objective was to evaluate a novel point‐of‐care immunochromatographic strip kit (STRIP) for Ca blood typing in horses. We hypothesized that the STRIP method would be an accurate method for Ca blood typing.
Materials and Methods
Animals
A total of 38 horses housed at the University of California‐Davis Center for Equine Health were used in this study. All protocols were approved under the University of California‐Davis Institutional Animal Care and Use Committee, and horses were housed in accordance with federal guidelines for the humane care and use of laboratory animals. Blood samples (20 mL) were collected from 1 jugular vein from each horse into no‐additive tubes, tubes containing K3‐ethylenediaminetetraacetic acid (EDTA),1 and tubes containing acid‐citrate dextrose (ACD).2 All analyses were performed within 24 hours of sample collection. All horses previously had been blood typed (for blood groups A, C, D, K, P, Q, and U) and screened for anti‐erythrocyte hemolysin and agglutinin antibodies (against Aa, Ab, Ac, Ad, Af, Ca, Da, Dg, Dk, Ka, Pa, Pb, Pc, Qa, Qb, Qc, and Ua) within the last 12 months by the University of California‐Davis Veterinary Medical Teaching Hospital Hematology Laboratory3 by previously described standard antisera and tube methods.14
All laboratory procedures were performed by 1 individual who was not blinded to the other test results (DL). This ensured consistent performance and interpretation for all assays and samples, although agreement between different users was not assessed. To ensure the horses were not anemic, packed cell volume (PCV) and total solids were determined by standard microcentrifuge and refractometer techniques. Crossmatch donor‐recipient pairs were selected arbitrarily.
Immunochromatographic Strip Ca Typing Method (STRIP)
An immunochromatographic strip test kit4 for Ca typing was used according to manufacturer instructions. Briefly, 3 drops of diluent were placed into a well of a 96‐well plate. Ten μL of EDTA blood was added and mixed with the diluent for 15 seconds. The tip of an immunochromatographic strip impregnated with a Ca and control monoclonal antibody at different positions was placed into the well for 2 minutes, permitting the RBC suspension to diffuse to the top of the strip. The resultant line at the Ca position on the strip was graded on a scale from 0 to 4+ (0 being negative and 4+ being equally strong to the control band). A test was considered valid when a red band appeared at the control site (C). All results were archived by photography.
Crossmatch Methods
Tube Agglutination Method (TUBE)
Tube agglutination was performed by standard techniques.15 Briefly, a washed RBC suspension of 4% was made for each donor and recipient. For the major crossmatch, 2 drops of recipient serum and 2 drops of donor RBC suspension were mixed in polystyrene test tubes,5 incubated at 37°C for 15 minutes and centrifuged at 1000 × g for 15 seconds. Tubes then were lightly agitated by tapping against the operator's index finger for approximately 2 seconds, and the degree of agglutination was graded both macroscopically and microscopically according to the following scale: 0, no macroscopic or microscopic agglutination; ±, only 1 very small agglutinate within the suspension seen macroscopically and only 1 small agglutinate seen microscopically per low power field (10× magnification); 1+, few small agglutinates seen macroscopically or 2–5 small agglutinates per field; 2+, some larger agglutinates interspersed with small agglutinates or approximately 2 large agglutinates per field; 3+, a few large agglutinates in suspension or 2–5 large agglutinates per field; 4+, 1 large agglutinate macroscopically or numerous large agglutinates per field. A grade of ≥1+ was considered a positive (incompatible) test result.
Hemolysis was determined by mixing 2 drops of recipient serum, 2 drops of rabbit complement,6 and 2 drops of donor RBC suspension in a tube.5 This suspension was incubated at 37°C for 90 minutes and then centrifuged at 1000 × g for 90 seconds. The degree of hemolysis was graded macroscopically as present (+) or absent (−) based on a red appearance of the supernatant. For all crossmatch pairs, an auto‐control (recipient red blood cells‐recipient serum) was performed and was negative for auto‐hemolysins and autoagglutinins, and no serum samples appeared hemolyzed before analysis.
Gel Column Method (GEL)
The gel matrix column test was performed by manufacturer guidelines.7 Briefly, a washed RBC suspension of 1% was made for each donor and recipient. Each gel card contains 6 polypropylene gel columns. For the major crossmatch, 25 μL of recipient serum and 50 μL of washed donor RBC suspension were added to the chamber at the top of the gel. For the detection of hemolysis, 25 μL of rabbit complement also was added to the chamber on top of the gel. The gel cards were incubated8 for 15 minutes at 37°C and then centrifuged9 for 15 minutes at 80 × g. For detection of hemolysis, the gel cards were incubated8 for 90 minutes at 37°C and then centrifuged9 for 15 minutes at 80 × g. Degree of RBC retention in the gel was graded according to the following scale: 0, all RBCs at the bottom of the gel; 1+, few RBC agglutinates in the lower half of the gel but most RBCs at the bottom of the gel; 2+, RBC agglutinates dispersed throughout the gel, 3+, RBC agglutinates throughout gel and RBCs on upper surface; 4+, all RBCs on top of gel (Fig 1). A RBC retention of ≥1+ was considered positive (incompatible). Hemolysis was graded as present (+) or absent (−) based on a red discoloration of the solution in the chamber at the top of the gel (Fig 1). For all crossmatch pairs, an auto‐control (recipient cells‐recipient serum) was performed and was found to be negative for auto‐hemolysins and autoagglutinins.
Figure 1.
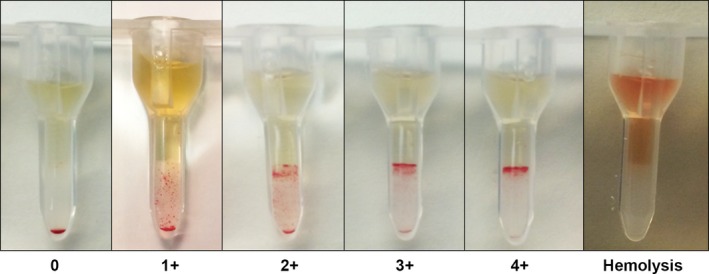
Gel column method for equine blood crossmatch. Gel columns from left to right show increasing degrees (0 to 4+) of agglutination incompatibility of equine blood crossmatches. The far right tube shows incompatibility characterized by hemolysis.
Predicted Blood Type Compatibility
Expected compatibility results were predicted by previous blood type and antibody testing results (i.e, the recipient plasma was negative/compatible or positive/incompatible for alloantibodies against one or more of the donor blood types).
Statistical Analyses
All statistical analyses were conducted by a standard statistical software package.10 A 2‐step approach to analysis was utilized. Initially, polychoric correlation was used to assess the pairwise association between the various crossmatch tests. Standard methods of performing pairwise correlation, such as Pearson's correlation, assume that the variables are continuous and follow a multivariate normal distribution. In the case of variables that are dichotomous or ordinal categorical variables, as was the case for our data, a correlation analysis by a polychoric correlation is preferred.16 If correlation was below an acceptable value of ρ = 0.5, we deemed these variables uncorrelated, and hence, there was no need for conducting further analyses.
If good correlation was found, additional statistical analyses by logistic regression and receiver operating characteristics (ROC) analyses then were used to assess the accuracy of the novel crossmatch assays. We used the standard macroscopic TUBE as our reference variable, because it is the accepted methodology for crossmatch assessments in horses at this time. The results of the TUBE were converted to a dichotomous categorical variable, where values ≤+0.5 agglutination were considered to be equal to 0 (compatible crossmatch), and values >+0.5 were converted to a value of 1 (incompatible crossmatch). Logistic regression was used to assess the specificity and sensitivity of test methods in comparison with our reference variable. Because sensitivity and specificity estimation rely on the determination of a single cut‐point to classify test results, a nonparametric area under the curve (AUC) ROC was used to provide a complete description of the classification accuracy.17 To assess breed effect on all crossmatch methodologies, we used logistic regression assessing the effect of breed of the donor and recipient animal on compatibility, followed by posthoc pairwise comparisons across the levels of factor variables.
Results
Horses
The study population included 19 Thoroughbreds (TB), 13 Quarter Horses (QH), 4 Standardbreds (STBD), and 2 Warmbloods (WB). The median age was 18 years (range, 9–24 years). There were 24 mares, 13 geldings, and 1 stallion. All horses were healthy, and the mean PCV and total solids were 34% (range, 28–40%) and 7.2 g/dL (range, 6.2–8.2 g/dL), respectively.
Ca Blood Typing by STRIP
Among the 38 horses evaluated, 35 horses were Ca+ by STRIP, showing a 2+ test band, and 3 horses were Ca‐ (0 band). The results were completely concordant with previous polyclonal blood typing results for the Ca antigen (Table 1 and Fig 2). The 3 Ca‐ horses included 2 TBs and 1 QH.
Table 1.
Sensitivity, specificity, predictive values, and polychoric correlation for equine crossmatch and blood typing methods compared to standard tube methods
| Polychoric Correlation | Sensitivity (%) | 95% CI | Specificity (%) | 95% CI | PPV (%) | 95% CI | NPV (%) | 95% CI | LR+ | 95% CI | LR− | 95% CI | AUC ROC | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crossmatcha | |||||||||||||||
| TUBE vs. MICRO | 0.97 | 85.71 | 73.78–93.62 | 96.67 | 90.57–99.31 | 94.12 | 83.76–98.77 | 91.58 | 84.08–96.26 | 25.74 | 8.40–78.63 | 0.15 | 0.08–0.28 | 0.91 | 0.86–0.96 |
| TUBE vs. GEL (Agglutination) | 0.882 | 91.07 | 80.38–97.04 | 82.22 | 72.74–89.48 | 76.12 | 64.14–85.69 | 93.67 | 85.84–97.91 | 5.12 | 3.26–8.05 | 0.11 | 0.05–0.25 | 0.9 | 0.85–0.95 |
| TUBE vs. GEL (Hemolysin) | 0.834 | 28.57 | 13.22–48.67 | 99.15 | 95.37–99.98 | 88.89 | 51.75–99.72 | 85.4 | 78.36–90.85 | 33.71 | 4.39–260.00 | 0.72 | 0.57–0.91 | 0.64 | 0.55–0.72 |
| TUBE vs. PREDICTED | 0.943 | 69.64 | 55.90–81.22 | 98.89 | 93.96–99.97 | 97.5 | 86.84–99.94 | 83.96 | 75.57–90.37 | 62.68 | 8.86–440.00 | 0.31 | 0.21–0.46 | 0.85 | 0.78–0.90 |
| Ca Blood Typingc | |||||||||||||||
| Standard vs. STRIP | 1 | 100 | 87.66–1b | 100 | 96.92–1b | 100 | 87.66–1b | 100 | 96.92–1b | Inf | N/A | 0.00 | N/A | 1 | 1.00–1.00 |
| bOne‐sided 97.5% CI | |||||||||||||||
aTest method as compared to standard tube crossmatch as reference value for 146 recipient‐donor pairs. bOne‐sided 97.5% confidence interval (CI). TUBE, tube crossmatch by standard gross macroscopic assessment of agglutination. MICRO, microscopic assessment of agglutination, tube method. GEL, crossmatch by gel column technique. PREDICTED, compatibility as predicted by prior blood type and alloantibody assays. Standard, Standard polyclonal blood typing assay. STRIP, immunochromatographic strip Ca blood typing kit. cImmunochromatographic strip method as compared to standard blood typing for Ca antigen as reference value. A value of 1 for polychoric correlation indicates perfect correlation. Prevalence of incompatible blood by TUBE agglutination was 38% (56/146). Prevalence of incompatible blood by TUBE hemolysis was 19% (28/146). Prevalence of Ca+ blood type was 92% (35/38).
Figure 2.

Equine Ca blood typing by immunochromatographic strip method. (A) Immunochromatographic strip showing a Ca+ result at the test line (Ca). This horse was also positive for Ca blood type by standard equine blood typing. (B) Immunochromatographic strip showing a Ca− result at test line. This horse was also negative for Ca blood type by standard equine blood typing. C = Control line—positive for all horses.
TUBE versus GEL Crossmatch
Major crossmatch tests were prepared for 146 recipient‐donor pairs by TUBE and GEL. Of these, 56 crossmatches were incompatible and 90 were compatible when using TUBE, which then served as the reference. Distribution of incompatibility scores varied slightly (Fig 3). TUBE crossmatch incompatibility scores were as follows: 0 (n = 85), +/‐ (5), 1+ (17), 2+ (12), 3+ (20), and 4+ (7). Gel column agglutination incompatibility scores were distributed as follows: 0 (79), 1+ (21), 2+ (13), 3+ (19), 4+ (14). The GEL results were highly correlated with the TUBE results (Table 1). The GEL crossmatch showed excellent discrimination as compared to TUBE (Fig 4A). The GEL was both sensitive and specific for predicting crossmatch compatibility (Table 1).
Figure 3.
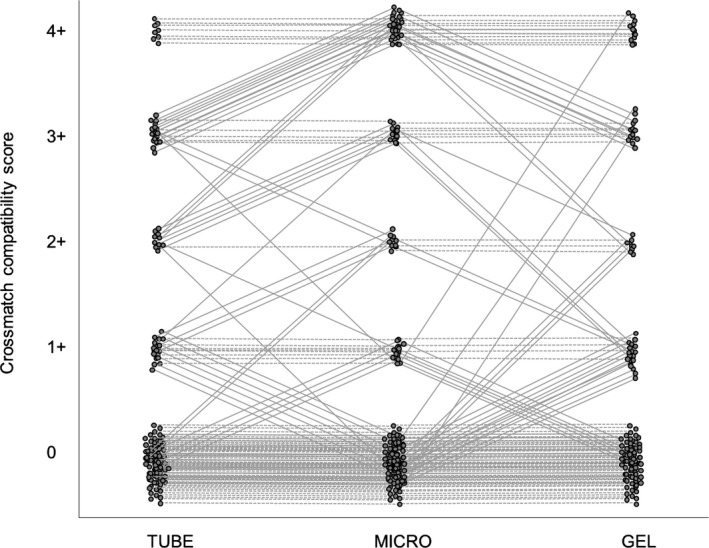
Comparison of equine crossmatch compatibility scores across 3 techniques: macroscopic evaluation of agglutination by the tube agglutination technique (TUBE), microscopic evaluation of agglutination by the tube technique (MICRO), and gel column agglutination (GEL) in 146 recipient‐donor pairs. The dashed horizontal lines indicate agreement between techniques, and the slanted solid lines demonstrate discordant results.
Figure 4.
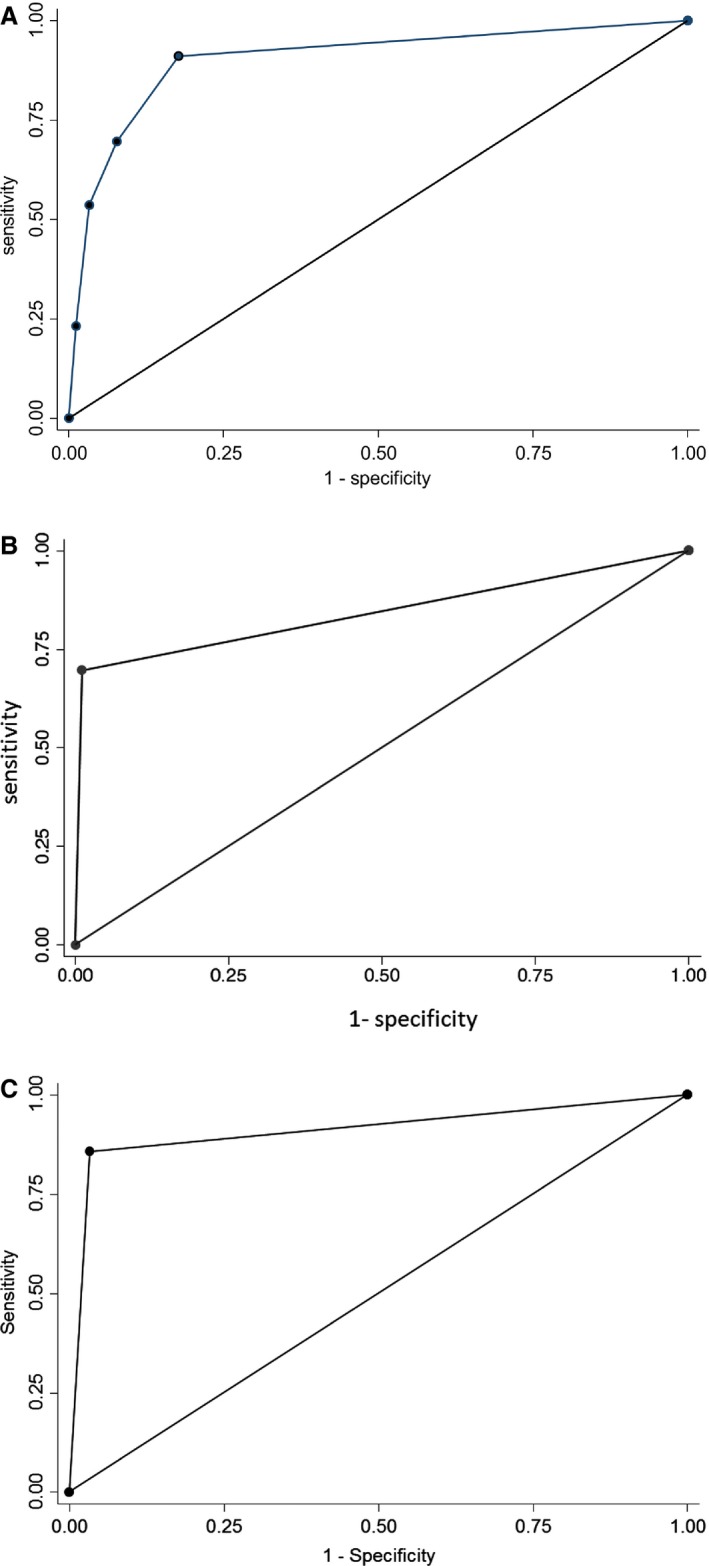
Nonparametric receiver operating characteristics (ROC) for evaluation of equine blood compatibility (146 recipient‐donor pairs) for standard tube macroscopic agglutination vs. (A) gel column agglutination crossmatch techniques (area under ROC curve = 0.903); (B) predicted compatibility based on blood type (area under ROC curve = 0.843); and (C) tube microscopic agglutination crossmatch techniques (area under ROC curve = 0.912).
Hemolysis was seen in 28 and 9 of 146 major crossmatches based on TUBE and on GEL, respectively. The hemolysis results by GEL and TUBE were well correlated. Hemolysis with GEL showed borderline acceptable discrimination of TUBE hemolysis results with an AUC‐ROC of 0.639 and had very low sensitivity, but excellent specificity (Table 1).
Predicted versus Crossmatch Compatibility (TUBE, GEL)
Based on the previous blood type and antibody testing results, 40 and 106 crossmatch pairs were predicted to be incompatible and compatible, respectively; 39 of these 40 were crossmatch incompatible by TUBE (agglutination, hemolysis, or both). These blood incompatibilities were attributed to alloantibodies most commonly against Aa (25 of 40) and Ca (19 of 40), with 16 of 40 having predicted incompatibility based on multiple antibodies. In addition, 17 pairs that were predicted to be compatible were found incompatible by TUBE. Predicted incompatibility showed strong correlation with TUBE (Table 1) and excellent discrimination of TUBE agglutination (Fig 4B) and of GEL agglutination (AUC‐ROC of 0.897). The PREDICTED compatibility was moderately sensitive when compared to TUBE but highly specific (Table 1).
Two crossmatch pairs where GEL and TUBE exhibited high incompatibility when PREDICTED compatible were investigated. The recipient serum (containing anti‐Aa and anti‐Ca antibodies) was incubated with known incompatible RBCs (Aa+ and Ca+ donor) to bind possible unidentified antibodies to these RBCs and thus remove them from further reactions. After 1 round of incubation and cell washing, this resulted in compatible crossmatch reactions for both pairs, suggesting likely elution of unidentified alloantibodies.
Macroscopic versus Microscopic TUBE
The macro‐ and micro‐scopic evaluations by TUBE were highly correlated. The microscopic TUBE score had outstanding discrimination of macroscopic TUBE agglutination (Fig 4C) and had good sensitivity and specificity (Table 1). Microscopic TUBE scores were distributed as follows: 0 (n = 77), +/‐ (18), 1+ (13), 2+ (5), 3+ (10), and 4+ (23).
Breed Effect on Crossmatch Pairs
When evaluating effects of recipient and donor breed, crossmatch pairs involving the 2 WB horses were found more likely compatible compared to the other breeds (TB, QH, STBD), both as donor and recipient across methods. Of 120 possible breed recipient‐donor pairings, those pairings involving the 2 WB horses were more likely compatible (P = .002–.022).
Discussion
Our study evaluates the accuracy among several methods of equine blood compatibility, including gross and microscopic assessment of agglutination and hemolysis by macroscopic standard tube agglutination as the reference method, and comparing these results to gel column agglutination and predicted compatibility by blood typing and alloantibody assay results. Initial correlation analysis showed that the alternative methodologies are highly associated with standard tube agglutination methodology. Our findings indicate that gel column agglutination is a suitable method for crossmatch evaluation of pretransfusion equine blood compatibility. This method had high accuracy when compared to standard tube agglutination methods and with predictive compatibility based on prior blood type and alloantibody data. The standard tube agglutination method has been faulted for its low reproducibility, varied result interpretations among operators, and requirements for both education and time commitment of personnel.7 Evaluation of standard tube agglutination compatibility involves agitating (gently tapping a finger against the tube) the RBC pellet at the bottom of the tube to assess degree of agglutination. This method is labor‐intensive and shaking methods may differ among users, which may affect grading and the interpretation of results. Gel column methods have been suggested to enhance transfusion safety in human medicine18 and have proven highly accurate in human medicine with a sensitivity of 97.58% and specificity of 99.93%.18 Only a small volume of RBCs is needed and the gel column method is easy to read, with grading being largely independent of the skill of the reader. Another advantage of the gel column method is that it can be readily captured by taking an image to be reviewed at a later time, in contrast to the standard tube agglutination reaction which is stable for only a few minutes and hard to image.18
Although the gel column assay appears to be an excellent method for detecting agglutination, it failed to detect hemolysis in the majority of crossmatches performed that involved known hemolysins (based on standard blood typing results). However, this method was not designed for detecting hemolysins in any species. The lack of discrimination of gel column for hemolysis is likely associated with the difficulty of visually discerning subtle color changes. Only a very small amount of RBCs was used, and hemolysis was difficult to recognize in the well at the top of the gel column and could therefore easily be missed unless pronounced. In addition, the agglutination reaction could no longer be assessed in the presence of massive lysis because of a lack of intact RBCs. Inability to detect hemolysis in the gel column method may make it a less favorable method for use in horses. However, a recent study showed that crossmatch incompatibilities resulting in decreased lifespan of transfused RBCs were always present in the agglutination test whether hemolysins were detected or not. Thus, the agglutination crossmatch is likely adequate to predict RBC survival in the absence of hemolysin testing, a test made easier by the gel column method.19
The high accuracy of microscopic tube agglutination compatibility scores when compared to standard tube macroscopic agglutination seen in our study indicates that gross evaluation is adequate for determining compatibility and that microscopic evaluation may not be necessary. Many laboratories (both for horses and for small animals) already have chosen to forgo microscopic crossmatch evaluation, and the data in our study support that decision.
The immunochromatographic strip method proved to be a highly sensitive and specific test for the presence of the Ca blood antigen in the limited survey (only 35 Ca+ and 3 Ca− horses) reported here. Ours is the first equine typing test utilizing a monoclonal antibody which offers more consistency than antisera, which may differ with each batch. The band reaction was always 2+ and thus adequate, albeit it could be stronger as seen with typing strips for canine DEA 1 and feline AB typing.10, 11 Furthermore, the immunochromatographic strip was simple to perform and once commercialized may well become a preferred blood typing method. Type Ca has been suggested to be protective for neonatal isoerythrolysis.20, 21 Naturally occurring alloantibodies against Ca may suppress immune responses to other blood group antigens. Mares negative for Aa that have anti‐Ca alloantibodies do not produce antibodies to Aa RBCs of their foals if the foal also shows the Ca antigen.20, 21 Although the Ca immunochromatographic typing strip exhibited excellent sensitivity and specificity in our survey, it should be evaluated on a larger cohort of horses and breeds to assess its accuracy and utility in predicting Ca blood type compatibilities.
The larger number of incompatibilities identified by standard tube agglutination and gel column agglutination compared to the predicted compatibilities suggests there may be additional alloantibodies in equine blood than those currently evaluated by standard commercially available blood typing. Two recent studies evaluating RBC lifespan after allogeneic transfusion of crossmatch‐compatible blood confirmed that RBC survival time was shorter in horses than in other species.19, 22 This decreased RBC lifespan may be associated with previously unidentified naturally occurring alloantibodies, RBC antigens, or both in equine blood. Although it was previously thought that naturally occurring alloantibodies occur in only a small percentage of horses and that their reactivity with equine RBCs is weak,3 there may be a larger contribution of these alloantibodies to blood incompatibility than previously recognized. Twenty percent of STBD mares and 10% of TB mares have anti‐Ca alloantibodies without known exposure to Ca+ RBCs and a common environmental antigen may result in the production of anti‐Ca antibodies.23 In our study, 2 crossmatch pairs that were predicted to be compatible based on prior extended blood typing exhibited strong incompatibilities. This finding suggests there may be unidentified alloantibodies present in these 2 recipient horses. Crossmatch‐compatible equine blood transfusions likely exhibit a decreased half‐life within the recipient because of the lack of sensitivity of our current alloantibody screening techniques. This lack of sensitivity, however, may be because of the fact that the indirect antiglobulin test is not used to identify alloantibodies. Thus, the gel column crossmatch should be assessed before transfusing horses, and the survival of the transfused RBCs should be followed. Although no previous study in horses has reported so many crossmatch results as identified here, ours was a relatively small survey. The gel column crossmatch method used here should facilitate larger surveys and more standardized evaluation in horses.
All crossmatch results were indicative of possible incompatibilities in vitro, but no blood transfusions were performed in this study to evaluate in vivo blood compatibility. In 1 study, transfusion reactions occurred in 16% of transfused horses, although several of these transfusions included incompatible blood by either major or minor crossmatch.24 The clinical importance of many alloantibodies (against 30 putative antigens) has not been clearly established. For example, anti‐Ca antibodies do not always appear to produce adverse clinical effects, and, as previously mentioned, actually may be protective against neonatal isoerythrolysis.21 In human transfusion medicine, some alloantibodies have been shown to lead to crossmatch incompatibility without being clinically hemolytic.25, 26 The clinical importance of specific equine RBC alloantibodies should be further investigated.
The majority of predicted incompatibilities based on prior blood type determination in our study were because of alloantibodies against the Aa blood type, followed by the Ca blood type. Type Aa and Qa are reported to be the most antigenic RBC types and are responsible for the majority of equine neonatal isoerythrolysis cases.20 Although no horse is a truly compatible donor for all blood types, the closest to a “universal equine donor” might be 1 that is negative for the Aa and Qa antigens and lacking alloantibodies. The apparent protection against incompatibility seen in WB horses in this study is interesting and warrants further investigation, because this observation was limited to crossmatches involving only 2 WB horses. The findings in this study suggest that when compatibility testing is not available before a transfusion, an Aa‐, Qa‐, and Ca‐ WB gelding may be the best choice as a blood donor compared to other breeds and blood types.
Limitations of our study include small sample size and lack of blinding for results. One individual performed all of the assays and interpretations, and this individual was not blinded from the results of the other tests. In addition, all testing was performed in a laboratory setting and the immunochromatographic strip method has not been evaluated in a field setting.
In conclusion, although the gel column crossmatch fails to identify hemolysis that may be clinically relevant in equine blood transfusions, gel column agglutination is a suitable method for evaluating compatibility before equine blood transfusions, and has advantages over the standard tube crossmatch. In addition, a novel immunochromatographic Ca blood typing strip kit holds promise for stall‐side blood typing to be performed in the near future.
Acknowledgments
Conflict of Interest Declaration: Urs Giger has been a scientific advisor to DiaMed (now owned by Bio‐Rad) and Alvedia, and Ca typing kits were provided by Alvedia.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
All work was done at the University of California and the University of Pennsylvania Schools of Veterinary Medicine.
This study was supported in part by NIH OD 010939, and Ca typing kits were kindly provided by Alvedia, Limonest, France.
This study was presented orally as a research abstract at the 2016 ACVIM Forum, Denver, CO.
Footnotes
Covidien Monoject, Minneapolis, MN
BD Vacutainer, BD Diagnostics, Franklin Lakes, NJ
Hematology Laboratory, University of California, Davis, Davis, CA
Alvedia Equine Blood Typing Test, Alvedia, Limonest, France
Globe Scientific 12 × 75 mm round bottom polystyrene test tubes, Fisher Scientific, Pittsburgh, PA
Rabbit complement, unadsorbed, Pel‐Freez Biologicals, Rogers, AR
Bio‐Rad Laboratories, DiaMed GmbH, Cressier sur Morat, Switzerland
ID—Incubator, DiaMed Microtyping system, Cressier sur Morat, Switzerland
ID—Centrifuge, DiaMed Microtyping system, Cressier sur Morat, Switzerland
STATA 14, College Station, TX
References
- 1. Sandberg K. Guidelines for the Interpretation of Blood Typing Tests in Horses. Brisbane, Australia: International Society for Animal Genetics; July, 1996. [Google Scholar]
- 2. Brown D, Vap LM. Principles of blood transfusion and crossmatching In: Thrall MA, Weiser G, Allison RW, Campbell TW, eds. Veterinary Hematology and Clinical Chemistry, 2nd ed Ames, IA: Wiley‐Blackwell; 2012:205–222. [Google Scholar]
- 3. Bowling A. Red blood cell antigens and blood groups in the horse In: Feldman B, Zinkl J, Jain N, eds. Schalm's Veterinary Hematology. Baltimore, MD: Lippincott, Williams, & Wilkins; 2000:774–777. [Google Scholar]
- 4. Owens SD, Snipes J, Magdesian KG, Christopher MM. Evaluation of a rapid agglutination method for detection of equine red cell surface antigens (Ca and Aa) as part of pretransfusion testing. Vet Clin Pathol 2008;37:49–56. [DOI] [PubMed] [Google Scholar]
- 5. de Graaf‐Roelfsema E, van der Kolk JH, Boerma S, van Haeringen H. Non‐specific haemolytic alloantibody causing equine neonatal isoerythrolysis. Vet Rec 2007;161:202–204. [DOI] [PubMed] [Google Scholar]
- 6. Zaruby JF, Hearn P, Colling D. Neonatal isoerythrolysis in a foal, involving anti‐pa alloantibody. Equine Vet J 1992;24:71–73. [DOI] [PubMed] [Google Scholar]
- 7. Tocci LJ, Ewing PJ. Increasing patient safety in veterinary transfusion medicine: An overview of pretransfusion testing. J Vet Emerg Crit Care 2009;19:66–73. [DOI] [PubMed] [Google Scholar]
- 8. Seth M, Jackson KV, Giger U. Comparison of five blood‐typing methods for the feline AB blood group system. Am J Vet Res 2011;72:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seth M, Jackson KV, Winzelberg S, Giger U. Comparison of gel column, card, and cartridge techniques for dog erythrocyte antigen 1.1 blood typing. Am J Vet Res 2012;73:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stieger K, Palos H, Giger U. Comparison of various blood‐typing methods for the feline AB blood group system. Am J Vet Res 2005;66:1393–1399. [DOI] [PubMed] [Google Scholar]
- 11. Giger U, Stieger K, Palos H. Comparison of various canine blood‐typing methods. Am J Vet Res 2005;66:1386–1392. [DOI] [PubMed] [Google Scholar]
- 12. Weiss DJ, Wardrop KJ. Clinical blood typing and crossmatching In: Feldman B, Zinkl J, Jain N, eds. Schalm's Veterinary Hematology. Baltimore, MD: Lippincott, Williams, & Wilkins; 2000:1101–1105. [Google Scholar]
- 13. Kessler RJ, Reese J, Chang D, et al. Dog erythrocyte antigens 1.1, 1.2, 3, 4, 7, and dal blood typing and cross‐matching by gel column technique. Vet Clin Pathol 2010;39:306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stormont C, Suzuki Y, Rhode E. Serology of horse blood groups. Cornell Vet 1964;54:439–452. [PubMed] [Google Scholar]
- 15. Harris M, Nolen‐Walston R, Ashton W, et al. Effect of sample storage on blood crossmatching in horses. J Vet Intern Med 2012;26:662–667. [DOI] [PubMed] [Google Scholar]
- 16. Lee SY, Poon WY, Bentler PM. A two‐stage estimation of structural equation models with continuous and polytomous variables. Br J Math Stat Psychol 1995;48:339–358. [DOI] [PubMed] [Google Scholar]
- 17. Hosmer DW, Lemeshow S, Sturdivant RX. Assessing the Fit of the Model In: Applied Logistic Regression. 3rd ed Hoboken, NJ: John Wiley & Sons, Inc.; 2013:162. [Google Scholar]
- 18. Noumsi G. The role of automated gel column testing technology in enhancing transfusion safety. MLO Med Lab Obs 2014;46:34–36. [PubMed] [Google Scholar]
- 19. Tomlinson JE, Taberner E, Boston RC, et al. Survival time of cross‐match incompatible red blood cells in adult horses. J Vet Intern Med 2015;29:1683–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilkins PA. Disorders of Foals In: Reed SM, Bayly WM, Sellon DC, eds. Equine Internal Medicine. 2nd ed St. Louis, Mo: W.B. Saunders; 2004:1381–1439. [Google Scholar]
- 21. Bailey E, Albright DG, Henney PJ. Equine neonatal isoerythrolysis: Evidence for prevention by maternal antibodies to the ca blood group antigen. Am J Vet Res 1988;49:1218–1222. [PubMed] [Google Scholar]
- 22. Mudge MC, Walker NJ, Borjesson DL, et al. Post‐transfusion survival of biotin‐labeled allogeneic RBCs in adult horses. Vet Clin Pathol 2012;41:56–62. [DOI] [PubMed] [Google Scholar]
- 23. Bailey E. Prevalence of anti‐red blood cel antibodies in the serum and colostrum of mares and its relationship to neonatal isoerythrolysis. Am J Vet Res 1982;43:1917–1921. [PubMed] [Google Scholar]
- 24. Hurcombe SD, Mudge MC, Hinchcliff KW. Clinical and clinicopathologic variables in adult horses receiving blood transfusions: 31 cases (1999–2005). J Am Vet Med Assoc 2007;231:267–274. [DOI] [PubMed] [Google Scholar]
- 25. Waheed A, Kennedy MS, Gerhan S. Transfusion significance of lewis system antibodies. Report on a nationwide survey. Transfusion 1981;21:542–545. [DOI] [PubMed] [Google Scholar]
- 26. Issitt PD, Combs MR, Bredehoeft SJ, et al. Lack of clinical significance of “enzyme‐only” red cell alloantibodies. Transfusion 1993;33:284–293. [DOI] [PubMed] [Google Scholar]


