Abstract
Background
Because of the insensitivity of renal cell carcinoma (RCC) to both chemotherapy and radiotherapy, surgery remains the primary approach for anticancer treatment. However, patients who do not receive timely diagnoses may not be suitable for surgery, especially in the late phase of tumor development. Thus, the discovery of novel effective treatment is of great importance. Allyl isothiocyanate (AITC) can inhibit the proliferation and induce apoptosis in many cancer cells. In this paper, we report on an in vitro study to determine the effect of AITC on proliferation and apoptosis of RCC line GRC-1.
Material/Methods
CCK8 assay was used to detect cell proliferation under gradient concentrations of AITC. Flow cytometry was employed to evaluate cell apoptosis. Real-time fluorescent polymerase chain reaction quantified mRNA levels of Bax and Bcl-2 genes. Western blotting was further employed for protein expression assay.
Results
AITC inhibited GRC-1 cell proliferation and induced cell apoptosis in a dose-dependent manner; it also elevated Bax while suppressing Bcl-2 gene expression at both mRNA and protein levels. In general, increasing concentration of AITC decreased Bcl-2/Bax ratio.
Conclusions
The inhibitory effect of AITC on GRC-1 cells is exerted via cell apoptosis, in which the imbalance of Bcl-2/Bax plays a significant role.
MeSH Keywords: Alprenolol; Apoptosis Inducing Factor; Cellulose 1,4-beta-Cellobiosidase
Background
Among all malignant tumors in the urinary system, renal cell carcinoma (RCC) is the second most common one, after bladder carcinoma. Because of its inherent insensitivity to radiation and chemotherapy, RCC is usually treated by surgical resection. However, as a result of distal metastasis or venous invasion, many patients are already unsuitable for radical surgery. Additionally, of patients with successful surgery, 20% to 40% experienced recurrence and/or metastasis. Therefore, the development of alternative treatment is still of critical importance.
Isothiocyanate is a glucosinolate hydrolysis product that consists of different types based on various side chains, and about one-third of them are allyl isothiocyanate (AITC). Glucosinolate widely exists in cruciferous plants, such as cabbage and cauliflower and their byproducts. Some studies suggest that AITC, as a tumor-related chemical compound found in natural plant diets, is involved in various biological processes, including cell apoptosis induction, cell cycle interference, or invasion/metastasis inhibition in various tumors, such as colon cancer, bladder carcinoma, liver cancer, neuroblastoma malignant glioma, and prostate carcinoma. Experiments on animal models found that AITC inhibited the progression of bladder carcinoma during the urine into bladder tissues. An epidemiological survey also indicated the improvement of the survival rate of patients with bladder cancer after eating cruciferous vegetables. Because RCC also belongs to the urinary system cancers, we thus hypothesized that AITC might also regulate the pathogenesis and progression of RCC. No direct animal study or clinical report, however, has been made regarding the treatment efficacy of AITC in RCC. In this context, we selected RCC cell line GRC-1 and examined the cell proliferation and apoptosis after the treatment with gradient concentrations of AITC. Also, the expressions of apoptotic-associated genes, Bcl-2 and Bax, were also quantified, to elucidate the possible mechanism of AITC in RCC pathogenesis.
Material and Methods
Cell culture
Renal carcinoma cell line GRC-1 was purchased from the Institute of Urine, at Beijing Medical University. Cells were generated from a 40-year-old male patient with recurrent mixed cell carcinoma (mainly renal granular cell carcinoma) after left nephrectomy, pathological grading of G3. Cells were cultivated in RMPI1640 medium (Gibco, US) containing 10% fetal bovine serum (FBS; Gibco, US) in a humidified chamber with 5% CO2 at 37°C. Cells were passed every 2 to 3 days. Culture medium was first removed, then washed twice by phosphate buffered saline (PBS). Trypsin was added to digest cells for preparing single-cell suspensions, which were centrifuged at 1000 g for 5 min, and resuspended in 1-mL culture medium.
CCK8 proliferation assay
The proliferation of cells was tested by cell counting with hemocytometer and Trypan Blue. Cells (1×104) were seeded into 96-well plates for 12-hour acclimation. AITC gradient concentrations of 0, 7.5, 15, and 30 μM were added to experimental cells at different treatment times (24, 48, and 72 hours; n=5 each). After drug intervention, PBS was used to wash cells, followed by adding 0.1 mL CCK8 mixture (1: 10 dilution) and 2-hour incubation at 37°C. A microplate reader was then used to quantify absorbance value at 450 nm for calculating relative survival rate of cells:
Flow cytometry
Cell suspensions were added into 6-well plates for 12-hour acclimation. Gradient concentrations of AITC (0, 7.5, 15, and 30 μM) were then used for 24-hour treatment in triplicates. After incubation, cells were digested by trypsin, washed in PBS, and centrifuged at 1000 g for 5 min. The proliferation of cells was tested by cell counting with hemocytometer. Cells (1×106) were suspended, centrifuged, and resuspended in Annexin V-FITC binding buffer and propidium iodinate. After incubation in the dark for 20 min, the cell mixture was loaded for flow cytometry assay of apoptotic ratio.
Real time PCR
Total RNA was extracted by Trizol, and was quantified using ultraviolet spectrometer. RNA integrity was identified using 1% agarose gel electrophoresis. cDNA was synthesized using reverse transcription kit (Takara, Japan) following manual instruction. Real-time fluorescent quantitative PCR was performed in a cycler (Model ViiA7, ABI, US) using SYBR green mixture (Takara, Japan), with cDNA as the template. PCR conditions were: 95°C pre-denature for 10 min, followed by 40 cycles each consisting of 95°C denature for 15 sec, 60°C annealing for 60 sec, and 72°C elongation for 60 sec. Each experiment was performed in triplicates using β-actin as the internal reference.
Western blotting
After treatment with gradient AITC (0, 7.5, 15, and 30 μM) for 24 hours, cells were lysed and homogenized for extracting total protein in the supernatant. Coomasie Brilliant Blue staining was used to quantify protein level. Proteins were separated in SDS-PAGE and transferred to NC membrane under electrical field. The membrane was first blocked by 5% defatted milk powder for 2 hours, then washed in PBS. Primary antibody was added for overnight incubation, followed by washing and secondary antibody incubation (1 hour). ECL reagents were added to develop the membrane, which was then exposed under x-ray. GIS-2020D gel image analysis system was used to calculate optical density values of protein bands. Relative expression level was calculated against β-actin.
Statistical analysis
SPSS 13.0 software was used to process all collected data, of which those fitted normal distribution was expressed as mean ± standard deviation. Analysis of variance was used to compare means across multiple groups, followed by least significant difference (LSD) test. P<0.05 was considered statistically significant.
Results
GRC-1 cell proliferation
In the assay with different concentrations of AITC, the proliferation of GRC-1 cells was inhibited in both dose- and time-dependent manners. The treatment with 30 μM of AITC for 72 hours had the most potent effect (Table 1).
Table 1.
AITC’s effect on GRC-1 cell proliferation.
| AITC concentration | 24 h | 48 h | 72 h |
|---|---|---|---|
| 0 μM | 1.00±0.042 | 1.00±0.043 | 1.00±0.041 |
| 7.5 μM | 0.92±0.038* | 0.87±0.038* | 0.81±0.040* |
| 15 μM | 0.86±0.044* | 0.78±0.039* | 0.69±0.043* |
| 30 μM | 0.72±0.057* | 0.67±0.041* | 0.58±0.042* |
p<0.05 compared to 0 μM group.
GRC-1 cell apoptosis
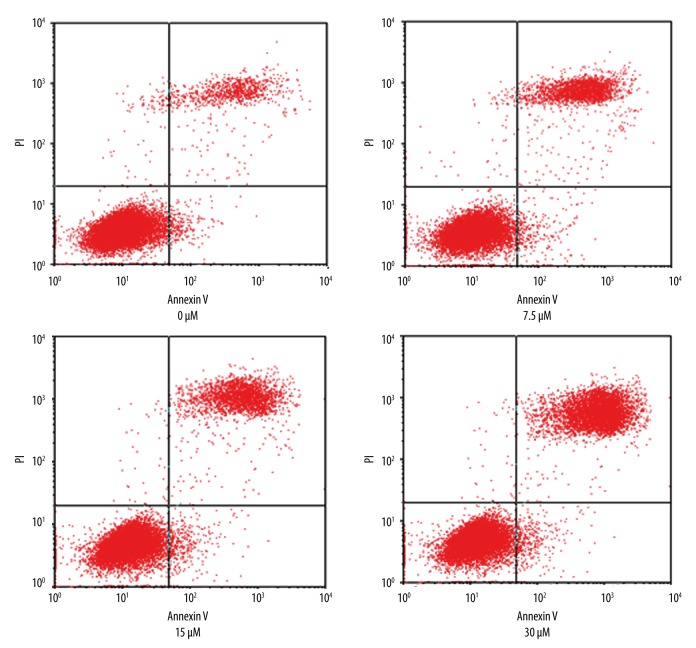
As shown in Figure 1, the apoptotic ratio of GRC-1 cells in control, 7.5-μM, 15-μM, and 30-μM groups were (7.08%±1.20), (13.28%±2.03), (19.46%±1.48), and (30.26%±1.58), respectively. The apoptotic ratio was increased by AITC in a dose-dependent manner.
Figure 1.
Cell apoptosis under allyl isothiocyanate.
Bax and Bcl-2 mRNA levels
The mRNA level of the Bcl-2 gene gradually decreased in AITC-treated cells as compared with controls (P<0.05). In contrast, the Bax mRNA level was up-regulated when concentration of AITC was increased (Figure 2).
Figure 2.
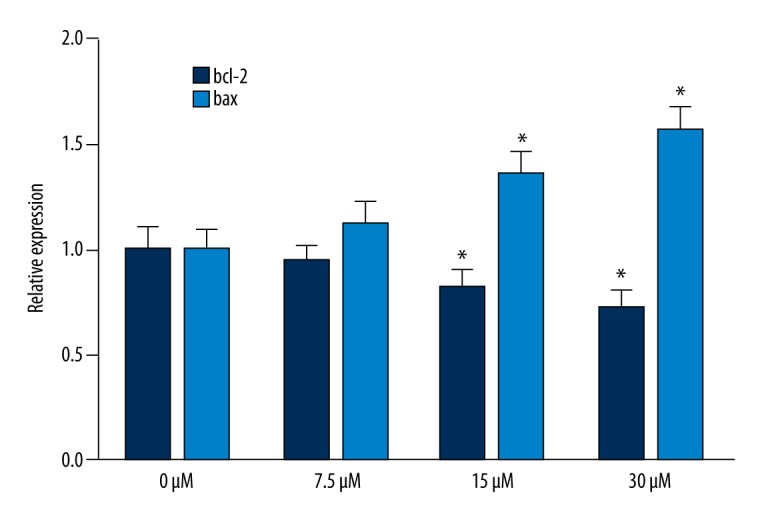
Bax and Bcl-2 mRNA levels. * P<0.05, compared with 0-μM group.
Bax and Bcl-2 protein expression levels
Consistent with Bax and Bcl-2 mRNA levels, the expression of Bcl-2 protein decreased as the concentration of AITC increased, whereas Bax protein level increased, indicating a decreasing Bcl-2/Bax ratio trend as AITC dosage increases (Table 2, Figure 3).
Table 2.
Bax and Bcl-2 protein level after AITC treatment.
| Protein | 0 μM | 7.5 μM | 15 μM | 30 μM | P value |
|---|---|---|---|---|---|
| Bax | 0.41±0.04 | 0.48±0.05* | 0.63±0.04* | 0.72±0.06* | <0.001 |
| Bcl-2 | 0.82±0.05 | 0.75±0.04* | 0.69±0.06* | 0.50±0.05* | <0.001 |
| Bcl-2/Bax | 2.00±0.14 | 1.56±0.15* | 1.10±0.11* | 0.69±0.12* | <0.001 |
p<0.05 compared to 0 μM group.
Figure 3.
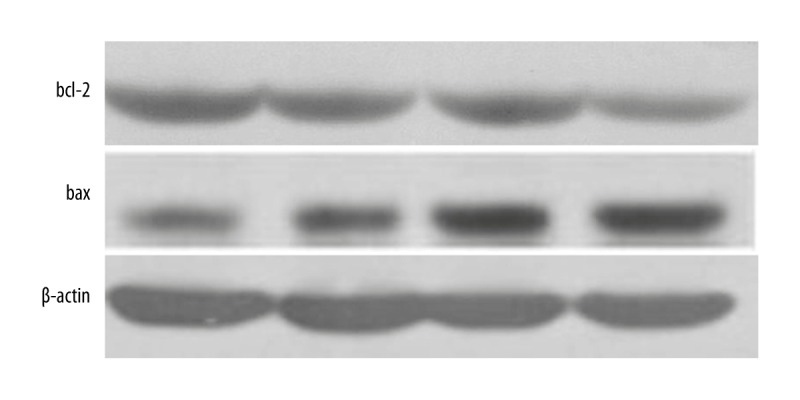
Bax and Bcl-2 protein expression levels.
Discussion
At present, the treatment of RCC includes surgery, chemotherapy, radiotherapy, immunotherapy, and targeted therapy; however, in some cases, RCC is not sensitive to radiotherapy or chemotherapy. Moreover, surgical treatment is mainly used in patients with early metastasis of RCC, which restricts the application. Immunotherapy and targeting therapy remain to be further developed. Therefore, development of a novel treatment for RCC is of great importance. Because of fewer associated side effects, medical plants have shown promising antitumor profiles compared with classical chemotherapy drugs. Both in vivo and in vitro studies have proved the antitumor effect of isothiocyanate [18,19]. AITC has been reported to inhibit the progression of bladder carcinoma [9]. Because RCC and bladder carcinoma are both urinary system cancers, we investigated whether AITC also regulates the pathogenesis and progression of RCC.
Our results showed a growing dearth of GRC-1 cells with elevated AITC concentration in a time-dependent manner, among which, 30-μM AITC treatments for 72 hours had potent inhibitory effects on cell proliferation (32%). The higher dosage of AITC also induced more cell apoptosis after 24 hours’ treatment, suggesting the inhibitory role of AITC may be owing to the induction of cell apoptosis. Evidence also revealed that AITC inhibited the RT4 cell proliferation by including cell apoptosis and interfering with cell cycle [10].
Bax and Bcl-2 belong to Bcl-2 family, which can modulate cell apoptosis [20]. Bcl-2 and Bax play as an antagonist pair. Bax itself promotes cell apoptosis and forms a dimer with Bcl-2 to limit the inhibitory effect of Bcl-2 on apoptosis [21]. The elevation of the Bcl-2/Bax ratio, therefore, was observed during the suppression of cell apoptosis [22], whereas the induction of apoptosis is always accompanied by the decline of the Bcl-2/Bax ratio [23]. Our study determined the effect of AITC on the expressions of Bax and Bcl-2 and found elevated Bax gene expression as well as descending Bcl-2 gene expression as the dosage of AITC increased, indicating a negative correlation between Bcl-2/Bax ratio and AITC concentration. Our results suggest that AITC inhibits the proliferation of GRC-1 cell line due to the imbalance between bcl2 and Bax, as is consistent with a previous study in RT4 cells [10].
Conclusions
For the first time, a study was performed on the effect of AITC on RCC cells, providing evidence of the inhibition on GRC-1 cell proliferation by AITC, possibly via Bcl-2/Bax imbalanced expression and further induced cell apoptosis. However, because the mechanism of AITC in inhibiting cell proliferation and inducing apoptosis is inherently complicated, the precise mechanism requires further explanation.
Footnotes
Disclosure of conflict of interest
The authors declare no competing financial or commercial interests in this manuscript.
Source of support: This work was supported by an applied basic research project of the Science and Technology Department in Sichuan (2013JY0173)
References
- 1.Amato RJ. Renal cell carcinoma: Review of novel single-agent therapeutics and combination regimens. Ann Oncol. 2005;16(1):7–15. doi: 10.1093/annonc/mdi002. [DOI] [PubMed] [Google Scholar]
- 2.Xu K, Thornalley PJ. Studies on the mechanism of the inhibition of human leukaemia cell growth by dietary isothiocyanates and their cysteine adducts in vitro. Biochem Pharmacol. 2000;60(2):221–31. doi: 10.1016/s0006-2952(00)00319-1. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharya A, Li Y, Wade KL, et al. Allyl isothiocyanate-rich mustard seed powder inhibits bladder cancer growth and muscle invasion. Carcinogenesis. 2010;31(12):2105–10. doi: 10.1093/carcin/bgq202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geng F, Tang L, Li Y, et al. Allyl isothiocyanate arrests cancer cells in mitosis, and mitotic arrest in turn leads to apoptosis via Bcl-2 protein phosphorylation. J Biol Chem. 2011;286(37):32259–67. doi: 10.1074/jbc.M111.278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao D, Srivastava SK, Lew KL, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24(5):891–97. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 6.Wu CL, Huang AC, Yang JS, et al. Benzyl isothiocyanate (BITC) and phenethyl isothiocyanate (PEITC)-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of caspase-3, mitochondria dysfunction and nitric oxide (NO) in human osteogenic sarcoma U-2 OS cells. J Orthop Res. 2011;29(8):1199–209. doi: 10.1002/jor.21350. [DOI] [PubMed] [Google Scholar]
- 7.Lau WS, Chen T, Wong YS. Allyl isothiocyanate induces G2/M arrest in human colorectal adenocarcinoma SW620 cells through down-regulation of Cdc25B and Cdc25C. Mol Med Rep. 2010;3(6):1023–30. doi: 10.3892/mmr.2010.363. [DOI] [PubMed] [Google Scholar]
- 8.Lai KC, Lu CC, Tang YJ, et al. Allyl isothiocyanate inhibits cell metastasis through suppression of the MAPK pathways in epidermal growth factorstimulated HT29 human colorectal adenocarcinoma cells. Oncol Rep. 2014;31(1):189–96. doi: 10.3892/or.2013.2865. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharya A, Li Y, Geng F, et al. The principal urinary metabolite of allyl isothiocyanate, N-acetyl-S-(N-allylthiocarbamoyl)cysteine, inhibits the growth and muscle invasion of bladder cancer. Carcinogenesis. 2012;33(2):394–98. doi: 10.1093/carcin/bgr283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savio AL, da Silva GN, Salvadori DM. Inhibition of bladder cancer cell proliferation by allyl isothiocyanate (mustard essential oil) Mutat Res. 2015;771:29–35. doi: 10.1016/j.mrfmmm.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Savio AL, da Silva GN, de Camargo EA, Salvadori DM. Cell cycle kinetics, apoptosis rates, DNA damage and TP53 gene expression in bladder cancer cells treated with allyl isothiocyanate (mustard essential oil) Mutat Res. 2014;762:40–46. doi: 10.1016/j.mrfmmm.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Hwang ES, Lee HJ. Allyl isothiocyanate and its N-acetylcysteine conjugate suppress metastasis via inhibition of invasion, migration, and matrix metalloproteinase-2/-9 activities in SK-Hep 1 human hepatoma cells. Exp Biol Med (Maywood) 2006;231(4):421–30. doi: 10.1177/153537020623100408. [DOI] [PubMed] [Google Scholar]
- 13.García A, Haza AI, Arranz N, et al. Protective effects of isothiocyanates alone or in combination with vitamin C towards N-nitrosodibutylamine or N-nitrosopiperidine-induced oxidative DNA damage in the single-cell gel electrophoresis (SCGE)/HepG2 assay. J Appl Toxicol. 2008;28(2):196–204. doi: 10.1002/jat.1270. [DOI] [PubMed] [Google Scholar]
- 14.Louhivuori LM, Bart G, Larsson KP, et al. Differentiation dependent expression of TRPA1 and TRPM8 channels in IMR-32 human neuroblastoma cells. J Cell Physiol. 2009;221(1):67–74. doi: 10.1002/jcp.21828. [DOI] [PubMed] [Google Scholar]
- 15.Chen NG, Chen KT, Lu CC, et al. Allyl isothiocyanate triggers G2/M phase arrest and apoptosis in human brain malignant glioma GBM 8401 cells through a mitochondria-dependent pathway. Oncol Rep. 2010;24(2):449–55. doi: 10.3892/or_00000878. [DOI] [PubMed] [Google Scholar]
- 16.Xu C, Shen G, Yuan X, et al. ERK and JNK signaling pathways are involved in the regulation of activator protein 1 and cell death elicited by three isothiocyanates in human prostate cancer PC-3 cells. Carcinogenesis. 2006;27(3):437–45. doi: 10.1093/carcin/bgi251. [DOI] [PubMed] [Google Scholar]
- 17.Tang L, Zirpoli GR, Guru K, et al. Intake of cruciferous vegetables modifies bladder cancer survival. Cancer Epidemiol Biomarkers Prev. 2010;19(7):1806–11. doi: 10.1158/1055-9965.EPI-10-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Y, Zhuang JX, Wang Q, et al. Inhibitory effect of benzyl isothiocyanate on proliferation in vitro of human glioma cells. Asian Pac J Cancer Prev. 2013;14(4):2607–10. doi: 10.7314/apjcp.2013.14.4.2607. [DOI] [PubMed] [Google Scholar]
- 19.Gupta P, Kim B, Kim SH, Srivastava SK. Molecular targets of isothiocyanates in cancer: Recent advances. Mol Nutr Food Res. 2014;58(8):1685–707. doi: 10.1002/mnfr.201300684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cory S, Adams JM. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2(9):647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 21.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74(4):609–19. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 22.Fan Y, Yang F, Cao X, et al. Gab1 regulates SDF-1-induced progression via inhibition of apoptosis pathway induced by PI3K/AKT/Bcl-2/BAX pathway in human chondrosarcoma. Tumour Biol. 2015 doi: 10.1007/s13277-015-3815-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Cheng F, Yang Y, Zhang L, et al. A natural triterpene derivative from Euphorbia kansui inhibits cell proliferation and induces apoptosis against rat intestinal epithelioid cell line in vitro. Int J Mol Sci. 2015;16(8):18956–75. doi: 10.3390/ijms160818956. [DOI] [PMC free article] [PubMed] [Google Scholar]