Abstract
Background
Persistent Organic Pollutants (POPs) are well-known environmental contaminants which are associated with chronic diseases. As foods are the major sources of human exposure to toxic pollutants, we developed an integrated dietary and education program to eliminate the chemical toxin throughout the human body. The present study evaluated effects of the dietary detoxification program on serum γ-glutamyltransferase (GGT), anthropometric data and metabolic biomarkers in adults.
Methods
Single-armed, pre-post study was conducted from June 2013 to June 2015 at a health examination center and a public health center in Seoul, Korea. Sixty eight subjects (mean age of 52.4 years) were recruited. Subjects participated 20 hours’ dietary education sessions. On-line coaching with SNS was performed to enhance participants’ proper protocol compliance. Physical and laboratory examinations were assessed at week 0 and 3.
Results
Changes of the serum GGT were correlated with reductions of the body fat percentage (r = .379, p = .001), body fat mass (r = .435, p = .000) and fasting blood glucose (r = .423, p = .000). Serum GGT, weight, body fat percentage, body fat mass, waist circumference, LDL-cholesterol, HDL-cholesterol, triglyceride, total cholesterol, and blood pressure of all participants were reduced with statistical significance in 3 weeks. In metabolic syndrome group, total cholesterol (p = .049), fasting blood glucose (p = .002), and systolic blood pressure (p = .001) were significantly reduced comparison to non-metabolic syndrome group.
Conclusion
This dietary detoxification program might decrease serum GGT which indicated the overall toxic burden in the body. Anthropometric data and metabolic biomarkers were improved. The integrated dietary and education detoxification program seemed to be a protective intervention for elimination of toxicants from the body.
Keywords: Persistent organic pollutant, Toxins, Detoxification, γ-glutamyltransferase, metabolic syndrome
INTRODUCTION
Persistent Organic Pollutants (POPs) are well-known environmental contaminants including polychlorinated bisphenyls (PCBs) and organochlorine pesticides (OCPs). These chemical compounds bioaccumulate in adipose tissue and come into toxins in body [1–3]. Furthermore, POPs have long-lasting adverse effects in our body from food chains. POPs increase reactive oxygen species (ROS), and lead to cell inflammations and oxidative stress [4–6]. It has been documented that POPs are associated with chronic diseases such as cancers, cardiovascular diseases, neuro-degenerative disease, and respiratory diseases [7,8]. Particularly, there are emerging evidences that these xenobiotics alter metabolic mechanism and elevate insulin resistance. It can be resulted in obesity, dyslipidemia, and type 2 diabetes [9–14]. In other words, POPs aggravate many chronic conditions through ingestion, and respiration. To our healthy life, it is important to reduce accumulating chemical agents in the human body.
Unfortunately, people are consistently exposed to various chemicals and these compounds accumulate in our body for several decades [15]. Accordingly, it is vital to avoid chemical materials exposure as well as to remove from the body. However, few studies on reducing POPs effectively from the body have been found in the literature. Traditionally, detoxification, toxin elimination intervention, has become well-used therapy to clean of intestine, alleviate toxic symptoms and lose weight [16]. As the pathway of removal of chemical agents is through urine and fecal elimination, detoxification approach diverse methods using fasting, laxatives and enzyme for specific period [1]. Though, it is preferable to natural methods than strict calorie restriction, intestine cleansing and using certain food or enzyme to discard noxious chemicals for health. As foods are the major sources of human toxic pollutants, detoxification intervention is an effective way of intaking foods containing lower POPs and decomposing toxin from the body naturally [17]. Therefore, we developed an integrated dietary and education program for toxin elimination.
Although these extensive therapies widespread utilizing remove toxins, it is controversy on effects and remedies of detoxification [1,18]. Since physiological biomarkers which reflect body toxin level and objective outcome indicators were not measured, it is insufficient to prove the effects of intervention to remove toxins [18]. Thus, physiological indicators which confirmed to body’s toxin level are necessary to measure the outcomes. It is widely established that serum γ-glutamyltransferase (GGT) is a biomarker of alcohol consumption, heart disease, hypertension and type 2 diabetes [19–21]. In addition, several studies suggested that increased GGT within its normal range can be predicted environmental pollutants [22,23]. Glutathione as an antioxidant combine xenobiotics intracellular and move toward extracellular to metabolite (phase II). GGT which located in cell membrane get involved in glutathione activities which conjugated various environmental pollutants [24]. Thus, the overall burden of toxicants can measure by GGT. Considering numerous synthetic chemicals to which we are exposed, measuring of certain manufactured materials does not understand to identify accumulating toxins in human body.
As environmental pollutants bioaccumulate in fatty tissue, it is essential to investigate the relationship between serum GGT and body fat indicators. Therefore, the present study was conducted to explore the effects of dietary detoxification program on serum GGT, weight, body fat percentage, body fat mass, waist circumference, lipid profiles, blood pressure and fasting blood glucose in adults.
MATERIALS AND METHODS
1. Study setting, design and sampling
This prospective, single-armed, pre-post study was conducted from June 2013 to June 2015 at a health examination center and a public health center in Seoul, Korea. Participants who were willing to join the dietary detoxification program were recruited through advertisements.
The inclusion criteria were: (1) age of 20–75 years, (2) able to follow a dietary intervention during 3 weeks, (3) physical conditions confirmed by doctors. The exclusion criteria were: (1) physically hard persons who were not able to perform the dietary detoxification program including cancer patients, nutritional problems, and taking medications for severe diseases, (2) not favorably communicate with cognitive impairment, (3) involved another dietary treatment.
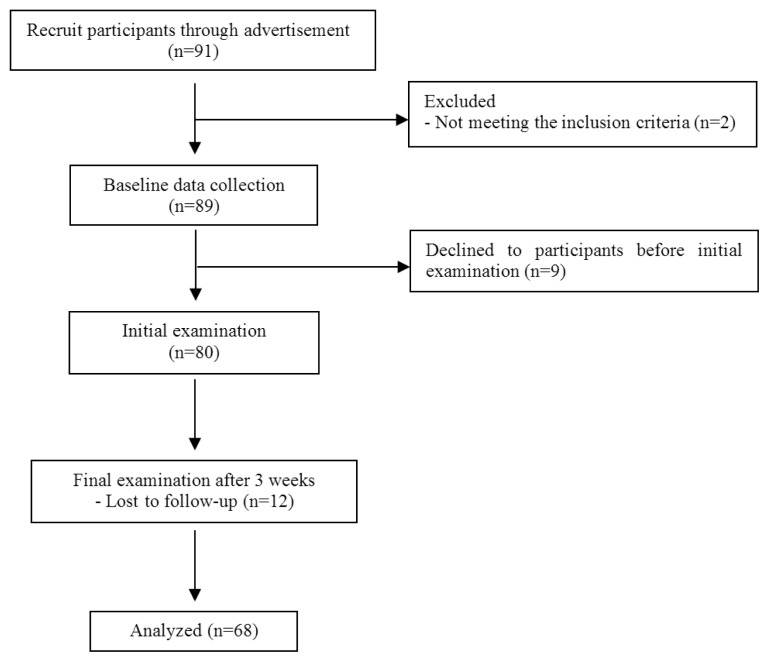
All participants (n = 91) were recruited through advertisements. Among of them screening test was done by inclusion and exclusion criteria. Eligible subjects took initial and final physical examination at 0 and 3 weeks. Excluding insufficient and dropped data during 3 weeks, finally 68 data was analyzed for the study (Fig. 1).
Fig. 1.
Flows of the study.
In additional analysis, the participants were divided into 2 groups depending on metabolic syndrome criteria. Metabolic syndrome definition was three or more of the following: (1) increased waist circumference (≥90 cm in men, ≥85 cm in women), (2) increased triglycerides ≥150 mg/dL) or drug treatment for elevated triglycerides, (3) decreased HDL cholesterol level (<40 mg/dL in men, <50 mg/dL in women) or drug treatment aimed to increase HDL cholesterol; (4) elevated blood pressure, defined as systolic blood pressure (SBP) ≥130 mmHg or diastolic blood pressure (DBP) ≥85 mmHg, or antihypertensive drug treatment; and (5) elevated fasting blood glucose >100 mg/dL or use of blood glucose lowering agents [25].
2. Dietary detoxification program
1) Toxin elimination diet
This program is a comprehensive toxin elimination program for 3 weeks. Intake foods are (1) whole grains, (2) brown rice, (3) legumes, (4) nuts, (5) white fish, (6) non-antibiotic, cage-free poultry, (7) vegetables and fruits, (8) seaweed [26]. White fish and non-antibiotic, cage-free poultry that contain relatively low POPs among animal protein source foods were allowed by a comprehensive literature review. Foods are avoided including (1) polished grains, (2) red meats and eggs, (3) processed foods, (4) foods with artificial flavor enhancer, (5) alcohol, (6) instant foods. After dinner, food ingestion except water was avoided for lipolysis during 12 hours fasting. Drinking water 1–2 L during day time and having liquid diets for breakfast and dinner was encouraged.
2) Education
All subjects participated education session during 20 hours. The education sessions were composed of introduction of effect of toxic agents on the human body, the principles of dietary intervention, the ways of lifestyle modification, cooking recipes and proper manner of recording a diet diary. Additional or special exercises were prohibited during intervention periods. However, body stretching was recommended for a posture balance.
3) Self-monitoring
Self-monitoring is a useful method to aware oneself the goal to achieve and shows the process of change [27]. This continuous feedback is a key strategy to maintain dietary interventions. Participants recorded weight, meal menu, and activities every day. 24-hours food diary was written for nutritional analysis during 3 days.
4) Telephone and SNS follow-up
In addition to education, daily SNS follow-up was performed to enhance the protocol properly. All participants were given answering immediately about dietary intervention and the way of coping with difficult situations. Also, telephone checkup has done by weekly.
5) Group support
After 10 days from initial examination, we had a group meeting session to share their challenged problems and short-term goal achievement. Participants were given supportive message and expressed their feelings each other.
3. Data collection and measurement
To evaluate the effects of dietary detoxification program, physical and laboratory indicators were measured. Physical examination including height, weight, waist circumference, body fat percentage, body fat mass, and blood pressure was assessed at week 0 and 3. For laboratory outcomes evaluation, serum GGT, LDL-cholesterol, HDL-cholesterol, triglyceride, total cholesterol, and fasting blood glucose, venous blood was collected in the morning after fasting 8 hours at initial and in 3 weeks.
The primary outcome was serum GGT. Secondary outcomes were weight, body fat percentage, body fat mass, waist circumference, lipid profiles, blood pressure and fasting blood glucose.
4. Data analysis
A descriptive analysis was done to summarize the characteristics and outcome variables using mean (standard deviation). Comparisons of the pre and post test were analyzed by using the paired t-test. Wilcoxon signed rank test was used to test the nonparametric. All tests were two-sided, and p-value <.05 were considered to present statistically significant differences. 23.0 SPSS software was used for the analysis. Nutritional analysis was used by CAN-Pro professionals version 4.0.
5. Ethical considerations
This study was approved by institutional review board of the Seoul National University (IRB-2013–100), and informed consent was obtained from all subjects.
RESULTS
The subjects had a mean age of 52.4 ± 11.7 years, females were 79.4%. All participants in this study had a mean body fat percentage 32.4 ± 8.0%, and their mean body fat mass was 21.7 ± 9.2 kg. The average of systolic blood pressure was 122.3 ± 16.0 mmHg, and diastolic blood pressure was 76.0 ± 10.3 mmHg. Fasting blood glucose average was 95.3 ± 23.7 mg/dL (Table 1).
Table 1.
General characteristics and anthropometric data in each group
| Characteristics | Total (N = 68) | Metabolic Syndrome (n = 18) | Non-metabolic Syndrome (n = 50) | p-value |
|---|---|---|---|---|
| Age (years) | 52.4 ± 11.5 (20–74) | 52.7 ± 10.6 | 51.3 ± 15.0 | 0.081 |
| Sex (male/female) | 14/54 | 5/13 | 9/41 | 0.498 |
| Height (cm) | 160.0 ± 6.9 | 161.2 ± 6.4 | 159.6 ± 7.1 | 0.424 |
| Weight (kg) | 65.1 ± 14.9 | 72.1 ± 15.8 | 62.5 ± 13.8 | 0.019 |
| Body fat percentage (%) | 32.4 ± 8.0 | 35.1 ± 6.0 | 31.5 ± 8.5 | 0.130 |
| Body fat mass (kg) | 21.7 ± 9.2 | 25.8 ± 9.0 | 20.2 ± 9.0 | 0.016 |
| Body Mass Index (kg/m2) | 25.3 ± 4.9 | 27.6 ± 4.9 | 24.5 ± 4.7 | 0.016 |
| Waist circumference (cm) | 84.5 ± 11.8 | 91.3 ± 10.0 | 82.2 ± 11.6 | 0.003 |
| Total cholesterol (mg/dL) | 196.0 ± 38.4 | 207.2 ± 32.5 | 192.0 ± 39.9 | 0.154 |
| LDL-cholesterol (mg/dL) | 111.8 ± 34.2 | 120.3 ± 32.4 | 108.8 ± 34.7 | 0.251 |
| HDL-cholesterol (mg/dL) | 58.8 ± 14.3 | 54.6 ± 17.4 | 60.3 ± 12.8 | 0.034 |
| Triglyceride (mg/dL) | 124.7 ± 68.3 | 173.7 ± 75.0 | 107.1 ± 56.9 | 0.000 |
| Systolic blood pressure (mmHg) | 122.3 ± 16.0 | 137.1 ± 15.3 | 116.9 ± 12.6 | 0.000 |
| Diastolic blood pressure (mmHg) | 76.0 ± 10.3 | 82.0 ± 12.0 | 73.8 ± 8.8 | 0.009 |
| Fasting blood glucose (mg/dL) | 95.3 ± 23.7 | 116.8 ± 36.1 | 87.5 ± 9.0 | 0.000 |
Mean ± SD or number.
A significant difference between groups, p-value for Mann-Whitney U test or chi-square test.
Table 2 shows the macro and micro nutrients intakes of the participants. Conventional dietary (before intervention) and toxin elimination diets intake energy was assessed 1400–1500 kcal that was not significant difference between pre and post intervention (p = .826). Carbohydrate, fat, cholesterol, and protein intakes were not statistical difference; however, their components ratio was significantly different. Dietary fiber (p = .014), vitamin C (p = .001), phosphorus (p = .013), potassium (p = .005) intakes were significantly increased in 3 weeks.
Table 2.
Comparison of macro and micro intake nutrients composition
| Nutrients | Conventional diets (before interventions) | Toxin elimination diets (after intervention) | p-value |
|---|---|---|---|
| Energy (kcal) | 1445.0 ± 340.8 | 1423.4 ± 230.7 | .826 |
| Carbohydrate (g) | 244.5 ± 70.9 | 255.8 ± 46.3 | .570 |
| Fat (g) | 30.3 ± 8.2 | 28.2 ± 8.4 | .445 |
| Animal fat (g) | 14.2 ± 6.4 | 6.4 ± 5.3 | .002 |
| Vegetable fat (g) | 16.1 ± 3.9 | 21.8 ± 7.8 | .007 |
| Cholesterol (g) | 233.5 ± 130.4 | 148.0 ± 107.9 | .062 |
| Total fatty acid (g) | 17.5 ± 7.5 | 13.3 ± 5.7 | .057 |
| Saturated Fatty acids (g) | 5.9 ± 4.2 | 3.3 ± 2.3 | .049 |
| Mono-Saturated Fatty acids (g) | 7.2 ± 4.3 | 5.0 ± 2.9 | .114 |
| Poly-Saturated Fatty acids (g) | 5.6 ± 2.4 | 6.2 ± 3.1 | .447 |
| Protein (g) | 52.9 ± 14.2 | 53.1 ± 9.6 | .971 |
| Plant protein (g) | 30.3 ± 6.0 | 40.5 ± 9.6 | .001 |
| Animal protein (g) | 22.6 ± 11.5 | 12.6 ± 9.8 | .020 |
| Dietary fiber (g) | 20.8 ± 8.2 | 27.0 ± 7.3 | .014 |
| Vitamin A (ug) | 990.6 ± 321.7 | 1226.6 ± 868.4 | .201 |
| Vitamin D (ug) | 2.4 ± 3.0 | 1.4 ± 1.2 | .237 |
| Vitamin E (ug) | 12.8 ± 5.0 | 14.3 ± 4.4 | .340 |
| Vitamin K (ug) | 233.2 ± 133.5 | 229.9 ± 161.8 | .939 |
| Vitamin C (ug) | 114.2 ± 52.6 | 172.4 ± 80.7 | .001 |
| Vitamin B6 (ug) | 1.6 ± 0.8 | 1.9 ± 0.4 | .157 |
| Vitamin B12 (ug) | 5.7 ± 4.1 | 4.0 ± 1.9 | .195 |
| Folate (ug) | 454.5 ± 107.1 | 534.6 ± 194.7 | .068 |
| Calcium (mg) | 410.4 ± 122.0 | 392.5 ± 129.4 | .624 |
| Phosphorus (mg) | 886.3 ± 207.3 | 1022.8 ± 166.0 | .013 |
| Sodium (mg) | 2963.2 ± 874.1 | 2450.3 ± 967.2 | .108 |
| Potassium (mg) | 2830.0 ± 1225.2 | 3965.0 ± 1289.7 | .005 |
| Magnesium (mg) | 84.6 ± 63.5 | 93.2 ± 35.4 | .593 |
Mean ± SD.
A significant difference between before and after intervention, p-value for independent t-test.
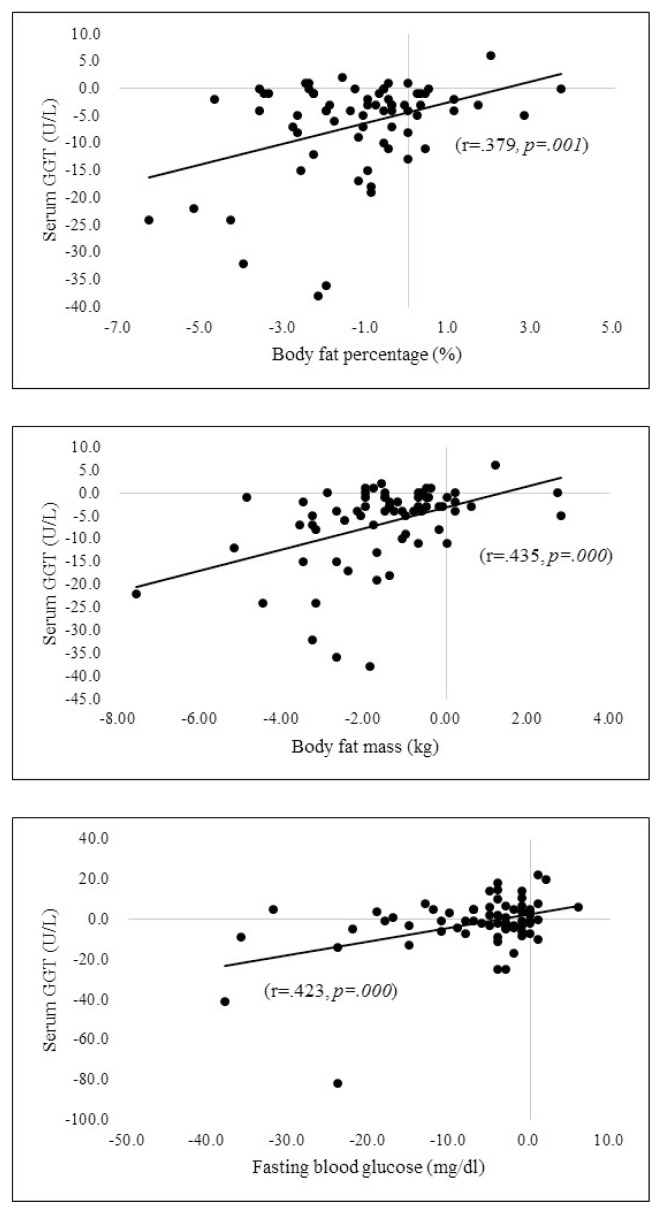
Fig. 2 demonstrates that changes of the serum GGT was correlated with reductions of the body fat percentage (r = .379, p = .001).body fat mass (r = .435, p = .000) and fasting blood glucose (r = .423, p = .000).
Fig. 2.
Correlations between changes in serum GGT, body fat percentage, body fat mass, and fasting blood glucose.
Table 3 is shown the average of serum GGT was 24.5 ± 16.6 U/L before the intervention. After 3 weeks, reduction of serum GGT with statistical significance in all participants (p = .000). Body weight (p = .000), body fat percentage (p = .000), body fat mass (p = .000), waist circumference (p = .000), LDL-cholesterol (p = .000), HDL-cholesterol (p = .003), triglyceride (p = .001), total cholesterol (p = .000), systolic blood pressure (p = .028), and diastolic blood pressure (p = .025) of all participants were reduced with statistical significance except fasting blood glucose (p = .304) in one group.
Table 3.
Clinical outcomes of the study participants between metabolic syndrome and non-metabolic syndrome group
| Clinical outcomes | Total (N = 68) | Metabolic Syndrome (n = 18) | Non-metabolic Syndrome (n = 50) | p-value | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| At baseline | At 3 weeks | At baseline | At 3 weeks | At baseline | At 3 weeks | ||
| Weight (kg) | 65.1 ± 14.9 | 62.8 ± 14.0* | 72.1 ± 15.8 | 69.2 ± 14.6* | 62.5 ± 13.8 | 60.5 ± 13.2* | 0.064 |
| Body fat percentage (%) | 32.4 ± 8.0 | 31.2 ± 7.8* | 35.1 ± 6.0 | 33.7 ± 6.7* | 31.5 ± 8.5 | 30.3 ± 8.1* | 0.626 |
| Body fat mass (kg) | 21.7 ± 9.2 | 20.1 ± 8.5* | 25.8 ± 9.0 | 23.8 ± 8.5* | 20.2 ± 9.0 | 18.8 ± 8.2* | 0.263 |
| Body Mass Index (kg/m2) | 25.3 ± 4.9 | 24.4 ± 4.6* | 27.6 ± 4.9 | 26.5 ± 4.6* | 24.5 ± 4.7 | 23.7 ± 4.4* | 0.073 |
| Waist circumference (cm) | 84.5 ± 11.8 | 81.8 ± 11.2* | 91.3 ± 10.0 | 88.9 ± 9.8* | 82.2 ± 11.6 | 79.4 ± 10.8* | 0.636 |
| Serum GGT(U/L) | 24.5 ± 16.6 | 17.9 ± 10.7* | 33.7 ± 21.6 | 24.7 ± 15.9* | 21.2 ± 13.1 | 15.5 ± 6.7* | 0.098 |
| Total cholesterol (mg/dl) | 196.0 ± 38.4 | 174.5 ± 30.9* | 207.2 ± 32.5 | 175.7 ± 33.1* | 192.0 ± 39.9 | 174.0 ± 30.4* | 0.049 |
| LDL-cholesterol (mg/dl) | 111.8 ± 34.2 | 98.9 ± 29.8* | 120.3 ± 32.4 | 100.3 ± 32.4* | 108.8 ± 34.7 | 98.3 ± 29.2* | 0.237 |
| HDL-cholesterol (mg/dl) | 58.8 ± 14.3 | 55.4 ± 13.9* | 54.6 ± 17.4 | 49.1 ± 13.6 | 60.3 ± 12.8 | 57.6 ± 13.5* | 0.504 |
| Triglyceride (mg/dl) | 124.7 ± 68.3 | 98.3 ± 52.7* | 173.7 ± 75.0 | 132.8 ± 73.4* | 107.1 ± 56.9 | 85.8 ± 36.5* | 0.131 |
| Systolic blood pressure (mmHg) | 122.3 ± 16.0 | 118.6 ± 14.7* | 137.1 ± 15.3 | 124.4 ± 18.7* | 116.9 ± 12.6 | 116.5 ± 12.5 | 0.001 |
| Diastolic blood pressure (mmHg) | 76.0 ± 10.3 | 73.3 ± 10.2* | 82.0 ± 12.0 | 76.8 ± 11.7* | 73.8 ± 8.8 | 72.1 ± 9.4 | 0.075 |
| Fasting blood glucose (mg/dl) | 95.3 ± 23.7 | 93.5 ± 16.1 | 116.8 ± 36.1 | 105.2 ± 25.0* | 87.5 ± 9.0 | 89.2 ± 8.2 | 0.002 |
Mean ± SD.
Between groups comparison, Mann-Whitney U test, p < 0.05.
Within group comparison, Wilcoxon signed rank test, p < 0.05.
In additional analysis, the subjects were divided into 2 groups depending on metabolic syndrome criteria. Metabolic syndrome group participants were 18, all the variables statistically significant decreased except HDL-cholesterol in 3 weeks. Total cholesterol (p = .049), fasting blood glucose (p = .002), and systolic blood pressure (p = .001) were significantly reduced comparison to non-metabolic syndrome group (Table 3).
DISCUSSION
Detoxification is one of the wide-spread integrative interventions. Some studies insisted that detoxification intervention can eliminate the body pollutants while, several physicians are concerned about the effects and warning of its harm [28,29]. Though some difficulties in standardized detoxification regimens, dietary detoxification intervention may effect on the health outcomes [16,30–32].
First, we verified the overall toxic burden by using serum GGT. In this study, statistically significant associations were investigated between the changes of fat tissue and serum GGT level. Several studies reported that POPs can elevate the insulin resistance and increase the prevalence of metabolic syndrome or diabetes [10–12]. Lim et al. explained that exposure to POPs may induce the mitochondrial dysfunction and metabolic syndrome [9]. Moon et al. reported that one of the representative POPs, bisphenol, induces glucose intolerance and insulin resistance in mice [33]. Lee et al. identified that some chemical pollutants are dose-response relationship with the incidence of type 2 diabetes [13]. Previous studies reported that serum GGT level may be a predictor of diabetes and hypertension and also is associated with dietary factors [34–36]. Toxin elimination diets and education program can be decreased the adipose tissue, and serum GGT. Although we did not directly measure the body toxins, it is necessary to research of POPs burdens measuring of many exposal chemicals in the body.
Second, serum GGT, weight, body fat percentage, body fat mass, waist circumference, lipids profiles, and blood pressure were significantly decreased in 3 weeks. These results suggest that a comprehensive dietary detoxification program can positively effect on reducing serum GGT, anthropometric data, lipids profiles, and blood pressure in all participants. Fasting blood glucose was not significantly decreased when we compared the changes in one group. In other hands, when it is focused on the only metabolic syndrome group, total cholesterol, fasting blood glucose, and systolic blood pressure were significantly reduced. Although sample size was small, this detoxification program may influence on decreasing cholesterol, blood glucose and blood pressure among insulin resistance subjects. Seven subjects had taken blood glucose lowering agents in metabolic syndrome group. Among of them, some subjects would decrease the number of glucose lowering drugs. Thus, this dietary detoxification program affected not only on lowering blood glucose level, but reducing taking medicines. Particularly, HDL-cholesterol slightly decreased in 3 weeks in post intervention (p = .055). A large scale 30-months follow-up study demonstrated that rapid decrease of fat might be associated with HDL-cholesterol reduction [37]. In our study, HDL-cholesterol decline seems a temporary phenomenon resulted in fat reduction. In addition, HDL to total cholesterol ratio is a practical indicator in clinical setting [38]. In this study, the change of HDL cholesterol/total cholesterol ratio, it was not significant difference between pre and post intervention.
Finally, even though this dietary detoxification did not restrict the calorie intakes, participants had a low-calorie diets comparing to Koreans’ dietary reference intakes. During toxin elimination diets, total intake energy and carbohydrate were almost similar to before intervention diets. A Literature review, low-calorie detoxifications diet decreased 15 toxicity scales and weight loss [16]. The vegetarian diets can be lowered several POPs level in type 2 diabetes [39]. However, a possible risk of detoxification diets could be nutritional imbalances, we need to consider of nutritional restriction diets [32]. There was no significant difference between pre and during macro-nutrients including carbohydrate, fat and protein in this study. The reason of decreased the body fat could be considered that animal fat and saturated fatty acids were decreased and dietary fiber was increased.
This dietary detoxification program could decrease serum GGT as a surrogate marker for overall toxic burden in the body. Anthropometric data and metabolic biomarkers were improved in adults. To our knowledge, detoxification may help to prevent the incidence of metabolic syndrome or type 2 diabetes. The integrated dietary and education detoxification program seemed to be a protective intervention for elimination of toxicants from the body.
REFERENCES
- 1.Genuis SJ. Elimination of persistent toxicants from the human body. Hum Exp Toxicol. 2011;30:3–18. doi: 10.1177/0960327110368417. [DOI] [PubMed] [Google Scholar]
- 2.Koch HM, Calafat AM. Human body burdens of chemicals used in plastic manufacture. Philos Trans R Soc B Biol Sci. 2009;364:2063–78. doi: 10.1098/rstb.2008.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merrill ML, Emond C, Kim MJ, Antignac J-P, Bizec BL, Clement K, et al. Toxicological function of adipose tissue: focus on persistent organic pollutants. Environ Health Perspect. 2013;121:162. doi: 10.1289/ehp.1205485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petriello M, Newsome B, Hennig B. Influence of nutrition in PCB-induced vascular inflammation. Environ Sci Pollut Res. 2014;21:6410–8. doi: 10.1007/s11356-013-1549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar J, Lind PM, Salihovic S, van Bavel B, Ingelsson E, Lind L. Persistent organic pollutants and inflammatory markers in a cross-sectional study of elderly Swedish people: the pivus cohort. ( Research) (Report) Environmental Health Perspectives. 2014;122:977. doi: 10.1289/ehp.1307613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costantini D, Meillère A, Carravieri A, Lecomte V, Sorci G, Faivre B, et al. Oxidative stress in relation to reproduction, contaminants, gender and age in a long-lived seabird. Oecologia. 2014;175:1107. doi: 10.1007/s00442-014-2975-x. [DOI] [PubMed] [Google Scholar]
- 7.Mostafalou S, Abdollahi M. Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol Appl Pharmacol. 2013;268:157–77. doi: 10.1016/j.taap.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 8.Rezg R, El-Fazaa S, Gharbi N, Mornagui B. Bisphenol A and human chronic diseases: Current evidences, possible mechanisms, and future perspectives. Environ Int. 2014;64:83–90. doi: 10.1016/j.envint.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Lim S, Cho YM, Park KS, Lee HK. Persistent organic pollutants, mitochondrial dysfunction, and metabolic syndrome. Ann N Y Acad Sci. 2010;12011:166–76. doi: 10.1111/j.1749-6632.2010.05622.x. [DOI] [PubMed] [Google Scholar]
- 10.Hyman MA. Environmental toxins, obesity, and diabetes: an emerging risk factor. Altern Ther Health Med. 2010;16:56. [PubMed] [Google Scholar]
- 11.Steven EK, Rebecca LH, Kristina MU. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 12.Lee D-H, Steffes M, Sjödin A, Jones R, Needham L, Jacobs D. Low Dose Organochlorine Pesticides and Polychlorinated Biphenyls Predict Obesity, Dyslipidemia, and Insulin Resistance among People Free of Diabetes. PLoS One. 2011;6:e15977. doi: 10.1371/journal.pone.0015977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee D-H, Lee I-K, Song K, Steffes M, Toscano W, Baker BA, et al. Strong Dose-Response Relation Between Serum Concentrations of Persistent Organic Pollutants and Diabetes: Results from the National Health and Examination Survey 1999–2002. Strong Dose-Response Relation Between Serum Concentrations of Persistent Organic Pollutants and Diabetes: Results from the National Health and Examination Survey 1999–2002. 2006;29:1638–44. doi: 10.2337/dc06-0543. [DOI] [PubMed] [Google Scholar]
- 14.Kuo C-C, Moon K, Thayer K, Navas-Acien A. Environmental Chemicals and Type 2 Diabetes: An Updated Systematic Review of the Epidemiologic Evidence. Curr Diab Rep. 2013;13:831–49. doi: 10.1007/s11892-013-0432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee D-H, Jacobs DR. Is serum gamma-glutamyltransferase a marker of exposure to various environmental pollutants? Free Radic Res. 2009;43:533–7. doi: 10.1080/10715760902893324. [DOI] [PubMed] [Google Scholar]
- 16.Morrison J, Iannucci A. Symptom Relief and Weight Loss From Adherence to a Meal Replacement-enhanced, Low-calorie Detoxification Diet. Int Med. 2012;11:42–7. [Google Scholar]
- 17.Andreas Moser G, McLachlan MS. The influence of dietary concentration on the absorption and excretion of persistent lipophilic organic pollutants in the human intestinal tract. Chemosphere. 2001;45:201–11. doi: 10.1016/S0045-6535(00)00551-8. [DOI] [PubMed] [Google Scholar]
- 18.Allen J, Montalto M, Lovejoy J, Weber W. Detoxification in naturopathic medicine: a survey. J Altern Complement Med. 2011;17:1175–80. doi: 10.1089/acm.2010.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onat A, Can G, Örnek E, Çiçek G, Ayhan E, Doğan Y. Serum γ-glutamyltransferase: independent predictor of risk of diabetes, hypertension, metabolic syndrome, and coronary disease. Obesity. 2012;20:842. doi: 10.1038/oby.2011.136. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Qiu L, Yan W-H, Cheng X-Q, Wu W, Guo X-Z, et al. Serum γ-glutamyltransferase and uric acid levels are associated with impaired fasting glucose in adults from Inner Mongolia, China. BMC Public Health. 2013;13:294. doi: 10.1186/1471-2458-13-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradley R, Fitzpatrick AL, Jenny NS, Lee D-H, Jacobs DR. Associations between total serum GGT activity and metabolic risk: MESA. Biomark Med. 2013;7:709–12. doi: 10.2217/bmm.13.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee D-H, Blomhoff R, Jacobs DR. Review Is Serum Gamma Glutamyltransferase a Marker of Oxidative Stress? Free Radic Res. 2004;38:535–9. doi: 10.1080/10715760410001694026. [DOI] [PubMed] [Google Scholar]
- 23.Lee DH, Jacobs DR. Serum γ-glutamyltransferase: new insights about an old enzyme. J Epidemiol Community Health. 2009;63:884–6. doi: 10.1136/jech.2008.083592. [DOI] [PubMed] [Google Scholar]
- 24.Lee DH, Steffes M, Jacobs D. Can persistent organic pollutants explain the association between serum γ-glutamyltransferase and type 2 diabetes? Diabetologia. 2008;51:402–7. doi: 10.1007/s00125-007-0896-5. [DOI] [PubMed] [Google Scholar]
- 25.Girman CJ, Dekker JM, Rhodes T, Nijpels G, Stehouwer CDA, Bouter LM, et al. An Exploratory Analysis of Criteria for the Metabolic Syndrome and Its Prediction of Long-term Cardiovascular Outcomes. Am J Epidemiol. 2005;162:438–47. doi: 10.1093/aje/kwi229. [DOI] [PubMed] [Google Scholar]
- 26.Kelly BC, Ikonomou MG, Blair JD, Morin AE, Gobas FAPC. Food web-specific biomagnification of persistent organic pollutants. Science. 2007;317:236–9. doi: 10.1126/science.1138275. [DOI] [PubMed] [Google Scholar]
- 27.Newman SP, Steed L, Mulligan K. In: Chronic physical illness: self-management and behavioural interventions. Newman Stanton, Steed Liz, Mulligan Kathleen, Newman SP, Steed L, Mulligan K., editors. Maidenhead, UK: 2009. [Google Scholar]
- 28.Fitzpatrick M. The meaning of detox. Lancet. 2003;361:94. doi: 10.1016/S0140-6736(03)12168-X. [DOI] [PubMed] [Google Scholar]
- 29.Dixon B. “Detox”, a mass delusion. Lancet Infect Dis. 2005;5:261. doi: 10.1016/S1473-3099(05)70094-3. [DOI] [PubMed] [Google Scholar]
- 30.Hennig B, Ettinger AS, Jandacek RJ, Koo S, McClain C, Seifried H, et al. Using Nutrition for Intervention and Prevention against Environmental Chemical Toxicity and Associated Diseases. Environ Health Perspect. 2007;115:493–5. doi: 10.1289/ehp.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sears ME, Genuis SJ. Environmental Determinants of Chronic Disease and Medical Approaches: Recognition, Avoidance, Supportive Therapy, and Detoxification. J Environ Public Health. 2012;2012:356798. doi: 10.1155/2012/356798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein AV, Kiat H. Detox diets for toxin elimination and weight management: a critical review of the evidence. J H Nutr. 2015;28:675–86. doi: 10.1111/jhn.12286. [DOI] [PubMed] [Google Scholar]
- 33.Moon MK, Jeong IK, Jung Oh T, Ahn HY, Kim HH, Park YJ, et al. Long-term oral exposure to bisphenol A induces glucose intolerance and insulin resistance. J Endocrinol. 2015;226:35–42. doi: 10.1530/JOE-14-0714. [DOI] [PubMed] [Google Scholar]
- 34.Lee D-H, Jacobs DR, Jr, Gross M, Steffes M, Kiefe CI, Lewis CE, et al. γ-Glutamyltransferase is a predictor of incident diabetes and hypertension: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Clinical Chemistry. 2003;49:1358–66. doi: 10.1373/49.8.1358. [DOI] [PubMed] [Google Scholar]
- 35.Lee DH, Silventoinen K, Jacobs DR, Jousilahti P, Tuomileto J. gamma-Glutamyltransferase, obesity, and the risk of type 2 diabetes: observational cohort study among 20,158 middle-aged men and women. J Clin Endocrinol Metab. 2004;89:5410–4. doi: 10.1210/jc.2004-0505. [DOI] [PubMed] [Google Scholar]
- 36.Lee D-H, Steffen LM, Jacobs DR. Association between serum γ-glutamyltransferase and dietary factors: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2004;79:600–5. doi: 10.1093/ajcn/79.4.600. [DOI] [PubMed] [Google Scholar]
- 37.Yatsuya H, Jeffery RW, Erickson DJ, Welsh EM, Flood AP, Jaeb MA, et al. Sex-specific HDL cholesterol changes with weight loss and their association with anthropometric variables: the LIFE study. Obesity (Silver Spring) 2011;19:429–35. doi: 10.1038/oby.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lemieux I, Lamarche B, Couillard C, Pascot A, Cantin B, Bergeron J, et al. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: the Quebec Cardiovascular Study. Arch Intern Med. 2001;161:2685–92. doi: 10.1001/archinte.161.22.2685. [DOI] [PubMed] [Google Scholar]
- 39.Kahleova H, Tonstad S, Rosmus J, Fisar P, Mari A, Hill M, et al. The effect of a vegetarian versus conventional hypocaloric diet on serum concentrations of persistent organic pollutants in patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2016;26:430–8. doi: 10.1016/j.numecd.2016.01.008. [DOI] [PubMed] [Google Scholar]