Abstract
Background
We explored the effect of parathyroid hormone (PTH)-induced bone marrow stem cells (BMSCs) complexed with fibrin glue (FG) in the repair of articular cartilage injury in rabbits.
Material/Methods
Forty-eight rabbits randomized into four groups were subjected to articular surgery (cartilage loss). The PTH and non-PTH intervention groups included transplantation with PTH/BMSC/FG xenogeneic and BMSC/FG xenogeneic complexes, respectively, into the injured area. The injured group contained no transplant while the control group comprised rabbits without any articular injury. Samples were monitored for cartilage repair up to three months post-surgery. Immunohistochemistry as well as real-time fluorescent quantitative PCR and Western blot were used to analyze the expression of type II collagen and aggrecan in the repaired tissue.
Results
At 12 weeks post-surgery, the loss of articular cartilage in the PTH group was fully repaired by hyaline tissue. Typical cartilage lacunae and intact subchondral bone were found. The boundary separating the surrounding normal cartilage tissue disappeared. The gross and International Cartilage Repair Society (ICRS) histological ranking of the repaired tissue was significantly higher in the PTH intervention group than in the non-PTH intervention and injury groups (p<0.05) without any significant difference compared to the control group (p>0.05). Type II collagen and aggrecan stained positive and the average optical density, relative mRNA expression and protein-integrated optical density in the PTH group were higher than in non-PTH and injured groups (p<0.05) but not significantly different from the control group (p>0.05).
Conclusions
PTH/BMSC/FG xenogeneic complexes effectively repaired the loss of cartilage in rabbit knee injury.
MeSH Keywords: Cartilage, Articular, Mesenchymal Stem Cell Transplantation, Parathyroid Hormone
Background
The incidence of articular cartilage injury is about 5% in the normal population, and 22% to 50% in athletes [1]. Arthroscopic screening of patients increased the incidence to 63% [2,3]. Further, articular cartilage exhibits poor capacity for self-repair. Therefore, articular cartilage injury often leads to osteoarthritis [4,5]. Until now, several therapies have been used to clinically treat cartilage injury, with poor efficacy [6–9]. Advances in tissue engineering based on bone marrow mesenchymal stem cells (BMSCs) offer novel insight into the repair mechanisms of articular cartilage [10]. Although the use of autologous BMSCs may be partially effective, the low abundance of BMSCs in bone marrow and the procedures to differentiate BMSCs into chondroblasts are a challenge. Few autologous BMSCs are available, with limited ability to passage and proliferate or de-differentiate in vitro, with significant decline in activity with age and self-injury in the autologous donor site. Therefore, development or discovery of novel cartilage that not only fully meets the standards of growth and repair but also greatly reduces the requirement of autologous BMSCs is imperative.
Parathyroid hormone (PTH) is the ligand for G protein-coupled receptors. PTH plays a dual role in catabolism and anabolism, triggering a series of physiological and biochemical reactions to regulate calcium homeostasis. PTH is one of the key hormones that regulate bone and cartilage growth [11,12]. It not only affects BMSC differentiation but also inhibits hypertrophic differentiation of chondrocytes and stimulates their proliferation [13–18].
Until now, the role of xenogenic BMSCs in the repair of cartilage injury via tissue engineering has not been reported. Therefore, in this study, we investigated into the differentiation of rat BMSCs into chondroblasts in vitro by PTH. We compounded the PTH-induced BMSCs with fibrin glue (FG) to construct xenogeneic PTH/BMSC/FG composites for transplantation into rabbit knee with the articular cartilage injury. We assessed the cartilage repair. We report the synthesis of a novel material for cartilage tissue engineering and regenerative medicine.
Material and Methods
Experimental animals
Ten one-week-old Sprague Dawley (SD) rats were provided by Guangdong Medical Experimental Animal Center (animal quality license: SCXK (Yue) 2008-0002) regardless of gender. Forty-eight healthy adult male New Zealand white rabbits aged six to seven months and weighing 2 kg to 2.5 kg each, were provided by the Experimental Animal Center of Guangzhou University of Traditional Chinese Medicine (License: SCXK(Yue) 2013-0020).
Experimental procedures
BMSC isolation, culture, and identification
SD rats were euthanized and immediately immersed in povidone-iodine for 10 minutes. Rat femurs were isolated from the demised SD rats under a sterile biological Class II-B safety cabinet (Altai Laboratory Equipment Co., Ltd.). The soft tissue surrounding the femurs was removed, following which they were rinsed in phosphate buffered saline (PBS, Institute of Biomedical Engineering, The Chinese Academy of Medical Sciences, China) containing penicillin and streptomycin (100 U/mL) for a total of three washes. After the epiphyseal region was dissected, the marrow cavity was exposed and repeatedly washed using 1 mL syringes filled with L-DMEM (Gibco, USA) medium containing 15% fetal bovine serum (FBS, Hyclone). The flushing fluid was added to the single cell suspension, incubated in 25 cm2 plastic culture flasks, supplemented with 3 mL L-DMEM medium containing 15% FBS, and cultured in an incubator (Thermo, USA) at 37°C and 5% CO2, and saturated humidity. The culture medium was changed every two to three days. The culture was terminated when 80% of the cells completed trypsin digestion and passage. BMSCs were collected after passage four, and prepared as cell suspensions. The CD44 (LifeSpan Biosciences, St. Louis, USA) expression in suspensions was detected by flow cytometry (Tanon, Shanghai).
PTH-induced differentiation of rat BMSCs into chondroblasts
Rat BMSCs (passage 4) were formulated into single cell suspension at a concentration of 1×104 cells/mL and seeded into 36 culture plates specifically for laser confocal microscopy, using 1 mL per plate. These plates were randomized into experimental and control groups, containing 18 plates each. In the experimental group, chondroblast medium containing 10 nM PTH (1–34) (Biovision, USA), was used for culture and chondroblast medium without PTH (1–34) was used as the control group. Media were changed every three days. Six samples from each group were collected on days 4, 7 and 14. Six non-overlapping fields were selected under laser confocal microscope (Carl Zeiss, Germany) to examine the expression of type II collagen and aggrecan. The average fluorescence intensity was measured.
Preparation of PTH/BMSCs/FG composites
First, 2.5 mL BMSC culture media were supplemented with 100 mg fibrinogen (100 mg, Sigma, USA) and mixed well to obtain the E solution as the suspension medium. The E solution was used to prepare PTH-induced (E1) and non-PTH-induced (E2) BMSC suspension (1×106 cells/mL). The F solution containing 40 mmol/L CaCl2 (Abcam, USA), 50 IU/mL thrombin (Sigma, USA) and 20 mg/mL tranexamic acid (Abcam, USA) was used to solidify the fibrin-BMSC suspension [19,20]. Then, 250 μL each of E1 and E2 cell suspension and F solution were added to 24-well plates and mixed well. The mixture quickly turned into a milky jelly and was labeled. Under the inverted phase contrast microscope (Nikon, Japan), BMSCs appeared as spherical and evenly distributed cells in the support materials. Eight hours after the BMSCs were glued to the FG support, the composites were transplanted into the rabbit knee sites to treat cartilage loss.
Experimental grouping
Forty-eight healthy adult New Zealand white rabbits weighing between 2 kg and 2.5 kg were selected and randomized into 4, 8, and 12 week groups, with 16 rabbits in each group. Both knees were used for this study. Within each group, 16 rabbits were randomized to PTH intervention, non-PTH intervention, injured, and control groups. After the randomization, the rabbits were maintained in separate cages for 14 days.
Preparation of rabbit models for knee articular cartilage loss
After weighing, the New Zealand white rabbits in the PTH intervention, the non-PTH intervention and the injury groups were anesthetized using Sumianxin II (0.2 mL/kg IM) (ZSGB-bio, Beijing) and 3% pentobarbital sodium (0.5 mL/kg IV) (ZSGB-bio, Beijing) for surgery. Sanitization was performed and a sterile sheet was used. A 2 cm longitudinal incision was made on the knee externally. The medial patella was dislocated to expose the femoral intercondylar fossa. A 4-mm diameter drill was used to create a 3-mm-deep cartilage defect. The PTH intervention group was transplanted with PTH-induced rat BMSC complexes into the injured area. The non-PTH intervention group included rabbits with non-PTH-induced rat BMSC complexes transplanted into the injured area. No material was transplanted into the injured area in the injury group while no knee injury was induced in the normal control group. Penicillin (40×104 IU) was intramuscularly injected once a day into each animal, for 5 days.
Postoperative process
The animals were sacrificed at 4, 8 and 12 weeks after surgery. Samples were collected and the gross observation and histological ranking were evaluated according to the International Cartilage Repair Society (ICRS) ranking scale (Table 1A, 1B) [21]. The immunohistochemical staining of type II collagen and aggrecan was analyzed based on the average optical density in the medical imaging system (Olympus, Japan). Real-time fluorescent quantitative PCR and Western blot were used to examine the expression of type II collagen and aggrecan in the repaired tissue samples. The average integrated optical density of protein was analyzed using gel imaging (Tanon, Shanghai). Each sample was measured three times on average. The whole experimental procedure was strictly based on the Guidance Suggestions for the Care and Use of Laboratory Animals [22].
Table 1A.
The ICRS ranking scale of the gross observation.
| Gross appearance | Points |
|---|---|
| I Degree of defect repair | |
| In level with surrounding cartilage | 4 |
| 75% repair of defect depth | 3 |
| 50% repair of defect depth | 2 |
| 25% repair of defect depth | 1 |
| 00% repair of defect depth | 0 |
| II Integration to border zone | |
| Complete integration with surrounding cartilage | 4 |
| Demarcating border <1 mm | 3 |
| 3/4 of graft integrated, 1/4 with a notable border >1 mm width | 2 |
| 1/2 of graft integrated with surrounding cartilage, 1/2 with a notable border >1 mm | 1 |
| From no contact to 1/4 of graft integrated with surrounding cartilage | 0 |
| III Macroscopic appearance | |
| Intact smooth surface | 4 |
| Fibrillated surface | 3 |
| Small, scattered fissures or cracs | 2 |
| Several, small or few but large fissures | 1 |
| Total degeneration of grafted ared | 0 |
| Overall repair assessment | |
| Grade I normal | 12 |
| Grade II nearly normal | 11–8 |
| Grade III abnormal | 7–4 |
| Grade IV severely abnormal | 3–1 |
Table 1B.
The ICRS ranking scale of the histology observation.
| Histologic appearance | Points |
|---|---|
| I Surface | |
| Smooth/level with normal | 4 |
| Smooth | 3 |
| Irregular | 2 |
| Clefts | 1 |
| Clefts to bone | 0 |
| II Matrix | |
| Hyaline-like cartilage | 3 |
| Hyaline and fibrocartilage | 2 |
| Fibrocartilage | 1 |
| Nonchondrocytic cells | 0 |
| III Cell distribution | |
| Columnar | 3 |
| Mixed/columnar clusters | 2 |
| Clusters | 1 |
| Individual cells/disorganized | 0 |
| IV Subchondral bone | |
| Normal | 3 |
| Mostly reshaped | 2 |
| Necrosten/crude tissue | 1 |
| Irregular/fracture | 0 |
| V Integration with surrounding cartilage | |
| Complete | 2 |
| Minor disruption (<25% of area) | 1 |
| Major disruption (>25% of area) | 0 |
| VI Cartilage mineralization | |
| Normal | 3 |
| Abnormal/heterotopic calcification | 0 |
Statistical analysis
The data were presented as mean ± standard deviation (SD) representing the results from three independent experiments. The data were analyzed by SPSSl6.0 package (SPSS Inc., Chicago, IL, USA). The comparison of means between groups was tested by one-way ANOVA. The means from multiple samples and a single group with similar variance were compared by LSD-t test. If the variance differed, Dunnett T3 test was used. The graphs were plotted using Graphpad Prisma 5 (GraphPad Software, Inc. USA). A value of p<0.05 was considered statistically significant.
Results
BMSC growth
After 48 hour of BMSC culture, spindle-shaped or polygonal adherent cells were found. On day 3, the number of adherent cells increased. Cells showed polygonal protuberances and grew in a fibroblast-like colony. On day 10, at 90% confluency the cells were tightly packed in swirling or radial shapes, indicating possible cell passage. Cell passages were performed every 5 to 7 days. BMSCs were obtained with a higher purity after each passage. The BMSCs after passage 4 were of the highest purity. Flow cytometric analysis revealed a high CD44 expression (93.76%) in BMSCs at passage 4 (Figure 1).
Figure 1.
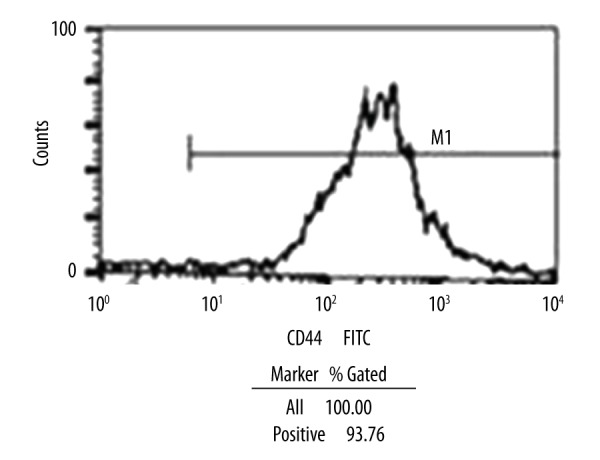
Expression of cell surface markers in cultured BMSCs as detected by flow cytometry. The surface marker CD44 was positively expressed by 93.7% of the cells.
PTH regulates differentiation of rat BMSCs into chondroblasts
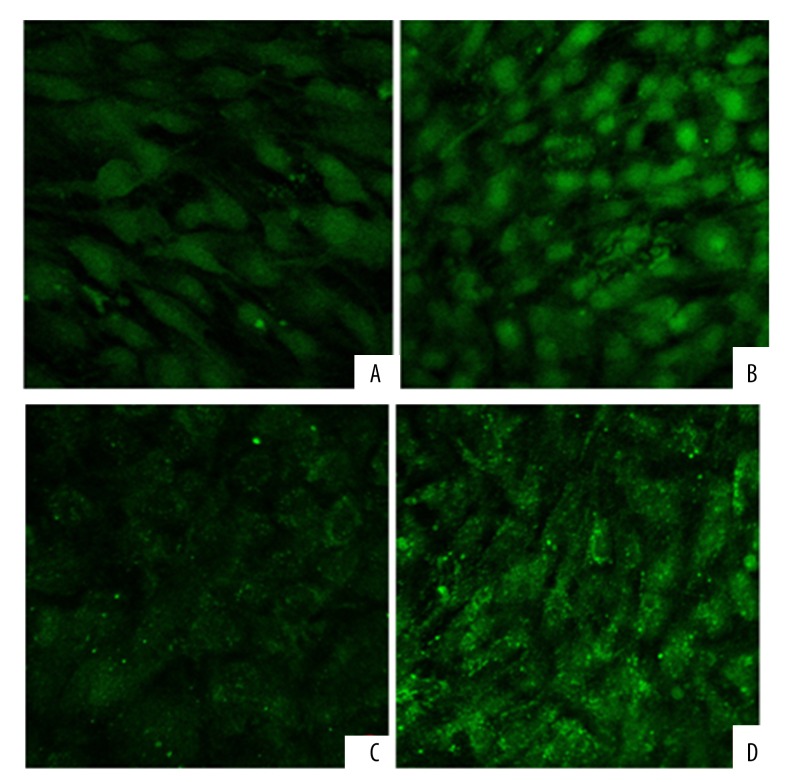
Laser confocal microscopy revealed that the green fluorescence of type II collagen and aggrecan was significantly lower in the control group than in the PTH-treated group (p<0.05) (Table 2A, 2B, Figure 2A–2D).
Table 2A.
Type II collagen fluorescence intensity of BMSCs in the experimental and control group.
| Group | n | Type II collagen fluorescence intensity | ||
|---|---|---|---|---|
| 4 d | 7 d | 14 d | ||
| Control group | 6 | 6.82±1.26 | 8.23±1.62 | 9.89±1.14 |
| Experimental group | 6 | 7.56±1.38 | 14.2±1.48*$ | 16.56±1.22*& |
Compared with control group,
p<0.05; compared with experimental group on the 4th day,
p<0.05 or on the 7th day,
p<0.05.
Table 2B.
Aggrecan fluorescence intensity of BMSCs in the experimental and control group.
| Group | n | Aggrecan fluorescence intensity | ||
|---|---|---|---|---|
| 4 d | 7 d | 14 d | ||
| Control group | 6 | 1.61±1.07 | 2.27±1.06 | 2.78±1.52 |
| Experimental group | 6 | 5.01±1.68* | 8.11±1.83*$ | 11.63±1.49*& |
Compared with control group,
p<0.05; compared with experimental group on the 4th day,
p<0.05 or on the 7th day,
p<0.05.
Figure 2.
The expression of type II collagen in BMSCs. The green fluorescence of type II collagen was significantly lower in the control group (A) than in the PTH-treated group (B). The expression of aggrecan in BMSCs. The green fluorescence of aggrecan was significantly lower in the control group (C) than in the PTH-treated group (D).
Animal experiments of cartilage repair
Gross observation
All the rabbits were regularly fed starting on day 3 after the surgery. The activities of all the animals were normal two weeks later, with no remarkable differences observed among the groups. No significant rejection or death was observed in the all animals. All surgical incisions were healed by first intention. No abnormal fluid or adhesions were observed in the articular lumen and no cartilage abrasion or osteophytes were seen around the injured area during the sample collection.
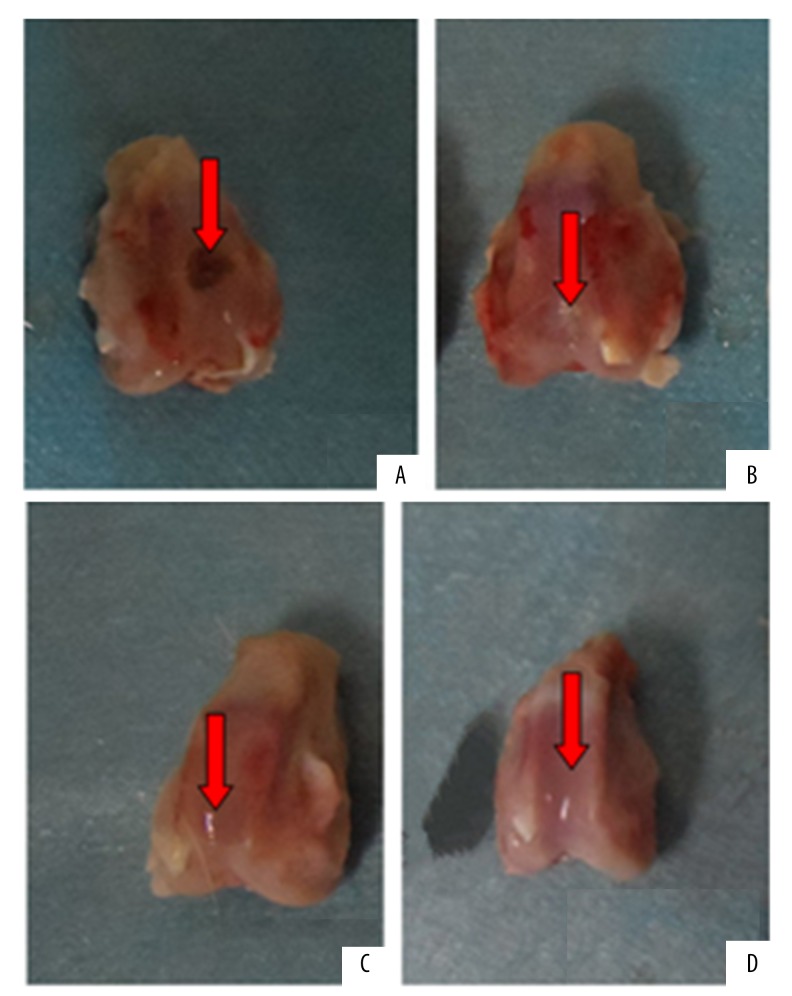
In the injury group at week 4 to 12 post-surgery, granulation was seen in the damaged cartilage area. The boundary with the surrounding cartilage was clearly visible (Figure 3A).
Figure 3.
General form of the postoperative 12w: (A) for the injury group, the restoration tissue was scar tissue; (B) for the non-PTH intervention group, the restoration tissue looked cartilage-like; (C) for the PTH intervention group, the restoration tissue was similar in color with the surrounding cartilage tissue; (D) for the normal control group, thre normal articular cartilage.
In the non-PTH intervention group, during week 4 to 8 post-surgery, the repaired tissue appeared gray with a rough surface and a clear boundary. At week 12 post-surgery the repaired tissue appeared cartilage-like with somewhat smooth surface and hazy boundary (Figure 3B).
In the PTH intervention group at week 4 to 8 post-surgery, the injured area was filled with hyaline tissue, with a relatively smooth surface and faint boundary. At week 12 post-surgery, the repaired tissue was similar in color with the surrounding cartilage presenting a smooth surface and indistinguishable boundary (Figure 3C).
The control group showed normal articular cartilage without significant degeneration during weeks 4 to 12 post-surgery (Figure 3D).
Two independent and unrelated pathologists evaluated the week 12 samples according to the ICRS scale for gross tissue repair and found that the PTH intervention group (PTH/BMSC/FG complex) scored significantly higher than the non-PTH intervention (BMSC/FG complex) and injury groups (p<0.05) with scores similar to the normal control group (p>0.05) (Table 3).
Table 3.
The week 12 samples according to the ICRS scale for gross tissue repair.
| Time | Injury group | Non-PTH intervention group | PTH intervention group | Normal control group |
|---|---|---|---|---|
| 12 w | 1.50±1.25 | 7.32±1.52 | 11.75±1.26* | 12.00±0.00 |
All data were expressed as mean ± standard deviation, n=6; Compared with injury group or Non-PTH intervention group,
p<0.05.
H&E staining
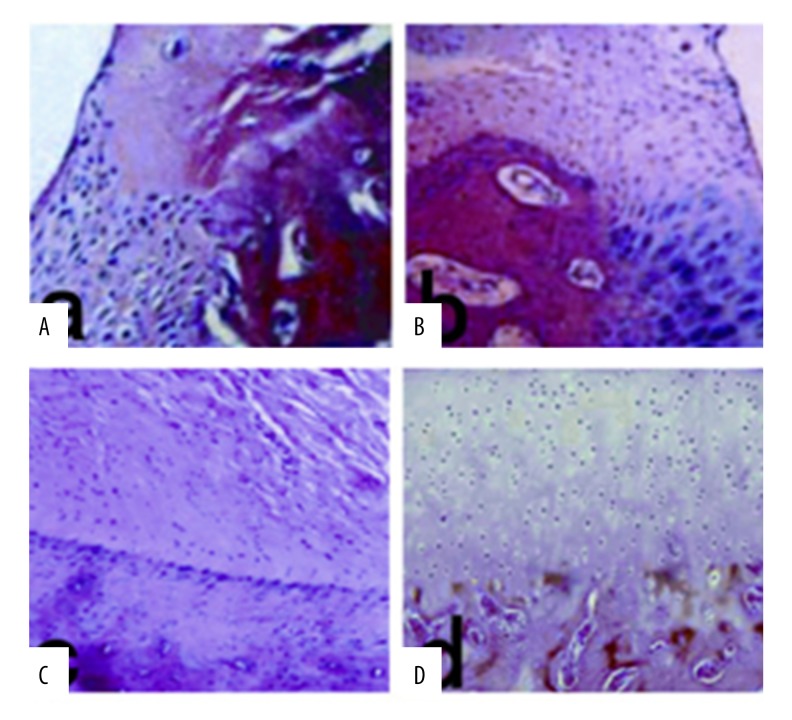
In the injury group, the injured area was repaired by fibroid tissue during week 4 to 12 post-surgery, and no chondrocytes were observed (Figure 4A).
Figure 4.
HE staining of the postoperative 12w (×100 magnification): (A) for the injury group, the restoration tissue without cartilage cells and cartilage lacunae; (B) for the non-PTH intervention group, the restoration tissue was seen more immature chondrocytes; (C) for the PTH intervention group, the injured area was completely restored by transparent tissue; (D) for the normal control group, the level of normal articular cartilage.
In the non-PTH intervention group, at week 4 post-surgery, the injured area was mainly repaired by fibroid tissue and a few abnormal chondrocytes were seen in the deep layer. At week 8 after surgery, chondrocytes were observed in the deep layer of the repaired tissue. During week 12 after surgery, additional immature chondrocytes were seen in the repaired tissue together with cartilage lacunae (Figure 4B).
In the PTH intervention group, at week 4 after surgery, the injured area was mainly repaired by fibrous cartilage-like tissue with low cell density. At week 8 after surgery, the injured area was mainly repaired by hyaline cartilage with high chondrocyte proliferation. At week 12 post-surgery, the typical columnar cartilage lacunae and intact subchondral bone were tightly connected with surrounding cartilage in the repaired tissue (Figure 4C).
In the normal control group, a hyaline cartilage with smooth surface and structured layer and clear architecture was observed (Figure 4D).
Two pathologists unrelated to this study evaluated the samples according to the ICRS scale for histological ranking and found that although the scores of the PTH intervention group (PTH/BMSC/FG complex) were lower than that of the normal control (p<0.05), they was significantly higher than that of non-PTH intervention (BMSC/FG complex) and injury groups (p<0.05). Eventually, they were close to the normal control group (p>0.05) by week 12 after surgery (p>0.05) (Table 4).
Table 4.
the samples according to the ICRS scale for histological ranking.
| Time | Injury group | Non-PTH intervention group | PTH intervention group | Normal control group |
|---|---|---|---|---|
| 4 w | 0.65±0.03 | 4.85±0.98 | 7.63±1.43*# | 18.00±0.00 |
| 8 w | 0.78±0.05 | 7.50±1.32 | 11.50±1.75*#$ | 18.00±0.00 |
| 12 w | 2.33±0.82 | 10.96±1.62 | 16.35±1.85*& | 18.00±0.00 |
All data were expressed as mean ± standard deviation, n=6; Compared with injury group or Non-PTH intervention group,
p<0.05; Compared with normal control group,
p<0.05; compared with PTH intervention group on the 4th week,
p<0.05 or on the 8th week,
p<0.05.
Immunohistochemistry
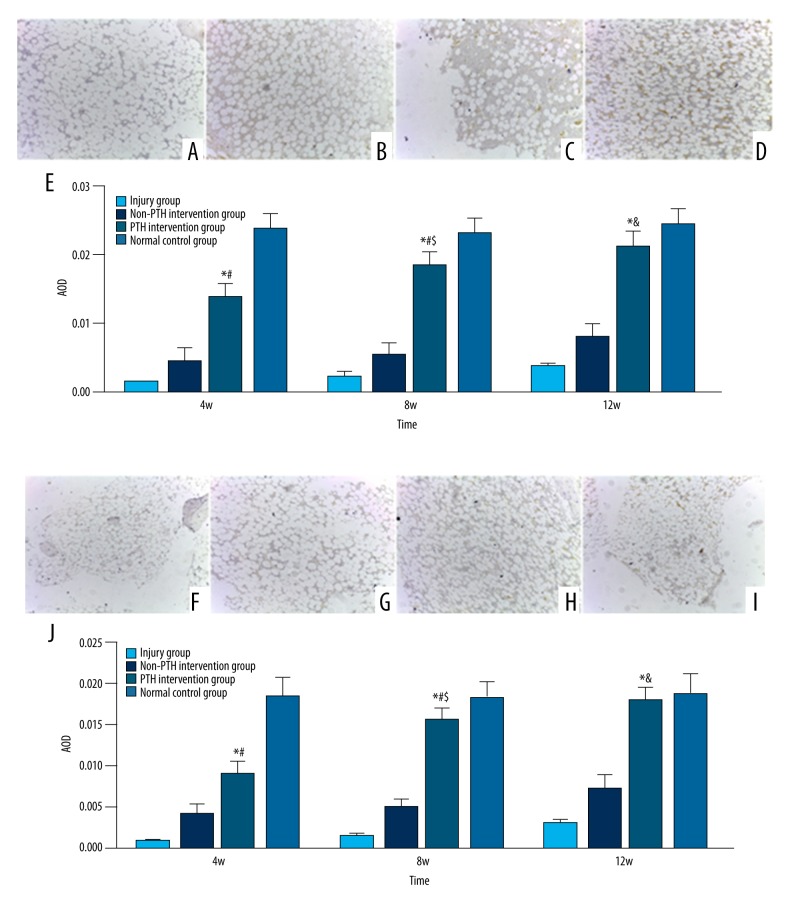
Immunohistochemistry of type II collagen and proteoglycan in each group was evaluated during the weeks 4, 8 and 12 after surgery and the average optical density was calculated. The results showed that the expression of type II collagen and aggrecan in the repaired tissue was negative at all time points in the injury group, but strongly positive in the normal control group. Samples in the PTH and non-PTH intervention groups stained positive for type II collagen and aggrecan by week 4 after surgery. The PTH intervention group showed stronger signal than the non-PTH group and the difference between PTH-intervention, non-PTH intervention, and injury groups was increasingly significant (p<0.05). By week 12, the expression of type II collagen and aggrecan in the repaired tissue from PTH intervention almost resembled the normal controls (p>0.05) (Figure 5A–5J)).
Figure 5.
Type II collagen immunochistochemical detection of the postoperative 12w restoration tissue (×100 magnification); (A) for the injury group, the staining was negative; (B) for the non-PTH intervention group, the staining was positive, but it was weaker than the PTH intervention group; (C) for the PTH intervention group, the staining was positive, and it was close to the normal control group; (D) for the normal control group, the staining was the best positive. (E) The average optical density of type II collagen in different groups. All date were expressed as mean ± standard deviation, n=6; Compared with injury group or non-PTH intervention group, * p<0.005; Compared with normal control group, # p<0.05; Compared with PTH intervention group on the 4th week, $ p<0.05 or on the 8th week, & p<0.05. Aggrecan immunochistochemical detection of the postoperative 12w restoration tissue (×100 magnification); (F) for the injury group, the staining was negative; (G) for the non-PTH intervention group, the staining was positive, but it was weaker than the PTH intervention group; (H) for the PTH intervention group, the staining was positive, and it was close to the normal control group; (I) for the normal control group, the staining was the best positive. (J) The average optical density of aggrecan in different groups. All date were expressed as mean ± standard deviation, n=6; Compared with injury group or non-PTH intervention group, * p<0.005; Compared with normal control group, # p<0.05; Compared with PTH intervention group on the 4th week, $ p<0.05 or on the 8th week, & p<0.05.
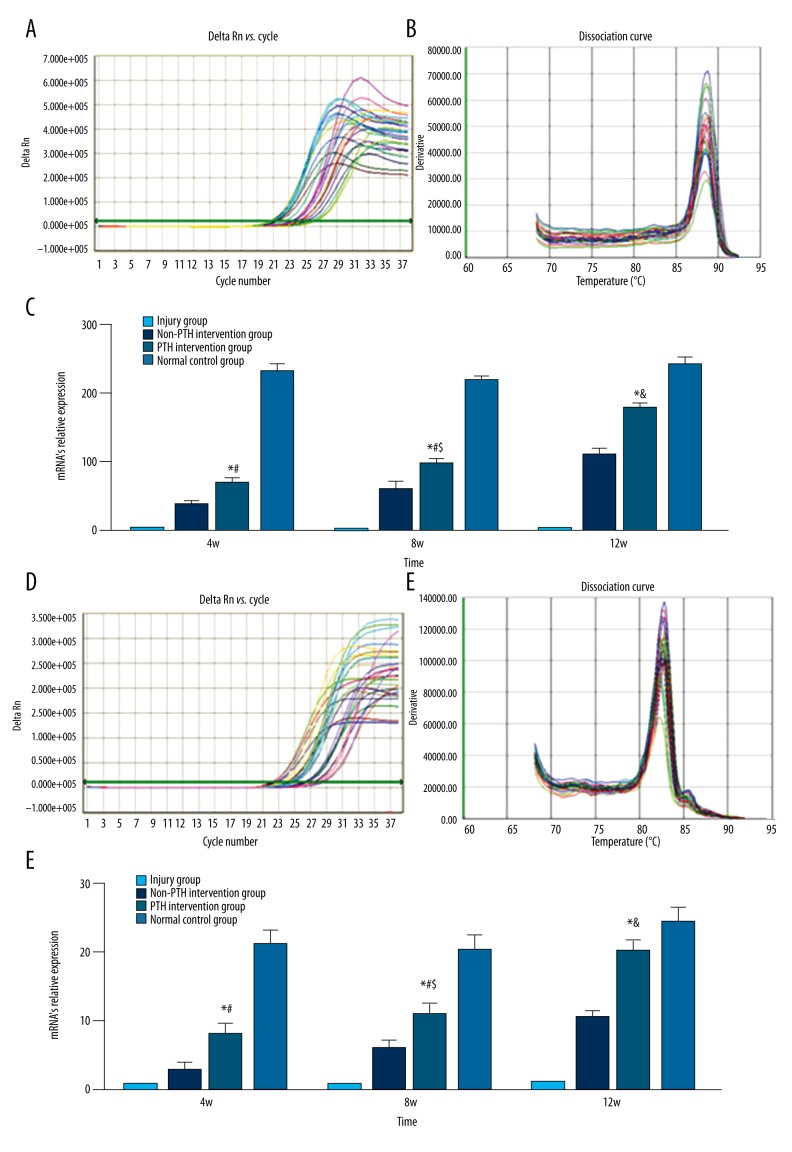
RT-PCR
Real-time fluorescent quantitative PCR was used to test the relative mRNA expression of type II collagen and aggrecan in the repaired tissue from each group. The results indicated that the mRNA levels of type II collagen and aggrecan in the repaired tissue were low and remained constant over time in the injury group. The non-PTH intervention group (BMSC/FG) showed regulated mRNA expression of type II collagen and aggrecan over time after surgery. The mRNA levels of type II collagen and aggrecan in PTH intervention group (PTH/BMSC/FG) were significantly higher than in the non-PTH intervention and injury groups at the same time point (p<0.05). By week 12, the type II collagen and aggrecan levels in the repaired tissue were equal to that of normal control group (p>0.05). The normal control group showed increased mRNA expression of both type II collagen and aggrecan at all time points (Figure 6A–6F).
Figure 6.
(A) Amplification curve of type II collagen; (B) dissociation curve of type II collagen. (C) The mRNA’s relative expression of type II collagen in different groups. All date were expressed as mean ± standard deviation, n=6; Compared with injury group or non-PTH intervention group, * p<0.005; Compared with normal control group, # p<0.05; Compared with PTH intervention group on the 4th week, $ p<0.05 or on the 8th week, & p<0.05. (D) Amplification curve of aggrecan; (E) dissociation curve of aggrecan. (F) The mRNA’s relative expression of aggrecan in different groups. All date were expressed as mean ± standard deviation, n=6; Compared with injury group or non-PTH intervention group, * p<0.005; Compared with normal control group, # p<0.05; Compared with PTH intervention group on the 4th week, $ p<0.05 or on the 8th week, & p<0.05.
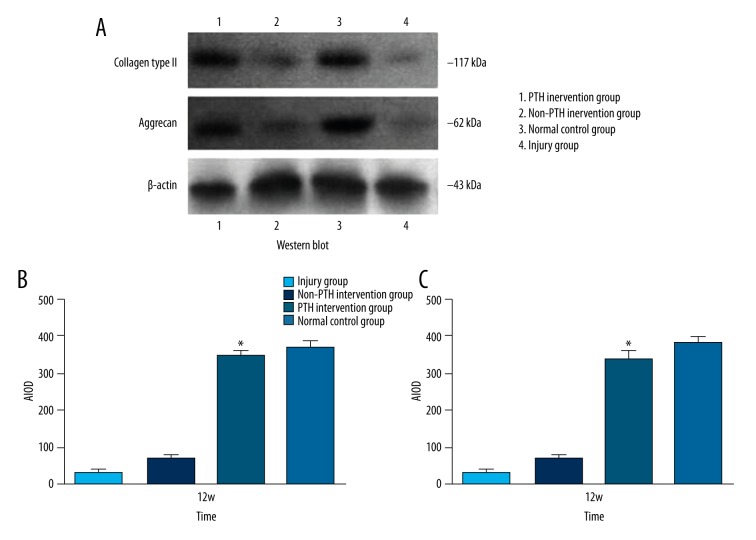
Western blot
Western blot revealed type II collagen and aggrecan expression in week 12 samples of each group. However, the injured group showed the lowest average integrated optical density of type II collagen and aggrecan while the normal control group expressed the highest level. The non-PTH intervention group (BMSC/FG) showed a higher average integrated optical density than the injury group. The PTH intervention group (PTH/BMSC/FG) showed a higher average integrated optical density than the injured and non-PTH intervention groups (p<0.05), which resembled the normal control group (p>0.05) (Figure 7A–7C).
Figure 7.
(A) Western blot electrophoregram. (B) The average integrated optical density of type II collagen in different groups. All data were expressed as mean ± standard deviation, n=6; Compared with injury group or non-PTH intervention group, * p<0.05. (C) The average integrated optical density of aggrecan in different groups. All data were expressed as mean ± standard deviation, n=6; Compared with injury group or non-PTH intervention group, * p<0.05.
Discussion
Articular cartilage injury has multiple etiologies. Autologous repair using articular cartilage grinding and microfracture, or transplantation of periosteum and perichondrium resulted in unsatisfactory histological outcomes [23–26]. The development of cartilage tissue engineering has opened new avenues for the repair of injured cartilage. BMSCs are the seed cells for cartilage tissue engineering, and are easily obtained from multiple sources compared with chondrocytes. As well, it is easy to maintain their phenotype in vitro. However, the abundance of BMSCs in bone marrow is low, about one in every 105 to 106 bone marrow nucleated cells [27]. Further, BMSC-derived chondrocytes are not identical to autologous chondrocytes. Allogeneic BMSCs obtained from individuals other than the patient adversely affect the donor and increase the surgical expense. They cannot meet the demand for seed cells in cartilage tissue engineering.
However, BMSCs do not induce immune rejection in vivo and play an immunosuppressive role [28–30]. Articular cartilage lacks nerve, blood, or lymph vessels, thereby resulting in weak antigenicity. In the absence of studies reporting the application of allogeneic BMSC seed cells for repair of cartilage injury, we used rat BMSCs to study the repair of knee articular cartilage injury in New Zealand white rabbits. Studies [31–36] reported that the efficiency of in vitro differentiation of BMSCs into chondrocytes was poor and the de-differentiation rate was high, which required cytokine induction. PTH, one of the bone-seeking hormones, is a polypeptide hormone synthesized and secreted by the chief cells in the parathyroid. PTH has been shown by various studies [11–14,37] to induce BMSCs proliferation, which is consistent with our preliminary results [38]. Our results indicate that PTH induced rat BMSC differentiation into chondroblasts and chondrocytes. Therefore, we performed xenogeneic cartilage transplantation using PTH-induced rat BMSCs complexed with the FG support to repair rabbit knee articular injury and evaluate postoperative outcomes.
In the PTH intervention group, the injured area was covered by repaired tissue four weeks after transplantation of PTH/BMSC/FG complex from both gross and histological perspectives. However, the surface was rough and dull, and the repaired tissue was dominated by fibrous granulation tissue. Eight weeks after surgery, the injured area was covered by tissue containing hyaline chondrocytes with smooth surface and occasional cartilage lacuna. However, the repaired tissue at the injured site in the non-PTH intervention group (BSMC/FG complex) was still dominated by fibrous tissue containing scattered chondrocytes. In the injured group, the cartilage loss was clearly visible with a clear boundary. By week 12 post-surgery, the injured area was fully repaired with smooth hyaline cartilage in the PTH group (PTH/BMSC/FG) manifesting tissue impedance and elasticity. Typical cartilage lacunae with columnar alignment, continuous subchondral tide line, and intact subchondral bone were seen in the repaired tissue. The boundary with the normal surrounding cartilage tissue disappeared and differed very little structurally compared with the articular cartilage in the control group. In the non-PTH intervention group, although the injured area was covered with hyaline cartilage tissue dominated by immature chondrocytes, it differed structurally from the control group. In the injured group, the cartilage loss was still visible and the injured area was only covered by fibrous tissue with scattered chondrocyte-like matrices. No chondrocytes or cartilage lacunae were seen and the boundary was clear. The data suggested that PTH/BMSC/FG complex promoted repair and regeneration of cartilage in a time-dependent manner.
Cartilage matrix has been reported to play an important role in supporting and protecting chondrocytes as well as in the regulation of information flow, adhesion, proliferation, and differentiation of chondrocytes [39,40]. Therefore, we used immunohistochemistry to analyze the synthesis of cartilage matrix in each group. By week 4 post-surgery, except for the injured group, all the experimental groups showed varying levels of cartilage matrix synthesis. The synthesis in PTH intervention group (PTH/BMSC/FG complex) was less than in the normal control group but was higher than in the non-PTH intervention (BMSC/FG complex). Eventually, all the groups other than the control group, showed increased synthesis of cartilage matrix. By week 12, no significant difference in the synthesis of cartilage matrix was found between the PTH intervention group (PTH/BMSC/FG complex) and the normal control. PTH intervention (PTH/BMSC/FG complex) was superior to non-PTH intervention and injury groups not only in the synthesis of cartilage matrix but also in the thickness of the regenerated cartilage, the structure of the subchondral bone, and the formation of tide lines. It differed little from the normal control in the structure of articular cartilage at week 12 post-surgery, indicating that PTH/BMSC/FG complex stimulated cartilage synthesis and promoted the repair.
Type II collagen is not only the major collagen component in cartilage matrix but also the specific extracellular matrix that is required to differentiate BMSCs into chondrocytes. Aggrecan stabilizes the structure of cartilage extracellular matrix and therefore, serves as an indispensable structural component [41]. In this study, we used RT-PCR and Western blot to identify the expression of type II collagen and aggrecan in the repaired and normal cartilage. Compared with the injured group, type II collagen and aggrecan mRNAs were detected four weeks after surgery in the PTH intervention (PTH/BMSC/FG complex) and non-PTH intervention groups (BMSC/FG complex) in a time-dependent manner with higher mRNA levels detected in the PTH group. By week 12 after surgery, both mRNA levels in the PTH intervention group were close to the normal control group, and were significantly higher than in the non-PTH intervention and the injured group. Western blot showed that the protein levels of type II collagen and aggrecan in the repaired tissue derived from the PTH intervention group were higher than in the non-PTH intervention and injured groups. They were not significantly different from those in the normal control group, suggesting that PTH/BMSC/FG complex played a critical role in the repair of injured articular cartilage.
No significant immune rejection was observed. Rabbits started normal eating on day 3 after surgery. No significant swelling, leakage, or abnormal activity was observed. BMSCs did not induce immune rejection in vivo and played a role in immunosuppression [28–30]. Lack of nerves, blood, or lymphatic vessels in the cartilage tissue contributed to poor antigenicity of cartilage. Our results showed that rat BMSCs induced no significant rejection or only mild immune reactions in rabbits. However, our pilot study only focused on the role of xenogeneic cartilage transplantation of PTH-induced rat BMSCs for the repair of articular cartilage injury in rabbit knee. Therefore, the study limitations relate to the short observation period. Long-term studies are needed to corroborate the preliminary findings reported here.
Conclusions
Our results suggested that the PTH/BMSC/FG complex comprising PTH-induced rat BMSCs complexed with FG, effectively promoted the repair and regeneration of articular cartilage injury in rabbit knee. The newborn cartilage secreted extracellular matrix and manifested normal function of hyaline cartilage.
Acknowledgement
We thank all the teachers and doctors of department of Orthopedic Surgery, Orthopedics implantation key lab of Guangdong Province, First Affiliated Hospital of Guangzhou Medical University.
Footnotes
Competing Interests
The authors declare that they have no competing interests.
Source of support: Departmental sources
References
- 1.Piasecki DP, Spindler KP, Warren T, et al. Intra articular injuries associated with anterior cruciate ligament tear: Findings at ligament reconstruction in high school and recreational athletes. Am J Sports Med. 2003;31:601–5. doi: 10.1177/03635465030310042101. [DOI] [PubMed] [Google Scholar]
- 2.Curl WW, Krome J, Gordon ES, et al. Cartilage injuries: A review of 31,516 knee arthroscopies. Arthroscopy. 1997;13:456–60. doi: 10.1016/s0749-8063(97)90124-9. [DOI] [PubMed] [Google Scholar]
- 3.Karin H, Eirik S, Torb S, et al. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;7:730–34. doi: 10.1053/jars.2002.32839. [DOI] [PubMed] [Google Scholar]
- 4.Hunter W. Of the structure and disease of articular cartilages. Philos Trans Lond. 1743;42:514–21. [Google Scholar]
- 5.Xu W-D, Wu Y-S, Zhang C-C. The First Edition. [Diagnosis and treatment of osteoarthrosis]. Shanghai: The Second Military Medical University Press; 2004. pp. 1–8. [on Chinese] [Google Scholar]
- 6.Nehrer S, Breinan HA, Ramappa A, et al. Chondrocyte-seeded collagen matrices implanted in a chondral defect in a canine model. Biomaterials. 1998;19:2313–28. doi: 10.1016/s0142-9612(98)00143-4. [DOI] [PubMed] [Google Scholar]
- 7.Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–95. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 8.Bin Z. [The research progress of articular cartilage defect repair strategies]. Journal of Biological Bone Materials and Clinical Research. 2007;4(6):25–28. [in Chinese] [Google Scholar]
- 9.Steadman R. Chondral defects in athletes. The 5th Panther Sports Medieine Symp; Pittsburgh, PA. 1992. [Google Scholar]
- 10.Jacker HJ, Amon I, Langer R, et al. Biological testing of biomaterials in the framework of permitted procedures. Z Exp Chir Transplant Kunstliche Organe. 1988;21(06):302–12. [PubMed] [Google Scholar]
- 11.Borba VZ, Macas NC. The use of PTH in the treatment of osteoporosis. Arq Bras Endocrinol Metabol. 2010;54(2):213–19. doi: 10.1590/s0004-27302010000200018. [DOI] [PubMed] [Google Scholar]
- 12.Adams SL, Cohen AJ, Lassová L. Integration of signaling pathways regulating chondrocyte differentiation during endochondral bone formation. J Cell Physiol. 2007;213(3):635–41. doi: 10.1002/jcp.21262. [DOI] [PubMed] [Google Scholar]
- 13.Hinoi E, Ueshima T, Hojo H, et al. Up-regulation of per mRNA expression by parathyroid hormone through a protein kinase A-CREB-dependent mechanism in chondrocytes. J Biol Chem. 2006;281(33):23632–42. doi: 10.1074/jbc.M512362200. [DOI] [PubMed] [Google Scholar]
- 14.Chang JK, Chang LH, Hung SH, et al. Parathyroid hormone 1–34 inhibits terminal differentiation of human articular chondrocytes and osteoarthritis progression in rats. Arthritis Rheum. 2009;60(10):3049–60. doi: 10.1002/art.24843. [DOI] [PubMed] [Google Scholar]
- 15.Liu Q, Wan Q, Yang R, et al. Effects of intermittent versus continuous parathyroid hormone administration on condylar chondrocyte proliferation and differentiation. Biochem Biophys Res Commun. 2012;424(1):182–88. doi: 10.1016/j.bbrc.2012.06.106. [DOI] [PubMed] [Google Scholar]
- 16.Di BG, Galderisi U, Fiorito C, et al. Dual role of parathyroid hormone in endothelial progenitor cells and marrow stromal mesenchymal stem cells. J Cell Physiol. 2010;222(2):474–80. doi: 10.1002/jcp.21976. [DOI] [PubMed] [Google Scholar]
- 17.Geng S, Zhou S, Glowacki J. Age-related decline in osteoblastogenesis and 1alpha-hydroxylase/CYP27B1 in human mesenchymal stem cells: Stimulation by parathyroid hormone. Aging Cell. 2011;10(6):962–71. doi: 10.1111/j.1474-9726.2011.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu B, Zhao X, Yang C, et al. Parathyroid hormone induces differentiation of mesenchymal stromal/stem cells by enhancing bone morphogenetic protein signaling. J Bone Miner Res. 2012;27(9):2001–14. doi: 10.1002/jbmr.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JX, Zhu LX, Jin AM, et al. In vitro experiment of aprotinin/tranexamic acid improved injectable frbrin glue. J Clin Rehab Tis Engi Res. 2008;12(49):9703–8. [Google Scholar]
- 20.Fussenegger M, Meinhart J, Hobling W, et al. Stabilized autologous fibrin-chondrocyte constructs for cartilage repair in vivo. Ann Plast Surg. 2003;51(5):493–98. doi: 10.1097/01.sap.0000067726.32731.E1. [DOI] [PubMed] [Google Scholar]
- 21.van den Borne MP, Raijmakers NJ, Vanlauwe J, et al. International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in Autologous Chondrocyte Implantation (ACI) and microfracture. Osteoarthritis Cartilage. 2007;15(12):1397–402. doi: 10.1016/j.joca.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 22.The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006. Sep 30, [in Chinese] [Google Scholar]
- 23.Baker B, Baker RO, Spadaro J. A study of electrochemical enhancement of articular cartilage repair. Clin Orthhop. 1974;102:251–67. doi: 10.1097/00003086-197407000-00029. [DOI] [PubMed] [Google Scholar]
- 24.Jackson L, Tahiri K, Montembault A, et al. Cell therapy in cartilage repair: Cellular and molecular bases. Soc Biol. 2008;202(4):313–21. doi: 10.1051/jbio:2008030. [DOI] [PubMed] [Google Scholar]
- 25.Yang SL, Harnish E, Leeuw T, et al. Compound screening platform using human induced pluripotent stem cells to identify small molecules that promote chondrogenesis. Protein Cell. 2012;3(12):934–42. doi: 10.1007/s13238-012-2107-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keeney M, Lai JH, Yang F. Recent progress in cartilage tissue engineering. Curr Opin Biotechnol. 2011;22(5):734–40. doi: 10.1016/j.copbio.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Ohgushi H, Calplan AI. Stem cell technology and biocramics: From cell to gene engineering. J Biomed Mater Res. 1990;48(6):913–27. doi: 10.1002/(sici)1097-4636(1999)48:6<913::aid-jbm22>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Tong ZC, Yang Z, Tong ZQ, et al. Study of inducing bone marrow-derived mesenchymal stem cells into chondrocytes in vitro. Zhongguo Gu Shang. 2008;21(5):362–64. [PubMed] [Google Scholar]
- 29.Nejadnik H, Hui JH, Feng Choong EP, et al. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: An observational cohort study. Am J Sports Med. 2010;38(6):1110–16. doi: 10.1177/0363546509359067. [DOI] [PubMed] [Google Scholar]
- 30.Maitra B, Szekely E, Gjini K, et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33(6):597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 31.Xue JX, Gong YY, Zhou GD, et al. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells induced by acellular cartilage sheets. Biomaterials. 2012;33(24):5832–40. doi: 10.1016/j.biomaterials.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 32.Kocamaz E, Gok D, Cetinkaya A, Tufan AC. Implication of C-type natriuretic peptide-3 signaling in glycosaminoglycan synthesis and chondrocyte hypertrophy during TGF-beta1 induced chondrogenic differentiation of chicken bone marrow-derived mesenchymal stem cells. J Mol Histol. 2012;43(5):497–508. doi: 10.1007/s10735-012-9430-2. [DOI] [PubMed] [Google Scholar]
- 33.Venkatesan JK, Ekici M, Madry H, et al. SOX9 gene transfer via safe, stable, replication-defective recombinant adeno-associated virus vectors as a novel, powerful tool to enhance the chondrogenic potential of human mesenchymal stem cells. Stem Cell Res Ther. 2012;3(3):22. doi: 10.1186/scrt113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang X, Sheng L, Xie F, Zhang Q. Differentiation of bone marrow-derived mesenchymal stem cells into chondrocytes using chondrocyte extract. Mol Med Rep. 2012;6(4):745–49. doi: 10.3892/mmr.2012.996. [DOI] [PubMed] [Google Scholar]
- 35.Xie X, Wang Y, Zhao C, et al. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials. 2012;33(29):7008–18. doi: 10.1016/j.biomaterials.2012.06.058. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy HE, Bara JJ, Brakspear K, et al. The comparison of equine articular cartilage progenitor cells and bone marrow-derived stromal cells as potential cell sources for cartilage repair in the horse. Vet J. 2012;192(3):345–51. doi: 10.1016/j.tvjl.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 37.Freed L, Martin I, Vunjak-Novakovic G. Frontiers in tissue engineering: In vitro modulation of chondrogenesis. Clin Orthop. 1999;367S:S46–58. [PubMed] [Google Scholar]
- 38.Chen Y, Bai B, Zhang S, et al. Effects of parathyroid hormone on calcium ions in rat bone marrow mesenchymal stem cells. Biomed Res Int. 2014;2014:258409. doi: 10.1155/2014/258409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novince CM, Entezami P, Wilson CG, et al. Impact of proteoglycan-4 and parathyroid hormone on articular cartilage. J Orthop Res. 2013;31(2):183–90. doi: 10.1002/jor.22207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andreasen PA, Kjøller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: A review. Int J Cancer. 1997;72(1):1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 41.Takao M, Uchio Y, Kakimaru H, et al. Arthroscopic drilling with debridement of remaining cartilage for osteochondral lesions of the talar dome in unstable ankles. Am J Sports Med. 2004;32(2):332–36. doi: 10.1177/0363546503261718. [DOI] [PubMed] [Google Scholar]