Abstract
Background
The purpose of our study was to investigate the association between polycystic ovary syndrome (PCOS) and hearing thresholds.
Material/Methods
Forty women diagnosed with PCOS (mean age, 24.33±6.38 years) and 40 healthy women controls (mean age, 26.38±6.75 years) were included in prospective study. Each case was tested with low (250, 500, 1000, and 2000 Hz), high (4000, 6000, and 8000 Hz) and extended high (EH) (9000–20000 Hz) frequency audiometry. The fasting plasma glucose, insulin, FSH, LH, total testosterone, and sex hormone-binding globulin were measured in all patients.
Results
The mean hearing thresholds at EH frequencies were statistically significantly higher in the PCOS group than in the control group (p=0.001 right ear and p=0.015 left ear). There were significant positive correlations among free testosterone index (FTI) values and hirsutism scores with EH frequency hearing thresholds.
Conclusions
At pure-tone audiometry (PTA) EH frequencies, we detected significantly higher hearing thresholds in PCOS patients than in controls. We also determined that elevated FTI and hirsutism score were positively correlated with elevated hearing thresholds in EH frequencies. These findings support that hyperandrogenism can play a role in the elevation of hearing thresholds in PCOS.
MeSH Keywords: Hearing Tests, Hirsutism, Hyperandrogenism, Polycystic Ovary Syndrome
Background
Sensorineural hearing loss (SNHL) is responsible for the majority of hearing losses. The main causes of SNHL are advanced age, the use of ototoxic medications, noise exposure, hereditary, and autoimmune diseases [1]. It has recently been reported that various other factors such as family history, smoking, alcohol consumption, head trauma, diseases such as diabetes, high blood pressure, and some renal pathologies also lead to changes in hearing [2–5].
Polycystic ovary syndrome (PCOS) is a common endocrine disease affecting at least 10% of females of reproductive age [6]. It is described by hyperandrogenism, menstrual irregularity, anovulation, infertility, and obesity [7]. It has also been associated with cardiovascular risk and early atherosclerosis [7–9]. In addition, PCOS is often accompanied by hyperinsulinemia, insulin resistance, dyslipidemia, and low-grade chronic inflammation that predispose to endothelial damage [10]. Also, high-frequency hearing is often affected sooner in diseases that cause endothelial damage [11]. High-frequency (4000–8000 Hz) hearing loss was first described in PCOS patients by Oghan and Coksuer [12]. Kucur et al. also identified EH frequency (8000–14000) hearing loss in PCOS patients [11]. However, these studies did not include any information about the association between hearing thresholds and the known parameters of PCOS such as IR, LH/FSH ratio, hirsutism score, and free testosterone index (FTI).
The aim of our study was to investigate the association between PCOS and hearing thresholds. In addition, the relationship among insulin resistance (IR), LH/FSH ratio, hirsutism score, and FTI with hearing thresholds were investigated.
Material and Methods
Subjects
This prospective study enrolled 80 consecutive cases that applied to the endocrinology and gynecology polyclinics of our hospital that met the inclusion criteria. Forty cases (mean age, 24.33±6.38 years; range 16–46 years) that were diagnosed with PCOS composed the PCOS group. Diagnosis of PCOS was according to Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group [13]. Forty age and body mass index (BMI) matched female volunteers (mean age, 26.38±6.75 years; range 16–42 years) composed the control group. The study design was confirmed by the local ethics committee (approval number: 11–26.12.2012) and informed consent was obtained from all of the women that participated in the study.
Exclusion criteria
For all participants, anamnesis was obtained, and otoscopic examination and pure-tone audiometric tests were performed. The exclusion criteria were: hyperprolactinemia, congenital adrenal hyperplasia, androgen-secreting tumors, thyroid disorders, Cushing syndrome, vertigo, tinnitus, middle ear diseases, major neurological and psychiatric disease, diabetes mellitus, chronic kidney failure, chronic liver failure, hypertension or hyperlipidemia and similar chronic diseases, history of previous ear surgery, family history of hearing loss, history of acoustic trauma, conductive hearing loss, subject to ototoxic substances, autoimmune diseases, cancer, exposure to radiation in the head or neck, pregnancy, smoking, alcohol use, ongoing infection or inflammation, and history of using any medication.
Audiometric evaluation and mean hearing levels
All patients had routine audiometric tests performed by the same expert audiometrist blinded to the study. The pure-tone audiometry (PTA) test was performed in a soundproof environment using an Interacoustic Clinical Audiometer (model AC40; Denmark). PTA-low (250, 500, 1000, and 2000 Hz), PTA-high (4000, 6000, and 8000 Hz) and PTA-extended high (9000, 10000, 11200, 12500, 14000, 16000, 18000, and 20000 Hz) frequencies were tested. Hearing threshold levels were measured in decibels (dB). In each case, the threshold level was calculated for both ears and at every frequency. The threshold levels at 250 Hz, 500 Hz, 1000 Hz, and 2000 Hz were identified as low-frequency audiometry, the thresholds at 4000 Hz, 6000 Hz, and 8000 Hz as high-frequency audiometry and the thresholds at 9000 Hz, 10000 Hz, 11200 Hz, 12500 Hz, 14000 Hz, 16000 Hz, 18000 Hz, and 20000 Hz as extended-high (EH) frequency audiometry and the mean value was calculated. The mean hearing thresholds of the PCOS group and control group at PTA-low, PTA-high, and PTA-EH frequencies were compared.
Morphometry
The physical examination of each of the women was performed by the same endocrinology specialist. After one night of fasting, height and weight were measured. BMI was calculated by dividing the weight by the square of height (kg/m2). The degree of hirsutism was determined using the Ferriman-Gallwey scoring (FGS) system [14].
Laboratory analyses
Blood samples were obtained during the follicular phase of the menstrual cycle after 12 hours fasting. The fasting plasma glucose levels (FPG) were evaluated using Roche Hitachi PP Modular Analyzer (Roche-Hitachi, Tokyo, Japan) by enzymatic colorimetric assay. Follicle-stimulating hormone (FSH), luteinizing hormone (LH), total testosterone (TT), sex hormone-binding globulin (SHBG), and insulin measurements were done using the chemiluminescent immunoassay method with an Immulite 2000 (Diagnostic Products, Los Angeles, CA, USA) analyzer. Insulin resistance was measured using the HOMA-IR (Homeostasis Model Assessment for Insulin Resistance) index [15]. The free testosterone index (FTI) was calculated according to the formula 100× serum testosterone (nmol/L)/SHBG (nmol/L) [16].
Statistical analysis
The descriptive statistics of the variables studied were given as mean, standard deviation, and minimum and maximum values. The student t-test was used to compare data between the control group and PCOS group. The Pearson correlation analysis was used to identify the linear associations between the variables. The statistical significance level was taken as 5%, and SPSS (version 21) was utilized for all statistical calculations.
Results
The demographic data and laboratory findings of the study groups are presented in Table 1. No difference was identified between the study groups with respect to age and BMI. Significantly higher FGS, FPG, insulin, HOMA-IR, TT, FTI, and LH/FSH measurements were identified in the PCOS group than in the control group (p=0.001, 0.009, 0.013, 0.005, 0.001, 0.001, and 0.003, respectively).
Table 1.
Demographic and laboratory findings in the study groups.
| Control (n=40) | Case (n=40) | p | |
|---|---|---|---|
| Age (year) | 26.38±6.75 | 24.33±6.38 | 0.167 |
| Height (m) | 1.64±0.06 | 1.62±0.05 | 0.228 |
| Weight (kg) | 66.73±15.43 | 62.38±10.16 | 0.140 |
| BMI (kg/m2) | 24.87±5.08 | 23.85±4.23 | 0.330 |
| FGS | 4.20±1.57 | 14.30±5.08 | <0.001 |
| FPG (mg/dL) | 88.83±9.54 | 94.95±10.76 | 0.009 |
| Insulin (μU/ml) | 9.90±3.75 | 14.06±9.61 | 0.013 |
| HOMA-IR | 2.19±0.92 | 3.28±2.18 | 0.005 |
| Total testosterone (ng/ml) | 0.73±0.28 | 1.37±0.53 | <0.001 |
| FTI | 1.72±0.94 | 3.99±3.22 | <0.001 |
| LH/FSH | 1.17±0.88 | 1.91±1.26 | 0.003 |
Data are mean ±SD. BMI – body mass index; FPG – fasting plasma glucose; HOMA-IR – homeostasis model assessment for insulin resistance; FGS – Ferriman-Gallwey score; FTI – free testosterone index; LH – futeinizing hormone; FSH – follicle-stimulating hormone.
The comparison of the low, high, and EH frequency hearing thresholds of the PCOS and control groups is presented in Table 2. There was no statistically significant difference between the mean hearing thresholds of the groups at low or high frequencies. On the other hand, the mean hearing thresholds at EH frequencies were statistically significantly higher in the PCOS group than in the control group (p=0.001 for the right ear and p=0.015 for the left ear).
Table 2.
Comparison of the case and control groups in terms of the average hearing thresholds at PTA-low, PTA-high and PTA-EH frequencies.
| Frequency range | Control (n=40) | Case (n=40) | p |
|---|---|---|---|
| PTA-low (right) | 12.48±2.33 | 11.45±3.59 | 0.134 |
| PTA-low (left) | 12.05±2.26 | 11.70±3.58 | 0.601 |
| PTA-high (right) | 13.21±2.65 | 14.03±5.10 | 0.372 |
| PTA-high (left) | 13.16±3.11 | 14.10±4.92 | 0.309 |
| PTA-EH (right) | 14.96±5.09 | 21.48±8.67 | 0.001 |
| PTA-EH (left) | 15.98±5.86 | 19.53±6.89 | 0.015 |
Data are mean ±SD. PTA – pure tone audiometry, EH – extended-high.
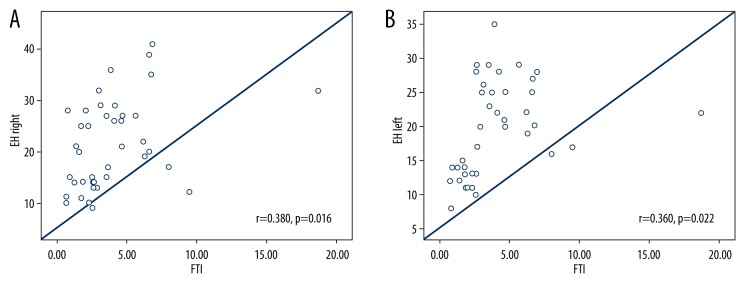
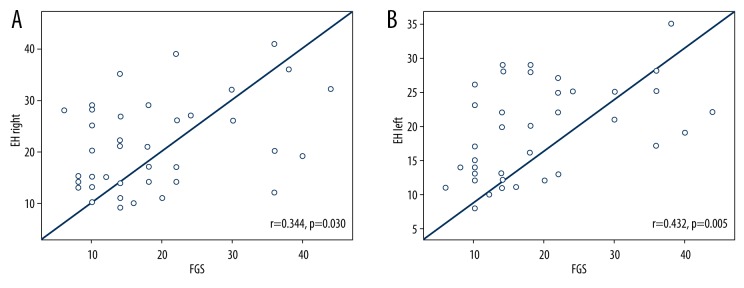
The correlation analysis of the PCOS group showed a positive correlation between the EH frequency hearing threshold levels and the FTI values (r=0.380, p=0.016 for the right ear and r=0.360, p=0.022 for the left ear; Figure 1A, 1B). Also, a significant positive correlation was identified between the hearing thresholds at EH frequencies and the FGS value (r=0.344, p=0.030 for the right ear and r=0.432, p=0.005 for the left ear; Figure 2A, 2B). But there was no significant correlation between the EH frequency and BMI, FPG, insulin, HOMA-IR, LH/FSH (p>0.005) in the PCOS group.
Figure 1.
(A) Correlation between EH-Right PTA measurements and FTI in PCOS group. (B) Correlation between EH-Left PTA measurements and FTI in PCOS group.
Figure 2.
(A) Correlation between EH-Right PTA measurements and FGS in PCOS group. (B) Correlation between EH-Left PTA measurements and FGS in PCOS group.
Comparison of the PCOS and control groups in terms of the average hearing thresholds at each frequency in PTA-EH measurements are shown in Table 3. Hearing thresholds at each frequency in PTA-EH were statistically significantly higher in the PCOS group than in the control group except for left ear 20000 Hz.
Table 3.
Comparison of the case and control groups in terms of the average hearing thresholds at each frequencies in PTA-EH measurements.
| Frequency (Hz) | Control (n=40) | Case (n=40) | p |
|---|---|---|---|
| 9000 | |||
| Right | 13.35±4.61 | 17.40±7.51 | 0.005 |
| Left | 13.58±4.83 | 16.18±5.76 | 0.032 |
| 10000 | |||
| Right | 14.20±4.57 | 18.95±8.33 | 0.002 |
| Left | 13.98±5.02 | 17.05±6.07 | 0.016 |
| 11200 | |||
| Right | 14.65±5.15 | 21.15±9.01 | <0.001 |
| Left | 15.15±6.01 | 18.68±6.62 | 0.015 |
| 12500 | |||
| Right | 16.08±5.64 | 23.63±9.63 | <0.001 |
| Left | 16.88±7.35 | 21.70±8.37 | 0.008 |
| 14000 | |||
| Right | 16.45±6.12 | 25.35±10.20 | <0.001 |
| Left | 18.38±7.71 | 22.50±8.99 | 0.031 |
| 16000 | |||
| Right | 16.15±5.84 | 24.70±10.18 | <0.001 |
| Left | 18.05±6.74 | 22.43±8.55 | 0.013 |
| 18000 | |||
| Right | 15.08±4.88 | 20.48±7.76 | <0.001 |
| Left | 16.15±5.45 | 19.28±6.32 | 0.020 |
| 20000 | |||
| Right | 14.80±4.70 | 20.15±8.32 | 0.001 |
| Left | 16.00±6.11 | 18.40±6.19 | 0.085 |
Bold values indicate statistical significance. Data are mean ±SD.
Discussion
There are various previous studies reporting that PCOS and hearing loss are associated at high (4000–8000 Hz) and EH (8000–14000 Hz) frequencies [11,12]. These studies do not provide any information about the presence of a relationship between hearing loss and known parameters of PCOS such as IR, elevated LH/FSH ratio, hirsutism score, and elevated FTI. To our knowledge, our study is the first study to investigate the association of hearing thresholds with parameters such as IR, LH/FSH ratio, hirsutism score, and FTI in PCOS cases.
In histological examination, Hwang et al. detected the presence of microvascular structures with leaner walls in the stria vascularis of the basal end of the cochlea in obese rats [17]. They also reported that markers such as tumor necrosis factor alpha, hypoxia-induced factor 1, caspase 3, poly (ADP-ribose) polymerase-1, nuclear factor kappa B, and apoptosis inducing factor are present in the basal end of the cochlea at high concentrations [17]. Various methods have been used to measure hearing thresholds [18,19]. In our study, the hearing threshold levels at EH frequencies (9000–20000 Hz) were statistically significantly higher in the PCOS group when compared to the control group. However, at low (250–2000 Hz) and high (4000–8000 Hz) frequencies, there was no significant difference between the hearing thresholds of the PCOS group and the control group. Our findings support other studies that also reported findings suggesting that EH frequencies which represent the basal end of the cochlea are often influenced sooner in disorders that cause endothelial and vascular damage [17,20,21]. In one other study, it was reported that chronic inflammation in PCOS could induce of hearing loss, particularly at EH frequencies [11]. As exposure to the multifactorial agents of PCOS continues over time, this impairment is likely to progress to involve low frequencies.
In previous studies, it was determined that hyperandrogenism was associated with elevated blood pressure, low HDL cholesterol, dysglycemia, insulin resistance, and abdominal obesity [22–27]. Endothelial damage and increased risk for cardiovascular disease in PCOS patients have also been reported [28]. Some of the biochemical and hormonal changes that occur in PCOS have been identified as responsible for atherosclerosis and endothelial dysfunction [11]. In light of all this information, it can be suggested that the hyperandrogenism in PCOS impairs microcirculation especially in the basal end of the cochlea through microangiopathy and leads to an elevation in hearing thresholds at EH frequencies. In our study, we detected the presence of a positive correlation between the FTI and FGS values and hearing thresholds at EH frequencies in the PCOS group. However, there was no significant correlation between BMI, FPG levels, insulin levels, IR, or LH/FSH ratio and hearing thresholds at EH frequencies. These results support the view that the elevation of testosterone levels (the fundamental cause of hirsutism in PCOS) may play a significant role in the pathophysiology of elevated hearing thresholds.
This study has some limitations. First, it is a cross-sectional study and therefore is incapable of assessing the changes that may occur in hearing levels over time; new longitudinal studies are required to investigate the causal role of PCOS in hearing loss. Second, the number of cases studied was small. New studies conducted with larger case populations are needed to elucidate the influence of PCOS and various risk factors on auditory functions.
Conclusions
At PTA-EH frequencies, we detected significantly higher hearing thresholds in PCOS patients than in controls. We also determined that elevated FTI and FPG levels were positively correlated with elevated hearing thresholds. These findings support the position that one of the most important factors playing a role in the elevation of hearing thresholds in PCOS is hyperandrogenism, which is a fundamental component of PCOS. A positive correlation was identified between elevated hearing thresholds and hirsutism scores (FGSs), which can easily be calculated during physical examination. We believe that PCOS-related hyperandrogenism plays an important role in sensorineural hearing loss and that this effect can be avoided by managing identified risk factors. Also, early hearing screening may help diagnose HL early, especially in PCOS cases with significant hyperandrogenism and hirsutism.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Source of support: Departmental sources
References
- 1.Li L, Chao T, Brant J, et al. Advances in nano-based inner ear delivery systems for the treatment of sensorineural hearing loss. Adv Drug Deliv Rev. 2016 doi: 10.1016/j.addr.2016.01.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shargorodsky J, Curhan SG, Eavey R, et al. A prospective study of cardiovascular risk factors and incident hearing loss in men. Laryngoscope. 2010;120:1887–91. doi: 10.1002/lary.21039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sogebi OA. Assessment of the risk factors for hearing loss in adult Nigerian population. Niger Med J. 2013;54:244–49. doi: 10.4103/0300-1652.119648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong JW, Jeon JH, Ku CR, et al. The prevalence and factors associated with hearing impairment in the Korean adults: the 2010–2012 Korea National Health and Nutrition Examination Survey (observational study) Medicine (Baltimore) 2015;94(10):e611. doi: 10.1097/MD.0000000000000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musani MA, Rauf A, Ahsan M, et al. Frequency and causes of hearing impairment in tertiary care center. J Pak Med Assoc. 2011;61:141–44. [PubMed] [Google Scholar]
- 6.Norman RJ, Dewailly D, Legro RS, et al. Polycystic ovary syndrome. Lancet. 2007;370:685–97. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 7.Pasquali R, Gambineri A, Pagotto U. The impact of obesity on reproduction in women with polycystic ovary syndrome. BJOG. 2006;113:1148–59. doi: 10.1111/j.1471-0528.2006.00990.x. [DOI] [PubMed] [Google Scholar]
- 8.Kelly CC, Lyall H, Petrie JR, et al. Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2001;86:2453–55. doi: 10.1210/jcem.86.6.7580. [DOI] [PubMed] [Google Scholar]
- 9.Kelly CJ, Speirs A, Gould GW, et al. Altered vascular function in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:742–46. doi: 10.1210/jcem.87.2.8199. [DOI] [PubMed] [Google Scholar]
- 10.Foltyn W, Strzelczyk J, Marek B, et al. Selected markers of endothelial dysfunction in women with polycystic ovary syndrome. Endokrynol Pol. 2011;62:243–48. [PubMed] [Google Scholar]
- 11.Kucur C, Kucur SK, Gozukara I, et al. Extended high frequency audiometry in polycystic ovary syndrome. ScientificWorldJournal. 2013;2013:482689. doi: 10.1155/2013/482689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oghan F, Coksuer H. Does hyperandrogenism have an effect on hearing loss in patients with polycystic ovary syndrome? Auris Nasus Larynx. 2012;39:365–68. doi: 10.1016/j.anl.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–47. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 15.Hwang JH, Hsu CJ, Liu TC, et al. Association of plasma adiponectin levels with hearing thresholds in adults. Clin Endocrinol (Oxf) 2011;75:614–20. doi: 10.1111/j.1365-2265.2011.04090.x. [DOI] [PubMed] [Google Scholar]
- 16.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 17.Hwang JH, Hsu CJ, Yu WH, et al. Diet-induced obesity exacerbates auditory degeneration via hypoxia, inflammation, and apoptosis signaling pathways in CD/1 mice. PLoS One. 2013;8(4):e60730. doi: 10.1371/journal.pone.0060730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatzopoulos S, Petruccelli J, Śliwa L, et al. Hearing threshold prediction with Auditory Steady State Responses and estimation of correction functions to compensate for differences with behavioral data, in adult subjects. Part 1: Audera and CHARTR EP devices. Med Sci Monit. 2012;18(7):MT47–53. doi: 10.12659/MSM.883195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatzopoulos S, Kochanek K, Sliwa L, et al. A pilot study on assessing hearing threshold using the Cochlea-Scan. Med Sci Monit. 2008;14(4):MT7–11. [PubMed] [Google Scholar]
- 20.Le Prell CG, Spankovich C, Lobariñas E, et al. Extended high-frequency thresholds in college students: Effects of music player use and other recreational noise. J Am Acad Audiol. 2013;24:725–39. doi: 10.3766/jaaa.24.8.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma D, Munjal SK, Panda NK. Extended high frequency audiometry in secretory otitis media. Indian J Otolaryngol Head Neck Surg. 2012;64:145–49. doi: 10.1007/s12070-012-0478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen MJ, Yang WS, Yang JH, et al. Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension. 2007;49:1442–47. doi: 10.1161/HYPERTENSIONAHA.106.083972. [DOI] [PubMed] [Google Scholar]
- 23.Muti P, Trevisan M, Panico S, et al. Body fat distribution, peripheral indicators of androgenic activity, and blood pressure in women. Ann Epidemiol. 1996;6:181–87. doi: 10.1016/1047-2797(96)00008-7. [DOI] [PubMed] [Google Scholar]
- 24.Chen MJ, Yang WS, Yang JH, et al. Low sex hormone-binding globulin is associated with low high-density lipoprotein cholesterol and metabolic syndrome in women with PCOS. Hum Reprod. 2006;21:2266–71. doi: 10.1093/humrep/del175. [DOI] [PubMed] [Google Scholar]
- 25.Valderhaug TG, Hertel JK, Nordstrand N, et al. The association between hyperandrogenemia and the metabolic syndrome in morbidly obese women. Diabetol Metab Syndr. 2015;7:46. doi: 10.1186/s13098-015-0040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmäng A, Larsson BM, Brzezinska Z, et al. Effects of short-term testosterone exposure on insulin sensitivity of muscles in female rats. Am J Physiol. 1992;262:851–55. doi: 10.1152/ajpendo.1992.262.6.E851. [DOI] [PubMed] [Google Scholar]
- 27.Nohara K, Laque A, Allard C, et al. Central mechanisms of adiposity in adult female mice with androgen excess. Obesity (Silver Spring) 2014;22:1477–84. doi: 10.1002/oby.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orio F, Palomba S, Colao A. Cardiovascular risk in women with polycystic ovary syndrome. Fertil Steril. 2006;86:20–21. doi: 10.1016/j.fertnstert.2006.03.003. [DOI] [PubMed] [Google Scholar]