Summary
Women with epilepsy (WWE) face specific challenges throughout their lifespan due to the effects of seizures and antiepileptic drugs on hormonal function, potentially affecting both sexual and reproductive health. This review article addresses the most common issues of practical relevance to clinicians treating WWE: epidemiology and clinical presentations (including catamenial epilepsy), contraception, reproductive and sexual dysfunction, pregnancy, lactation, menopause-related issues (including bone health), and mental health aspects. Awareness of these gender-specific issues and implementation/adaptation of effective interventions for WWE results in significantly improved health-related quality of life in this patient population.
Keywords: antiepileptic drugs, contraception, epilepsy, gender, pregnancy, seizures, women
Introduction
The term “epilepsy” defines a group of disorders characterized by an enduring predisposition of the brain to produce seizures. To reduce diagnostic ambiguity and promote a better understanding of the pathogenesis of epilepsy and seizures, the International League Against Epilepsy (ILAE) recently proposed a new definition of epilepsy (Malkan and Beran, 2014), which encompasses the following clinical scenarios: i) at least two unprovoked (or reflex) seizures occurring more than 24 hours apart; ii) one unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (at least 60%) after two unprovoked seizures, occurring over the next 10 years; iii) an established diagnosis of an epilepsy syndrome. Seizures can be either generalized (characterized by diffuse epileptiform discharges involving distributed neuronal networks bilaterally) or focal (discrete localization and involvement limited to one hemisphere). Focal seizures are further classified as motor/sensory and dyscognitive, whereas generalized seizures encompass tonic-clonic, absence, myoclonic, tonic, clonic or atonic seizures (Berg et al., 2010). As currently defined, epilepsy is considered to be resolved in patients who have remained seizure-free for at least 10 years and off antiepileptic drugs (AEDs) for at least five years (Fisher et al., 2014).
Epilepsy is a relatively common condition, with higher prevalence rates in developing countries. It is estimated that around 3% of people receive a diagnosis of epilepsy at some point in their lifetime, with 70% achieving remission (Rugg-Gunn and Sander, 2012). Although sex ratios in the epidemiology of epilepsy are not fully established, there appears to be a slight gender difference in the prevalence of different epilepsy types, such as idiopathic generalized epilepsy and childhood absence epilepsy (2–5 times more common in girls than boys) and juvenile myoclonic epilepsy (1.5 times more common in girls than boys) (Savic, 2014). In several developing countries there is still evidence of social stigma attached to women with epilepsy (WWE). In Nigeria, WWE have been found to have worse social and economic status in comparison with women not suffering from epilepsy (Komolafe et al., 2012). In a study conducted in India, more than half of WWE concealed their history of epilepsy prior to their wedding, fearing social stigma and breakdown of the marriage negotiations (Santosh et al., 2007). In general, WWE experience changes in seizure frequency and severity in relation to different phases in the reproductive cycle: during puberty, over the menstrual cycle, and during pregnancy and the menopause (Morrell, 1999). WWE could arguably be regarded as a distinct illness sub-population with a number of distinguishing features compared with men with similar diagnoses; and these features need to be considered. Management of epilepsy in women requires not only knowledge of epilepsy, but also recognition of the various roles and priorities women have in their lives (education, career development, child rearing, the role as carer within the extended family), and attention to gender-specific issues and their impact on patients’ wellbeing throughout life (Jackson, 2006). These elements are discussed further in the present article, which focuses in particular on catamenial epilepsy, hormonal interactions with AEDs, sexual dysfunction, contraception, pregnancy, menopause, bone health and mental health.
Catamenial epilepsy
Catamenial epilepsy refers to exacerbation of seizures during different phases of the menstrual cycle in women with pre-existing epilepsy. Catamenial epilepsy can affect one third to one half of WWE (Foldvary-Schaefer and Falcone, 2003; Morrell, 1999), and it is reported that up to one third of female patients with intractable complex partial seizures may have this condition (Herzog, 2006). Overall, WWE are 2.5 times more likely to have menstrual disorders than women in the general population (Herzog, 2006). Seizure exacerbation has been found to be more common in anovulatory than ovulatory cycles (Kim et al., 2010), and the occurrence of seizures during anovulatory cycles increases the risk of infertility. Additionally, anovulatory cycles tend to be associated with an increase in seizure frequency during non-menstrual phases, whilst ovulatory cycles can have one or two peaks in seizure frequency, around the time of menstruation and/or ovulation (Herzog et al., 1997).
The occurrence of changes in estrogen and progesterone levels is thought to be a key factor in catamenial epilepsy, with progesterone being considered an anticonvulsant hormone and estrogen a proconvulsant. The four phases of the menstrual cycle are the menstrual phase (day 25 of the first cycle to day 3 of the next cycle), the follicular phase (days 4–10), the ovulatory phase (days 11–16) and the luteal phase (day 17-day 3 of the next cycle).
Catamenial epilepsy is characterized by three patterns of increased seizure frequency: C1 (perimenstrual pattern: increased frequency of seizures from day 25 of the first cycle to day 3 of the next cycle), C2 (periovulatory pattern: increased seizure frequency on days 10–14) and C3 (luteal pattern: increased frequency of seizures from day 17 of the first cycle to day 3 of next cycle when compared with days 4–10, the follicular phase). C1 and C3 are associated with a decrease in progesterone levels, while C2 sees an increase in the level of estrogen (Harden and Pennell, 2013; Reddy, 2013). Frequency of generalized tonic-clonic seizures was found to be higher in anovulatory cycles, which were also associated with an increase in the serum estradiol/progesterone ratio (Herzog et al., 2011). Careful assessment of menstrual and seizure diaries and categorization of cycle type (ovulatory versus anovulatory) and duration are important steps in the diagnosis of catamenial epilepsy.
AEDs can also have an impact on, or association with, the menstrual cycle. Menstrual disorders in WWE as well as being adverse effects of AEDs, can also be due to seizure-induced disruption of neuroendocrine function. Strong evidence suggests that menstrual disorders, including polycystic ovary syndrome (PCOS), are found more frequently in WWE treated with valproate. Women younger than 20 years seem to be especially vulnerable (Morrell et al., 2008), although the effect of valproate on the menstrual cycle appears to be reversible when treatment is discontinued (Mikkonen et al., 2004). Menstrual disorders have also been found to be more common in patients taking valproate than carbamazepine (Isojärvi et al., 2005). Furthermore, the frequency of menstrual disorders appears to be particularly elevated among women with high seizure frequency and in those receiving polytherapy (Svalheim et al., 2003). There is limited evidence about the use of pharmacotherapy in managing catamenial epilepsy (Foldvary-Schaefer and Falcone, 2003). Increasing the dose of AEDs at the time of anticipated seizure occurrence is a possible treatment option. The use of intermittent clobazam, on days when seizures are anticipated, is the only treatment option shown to have benefit in a randomized controlled trial (Feely and Gibson, 1984). Alternative therapies include acetazolamide or progestogens given perimenstrually. For WWE who are not taking AEDs, the following can be considered: intermittent perimenstrual clobazam (5 to 30 mg/day), the oral contraceptive pill (OCP), depot progestogen therapy, or perimenstrual progestogen (Crawford, 2005). Results from a recent randomized controlled trial (Herzog et al., 2012) showed that WWE who present with the C1 catamenial seizure pattern may benefit from progesterone treatment (French, 2013).
Female hormones and contraception
There exist complex, multidirectional interactions between female hormones, seizures and AEDs. Most hormones act as neurosteroids and can thus modulate brain excitability via direct binding sites. Any changes in endogenous or exogenous hormone levels can affect the occurrence of seizures, either directly or via pharmacokinetic interactions that modify the plasma levels of AEDs (Harden, 2008).
The pharmacokinetic interactions between oral contraceptives and AEDs are bidirectional (Johnston and Crawford, 2014). A number of AEDs, including carbamazepine, oxcarbazepine, topiramate, phenobarbitone and phenytoin, are known to have cytochrome-inducing properties (CYP3A4 group). These cytochromes are also involved in the metabolism of estrogen and progesterone, which are the main components of the combined OCP. Therefore, concomitant administration of cytochrome-inducing AEDs and the OCP may reduce the efficacy and effectiveness of the OCP. WWE taking these AEDs together with hormonal forms of contraception should receive counseling about the possible risks. In clinical practice, patients can be advised to increase the dosage of estrogen (starting with 50 micrograms daily of ethinyl estradiol). As full oral contraceptive efficacy cannot be guaranteed even on a higher-dose OCP with normal cycles, WWE taking enzyme-inducing AEDs can be advised to use the emergency contraceptive pill at a higher dose after unprotected sexual intercourse. No clinically relevant interaction has been documented between sodium valproate, levetiracetam, ethosuximide, pregabalin, gabapentin, vigabatrin, tiagabine, benzodiazepines and any form of OCP. Patients taking the OCP can show reduced concentrations of lamotrigine, and lamotrigine plasma levels should therefore be monitored (Sabers et al., 2003). There are no reported interactions between lamotrigine and the progestogen-only contraceptive pill (Gaffield et al., 2011). Finally, medroxyprogesterone acetate depot injection, levonorgestrel-releasing intrauterine systems, and copper-containing intrauterine devices are methods of contraception not affected by enzyme-inducing AEDs (O’Brien and Guillebaud, 2010). Both depot preparations and non-hormonal methods of contraception can be recommended as safe options for WWE on AEDs (Dutton and Foldvary-Schaefer, 2008).
Reproductive and sexual dysfunction
The most common reproductive endocrine disorder in WWE is PCOS, a condition characterized by hyperandrogenism, multiple ovarian cysts, anovulatory cycles, hirsutism and obesity. The prevalence of PCOS in WWE has been estimated at between 4% and 19% (Lobo, 1995). Most studies suggest an increased incidence of PCOS in women taking valproate (Hu et al., 2011; Zhou et al., 2012) as opposed to carbamazepine or lamotrigine (Morrell et al., 2008).
Although most WWE have a normal sex life, areas of sexual dysfunction have been reported in this patient population. Complaints can range from decreased sex drive to inadequate orgasmic satisfaction. AEDs as a drug class are also associated with sexual dysfunction in their own right (Harden, 2008). In a study by Gil-Nagel et al. (2006), WWE reported improvements in sexual desire, pleasure and orgasm when they switched to or initiated treatment with lamotrigine. There have also been reports of seizures induced by orgasm in WWE (Ozkara et al., 2006). However, the etiology of sexual dysfunction in WWE is considered to be multifactorial, with contributions from both the physical and psychological domains: for example, depression associated with epilepsy is also a significant factor in WWE who have difficulties with sexual functioning (Cavanna et al., 2009; Zelená et al., 2011).
Pregnancy and lactation
Although WWE often express concerns about worsening seizure control during pregnancy, converging evidence from multiple studies shows that seizure activity during gestation is unchanged from pre-pregnancy baseline in more than half of cases (Harden et al., 2009a). In the European and International Registry of Antiepileptic Drugs in Pregnancy, 64% of WWE reported no change in seizure control from the first trimester to the following two trimesters, with 93% of these women being seizure free (EURAP Study Group, 2006). WWE who report changes in seizure control, are as likely to experience an improvement as a worsening (Harden et al., 2009a).
Concerns about increased risk of obstetric complications in WWE are not supported by available evidence (EURAP Study Group, 2006). Seizures during pregnancy should be treated to improve health-related quality of life, to prevent seizure-related injury, and to avoid status epilepticus. Uncertainty exists as to whether seizures in pregnant WWE represent a risk to the fetus. Case reports of both generalized and complex partial seizures occurring during labor have been associated with deceleration of fetal heart rate suggestive of fetal hypoxia, and with prolonged uterine contractions (Nei et al., 1998; Sahoo and Klein, 2005). Seizures resulting in falls can cause direct trauma to the gravid uterus, possibly resulting in complications such as bleeding, premature rupture of the membranes and labor, and fetal death. Abruptio placentae has been reported to occur in up to 5% of mild traumatic injuries and up to 50% of major blunt traumas during pregnancy (Pearlman et al., 1990). However, a recently published systematic review and meta-analysis on epilepsy in pregnancy and reproductive outcomes found that the odds of early preterm birth, gestational diabetes, fetal death or stillbirth, perinatal death, or admission to the neonatal intensive care unit did not differ between WWE and those without the disorder (Viale et al., 2015).
AED therapy in pregnancy needs to be carefully managed from preconception through to the intrapartum and post-partum stages (Voinescu and Pennell, 2015). Before pregnancy, WWE should be advised to discontinue AED therapy when appropriate, or use monotherapy rather than polytherapy whenever possible. Patients should be helped to find the lowest AED dose that will maintain seizure control. Pregnancy in WWE is mostly uneventful, as children are usually delivered healthy and there is no increased risk of obstetric complications (Crawford, 2005). However in a small proportion of patients complications can occur, due to multiple risk factors related to physiological changes during pregnancy. These include changes in circulating levels of steroids, increased blood volume and cardiac output, increase in glomerular filtration rate, postural hypotension, dilutional anemia, increased metabolic rate, and insulin resistance. As a result of complex interactions between these factors, seizure frequency can either increase or decrease during pregnancy. The pharmacokinetic profiles of AEDs also play an important role in changes in seizure frequency during pregnancy. The management of WWE during pregnancy should include monitoring of serum levels of AEDs, especially lamotrigine, carbamazepine and phenytoin. Lamotrigine and oxcarbazepine serum concentrations may be particularly prone to decline as a result of increased glucuronidation during pregnancy. WWE taking these AEDs throughout pregnancy have been found to require more dose increases and to suffer more convulsive seizures (EURAP Study Group, 2006; Vajda et al., 2006). After delivery, doses can be reduced to pre-pregnancy baseline levels to avoid toxicity but maintain seizure control.
Several AEDs are known to have teratogenic effects on the developing fetus (Table I). Converging evidence from registry studies indicates that teratogenic risks are higher with valproate, followed by carbamazepine and topiramate. Among other commonly prescribed AEDs, older generation agents such as phenobarbitone and phenytoin have been associated with higher risks compared with lamotrigine, levetiracetam, clonazepam and gabapentin (Vajda et al., 2014; Voinescu and Pennell, 2015).
Table I.
Most commonly reported teratogenic effects of antiepileptic drugs.
| Antiepileptic drug | Major congenital malformations |
|---|---|
| Phenytoin | Fetal hydantoin syndrome, congenital heart disease, facial clefts |
| Valproate | Neural tube defects, craniofacial, skeletal, cardiovascular, cerebral defects. Language problems |
| Carbamazepine | Neural tube defects, congenital heart defects, reduced growth, and hypospadias |
| Barbiturates | Congenital heart defects, craniofacial defects, limb abnormalities, growth deficiency |
| Benzodiazepines | Orofacial clefts |
| Lamotrigine | Weak evidence of non-syndromic facial cleft |
Evidence-based recommendations include the avoidance of valproate and AED polytherapy during pregnancy, especially during the first trimester, in order to reduce the risk of congenital malformations. Children born to WWE who were taking valproate throughout pregnancy have been found to have significantly lower IQ scores as compared to the children of WWE taking carbamazepine, phenytoin or lamotrigine (Harden et al., 2009a; Meador et al., 2009; Bromley et al., 2014). Moreover, available data show that children exposed to valproate in utero are at increased risk of autism spectrum disorder (approximately threefold) and childhood autism (approximately fivefold) compared with the general population (Christensen et al., 2013).
Children born to WWE on AED therapy are also considered to be twice as likely to be small for their gestational age (Harden et al., 2009a). Further recommendations include pre-conception folic acid supplementation in order to prevent congenital malformations in newborns and possibly improve neurodevelopmental outcomes (Harden et al., 2009b; Shannon et al., 2014). Safety data on the newer AEDs are quite sparse. In clinical practice, most women can be advised to continue their AED therapy, given the risk of seizures in pregnancy and the potential consequences for both mother and baby (Bromley et al., 2014).
With regard to lactation, both levetiracetam and primidone are thought to be transferred to breast milk in clinically significant amounts as compared to valproate, phenytoin and phenobarbital (Harden et al., 2009b). It is considered generally safe for WWE taking AEDs to breastfeed, given that only very small amounts of these drugs are excreted in breast milk. Finally, it has been recommended that vitamin K be administered parenterally to children born to WWE on AED treatment (National Institute for Health and Care Excellence, 2012).
Menopause-related issues
It is reported that in the menopause, 40% of WWE can experience a worsening of seizure frequency, whereas up to 27% may go into remission (Crawford, 2005). As a result of hormonal changes, the frequency of catamenial seizures may increase in the perimenopause and decrease at menopause. Hormone-replacement therapy is significantly associated with increased frequency of seizures in menopausal women (Harden et al., 1999).
Cytochrome-inducing AEDs are known to affect bone mineral density and are associated with bone disorders such as osteoporosis and fractures during and after menopause. Phenytoin has been found to be associated with decreased levels of bone-specific alkaline phosphatase, as well as increased bone turnover, which can predispose to fractures, especially to the neck of femur (Pack et al., 2008). Finally, topiramate use is associated with low parathyroid hormone levels and increased bone turnover in pre-menopausal women (Heo et al., 2011).
Mental health
Psychiatric comorbidity is high in patients with epilepsy (Jones et al., 2010; Kerr et al., 2011), often as a result of AED treatment (Cavanna et al., 2010; Eddy et al., 2012; Piedad et al., 2012). The overall prevalence rate of psychiatric conditions in epilepsy ranges between 20–30% and 50–60%, according to different estimates (Jones et al., 2010; Karouni et al., 2010). Depression has a particularly high prevalence rate in patients with epilepsy, and WWE are at increased risk of developing depression (Beghi et al., 2004). Moreover, symptoms of depression have been reported by WWE before and after pregnancy (Reiter et al., 2013). Postpartum depression rates are higher in WWE as compared to the general population and the choice of AED has not been found to modify the risk of developing postpartum depression (Galanti et al., 2009).
A recently published review of studies on psychiatric disease in the peripartum period in WWE identified peripartum depression, anxiety and fear of birth as the most clinically relevant conditions (Bjørk et al., 2015). Specifically, the point prevalence of depression from the second trimester to six months postpartum ranged from 16 to 35% in WWE (compared to 9–12% in controls), with the highest estimates recorded in pregnancy and in the perinatal period. Anxiety symptoms six months after delivery were reported by 10% of WWE and 5% of controls. Fear of birth symptoms were increased in primiparous WWE compared with controls, and previous psychiatric disease, sexual/physical abuse, AED polytherapy, and high seizure frequency were the main risk factors.
Psychosis in epilepsy tends to have a temporal relationship with seizures and is currently classified as peri-ictal, ictal, postictal and interictal (in the latter case there is no relationship with seizures). The first three clinical conditions are usually self-limiting and only occasionally require the use of psychotropic medication, whereas interictal psychosis (schizophrenia-like psychosis of epilepsy) often responds well to antipsychotic medication. Studies looking into psychiatric conditions in women have reported an increased risk of both affective disorder and psychosis (Jones et al., 2014), possibly due to the physiological changes associated with menstruation, pregnancy and menopause, as well as pharmacokinetic and pharmacodynamic changes associated with the above physiological states.
Although, in clinical practice, the use of psychotropic medications is generally considered safe in patients with epilepsy, there is an associated risk of reducing the seizure threshold; this risk varies according to the different pharmacological classes. The findings of most studies suggest that judicious use of psychotropic medication outweighs the risk of reducing the seizure threshold (Kanner, 2008). There is limited evidence to help clinicians in the choice of psychotropic medications in patients with epilepsy, and there does not seem to be enough evidence to restrict the use of any medication apart from clozapine in this vulnerable patient group (Lee et al., 2003). It has been shown that low starting doses and slow titration can be helpful in maximizing the therapeutic potential and reducing the risk of adverse effects. A recently published systematic literature review showed positive results for the use of antidepressants (Maguire et al., 2014) and inconclusive results for the use of antipsychotics (Farooq and Sherin, 2008) in patients with epilepsy. The choice of psychotropic medication in patients with epilepsy requires careful consideration, taking into account the effects of antidepressants and antipsychotics on seizure threshold, as well as their interaction potential with anticonvulsants. Among psychotropics, selective serotonin-reuptake inhibitors such as citalopram and sertraline have been shown to have little effect on seizure threshold and a low potential for interaction with anticonvulsants. Tricyclic anti-depressants are known to potentially lower the seizure threshold (Taylor et al., 2012). The dual serotonin and noradrenaline reuptake inhibitor venlafaxine is also considered a relatively safe option (Maguire et al., 2014). Anticonvulsants with less potential for interaction with antidepressants include lamotrigine, pregabalin, gabapentin and levetiracetam (Mula, 2008).
Antipsychotic use in epilepsy has a thinner evidence base when compared to the use of antidepressants. Apart from clozapine, which is considered a proconvulsant (especially at higher doses), there is limited evidence supporting the use of one drug over another. The use of atypical antipsychotics seems to be beneficial overall in patients with epilepsy and psychotic symptoms (Okazaki et al., 2014). Carbamazepine and phenytoin are known to decrease plasma levels of atypical antipsychotics and therefore correction of antipsychotic dosages should be considered. Lamotrigine and topiramate are not known to interact with antipsychotics (de Leon, 2004). Antipsychotics seem to have little effect on AED metabolism. Finally, there is limited evidence on the effects of psychotropic medications throughout pregnancy. Antidepressants have been reported to potentially cause serotonin discontinuation syndrome and mood stabilizers have been associated with an increased risk of fetal malformations, whereas the effects of antipsychotics appear to be mixed (Galbally et al., 2011).
A recent study examined gender-related differences in the use of psychopharmacological agents for the treatment of psychiatric comorbid conditions in patients with refractory epilepsy (Karouni et al., 2013). With regard to antidepressants and antipsychotics, women used escitalopram and quetiapine to a larger extent than men, while men more commonly used olanzapine and haloperidol, which suggests that the tolerability profiles of psychotropic medications can influence pharmacotherapy choices in WWE (Gidal et al., 2009).
Concluding remarks
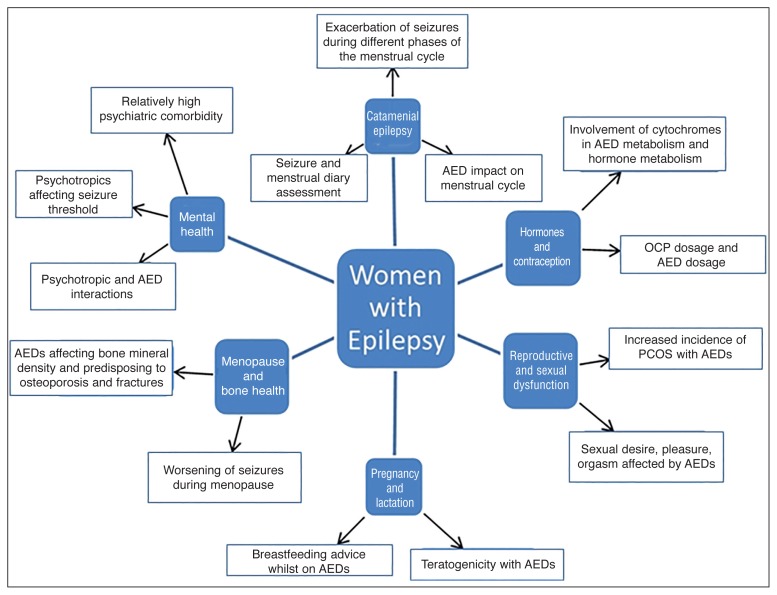
Epilepsy in women poses several challenges to clinicians, as it can affect different phases of the reproductive cycle: sexual development, pregnancy, breast-feeding and menopause (Fig. 1). Catamenial epilepsy can result in significant diagnostic difficulties and requires long-term monitoring of both seizure type and frequency. Epilepsy in women can also affect fetal growth, due to the teratogenic effects of AEDs and poor control of seizures.
Figure 1.
Summary of clinically relevant issues in women with epilepsy.
Abbreviations: AED=antiepileptic drug; PCOS=polycystic ovary syndrome; OCP=oral contraceptive pill
Sexual dysfunction and contraception are both important factors to consider in the management of WWE. It is therefore very important to educate and involve WWE in decision making about the choice of AED and offer preconception counseling. Mental health problems are particularly common in WWE, and although existing evidence on the safety and efficacy of psychotropic medications is still limited, most of the available psychotropic medications appear to be relatively safe if gradually titrated and appropriately used to address behavioral issues and therefore reduce the overall disease burden.
References
- Beghi E, Roncolato M, Visonà G. Depression and altered quality of life in women with epilepsy of childbearing age. Epilepsia. 2004;45:64–70. doi: 10.1111/j.0013-9580.2004.56502.x. [DOI] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Bjørk M, Veiby G, Engelsen B, Gilhus NE. Depression and anxiety during pregnancy and the postpartum period in women with epilepsy: a review of frequency, risks and recommendations for treatment. Seizure. 2015;28:39–45. doi: 10.1016/j.seizure.2015.02.016. [DOI] [PubMed] [Google Scholar]
- Bromley R, Weston J, Adab N, et al. Treatment for epilepsy in pregnancy: neurodevelopmental outcomes in the child. Cochrane Database Syst Rev. 2014;(10):CD010236. doi: 10.1002/14651858.CD010236.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Ali F, Rickards HE, et al. Behavioral and cognitive effects of anti-epileptic drugs. Discov Med. 2010;9:138–144. [PubMed] [Google Scholar]
- Cavanna AE, Cavanna S, Bertero L, et al. Depression in women with epilepsy: Clinical and neurobiological aspects. Funct Neurol. 2009;24:83–87. [PubMed] [Google Scholar]
- Christensen J, Grønborg TK, Sørensen MJ, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013;309:1696–1703. doi: 10.1001/jama.2013.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford P. Best practice guidelines for the management of women with epilepsy. Epilepsia. 2005;46(Suppl 9):117–124. doi: 10.1111/j.1528-1167.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- de Leon J. Psychopharmacology: atypical antipsychotic dosing: the effect of co-medication with anticonvulsants. Psychiatr Serv. 2004;55:125–128. doi: 10.1176/appi.ps.55.2.125. [DOI] [PubMed] [Google Scholar]
- Dutton C, Foldvary-Schaefer N. Contraception in women with epilepsy: pharmacokinetic interactions, contraceptive options, and management. Int Rev Neurobiol. 2008;83:113–134. doi: 10.1016/S0074-7742(08)00006-8. [DOI] [PubMed] [Google Scholar]
- Eddy CM, Rickards H, Cavanna AE. Behavioral adverse effects of antiepileptic drugs in epilepsy. J Clin Psychopharmacol. 2012;32:362–375. doi: 10.1097/JCP.0b013e318253a186. [DOI] [PubMed] [Google Scholar]
- EURAP Study Group. Seizure control and treatment in pregnancy: observations from the EURAP epilepsy pregnancy registry. Neurology. 2006;66:354–360. doi: 10.1212/01.wnl.0000195888.51845.80. [DOI] [PubMed] [Google Scholar]
- Farooq S, Sherin A. Interventions for psychotic symptoms concomitant with epilepsy. Cochrane Database Syst Rev. 2008;(4):CD006118. doi: 10.1002/14651858.CD006118.pub2. [DOI] [PubMed] [Google Scholar]
- Feely M, Gibson J. Intermittent clobazam for catamenial epilepsy: tolerance avoided. J Neurol Neurosurg Psychiatry. 1984;47:1279–1282. doi: 10.1136/jnnp.47.12.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- Foldvary-Schaefer N, Falcone T. Catamenial epilepsy: pathophysiology, diagnosis, and management. Neurology. 2003;61(Suppl 2):S2–15. doi: 10.1212/wnl.61.6_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- French JA. Treatment of catamenial epilepsy is still up in the air. Epilepsy Curr. 2013;13:71–72. doi: 10.5698/1535-7597-13.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffield ME, Culwell KR, Lee CR. The use of hormonal contraception among women taking anticonvulsant therapy. Contraception. 2011;83:16–29. doi: 10.1016/j.contraception.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Galanti M, Newport DJ, Pennell PB, et al. Postpartum depression in women with epilepsy: Influence of antiepileptic drugs in a prospective study. Epilepsy Behav. 2009;16:426–430. doi: 10.1016/j.yebeh.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbally M, Snellen M, Lewis AJ. A review of the use of psychotropic medication in pregnancy. Curr Opin Obstet Gynecol. 2011;23:408–414. doi: 10.1097/GCO.0b013e32834b92f3. [DOI] [PubMed] [Google Scholar]
- Gidal BE, French JA, Grossman P, et al. Assessment of potential drug interactions in patients with epilepsy: impact of age and sex. Neurology. 2009;72:419–425. doi: 10.1212/01.wnl.0000341789.77291.8d. [DOI] [PubMed] [Google Scholar]
- Gil-Nagel A, López-Muñoz F, Serratosa JM. Effect of lamotrigine on sexual function in patients with epilepsy. Seizure. 2006;15:142–149. doi: 10.1016/j.seizure.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Harden CL, Pennell PB. Neuroendocrine considerations in the treatment of men and women with epilepsy. Lancet Neurol. 2013;12:72–83. doi: 10.1016/S1474-4422(12)70239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden CL, Meador KJ, Pennell PB, et al. Practice parameter update: management issues for women with epilepsy - focus on pregnancy (an evidence-based review): teratogenesis and perinatal outcomes: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009a;73:133–141. doi: 10.1212/WNL.0b013e3181a6b312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden CL, Pennell PB, Koppel BS, et al. Management issues for women with epilepsy – focus on pregnancy (an evidence-based review): III Vitamin K, folic acid, blood levels, and breast-feeding: Report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2009b;50:1247–1255. doi: 10.1111/j.1528-1167.2009.02130.x. [DOI] [PubMed] [Google Scholar]
- Harden CL. Sexual dysfunction in women with epilepsy. Seizure. 2008;17:131–135. doi: 10.1016/j.seizure.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Harden CL, Pulver MC, Ravdin L, et al. The effect of menopause and perimenopause on the course of epilepsy. Epilepsia. 1999;40:1402–1407. doi: 10.1111/j.1528-1157.1999.tb02012.x. [DOI] [PubMed] [Google Scholar]
- Heo K, Rhee Y, Lee HW, et al. The effect of topiramate monotherapy on bone mineral density and markers of bone and mineral metabolism in premenopausal women with epilepsy. Epilepsia. 2011;52:1884–1889. doi: 10.1111/j.1528-1167.2011.03131.x. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Fowler KM, Smithson SD, et al. Progesterone vs placebo therapy for women with epilepsy: a randomized clinical trial. Neurology. 2012;78:1959–1966. doi: 10.1212/WNL.0b013e318259e1f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG, Fowler KM, Sperling MR, et al. Variation of seizure frequency with ovulatory status of menstrual cycles. Epilepsia. 2011;52:1843–1848. doi: 10.1111/j.1528-1167.2011.03194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG. Menstrual disorders in women with epilepsy. Neurology. 2006;66(Suppl 3):S23–28. doi: 10.1212/wnl.66.66_suppl_3.s23. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–1088. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Hu X, Wang J, Dong W, et al. A meta-analysis of polycystic ovary syndrome in women taking valproate for epilepsy. Epilepsy Res. 2011;97:73–82. doi: 10.1016/j.eplepsyres.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Jackson M. Epilepsy in women: A practical guide to management. Practical Neurology. 2006;6:166–179. [Google Scholar]
- Johnston CA, Crawford PM. Anti-epileptic drugs and hormonal treatments. Curr Treat Options Neurol. 2014;16:288. doi: 10.1007/s11940-014-0288-3. [DOI] [PubMed] [Google Scholar]
- Jones I, Chandra PS, Dazzan P, et al. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384:1789–1799. doi: 10.1016/S0140-6736(14)61278-2. [DOI] [PubMed] [Google Scholar]
- Jones R, Rickards H, Cavanna AE. The prevalence of psychiatric disorders in epilepsy: a critical review of the evidence. Funct Neurol. 2010;25:191–194. [PubMed] [Google Scholar]
- Kanner AM. The use of psychotropic drugs in epilepsy: what every neurologist should know. Semin Neurol. 2008;28:379–388. doi: 10.1055/s-2008-1079342. [DOI] [PubMed] [Google Scholar]
- Karouni M, Arulthas S, Larsson PG, et al. Psychiatric comorbidity in patients with epilepsy: a population-based study. Eur J Clin Pharmacol. 2010;66:1151–1160. doi: 10.1007/s00228-010-0861-y. [DOI] [PubMed] [Google Scholar]
- Karouni M, Henning O, Larsson PG, et al. Pharmacological treatment of psychiatric comorbidity in patients with refractory epilepsy. Epilepsy Behav. 2013;29:77–81. doi: 10.1016/j.yebeh.2013.06.031. [DOI] [PubMed] [Google Scholar]
- Kerr MP, Mensah S, Besag F, et al. International consensus clinical practice statements for the treatment of neuropsychiatric conditions associated with epilepsy. Epilepsia. 2011;52:2133–2138. doi: 10.1111/j.1528-1167.2011.03276.x. [DOI] [PubMed] [Google Scholar]
- Kim GH, Lee HW, Park H, et al. Seizure exacerbation and hormonal cycles in women with epilepsy. Epilepsy Res. 2010;90:214–220. doi: 10.1016/j.eplepsyres.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Komolafe MA, Sunmonu TA, Afolabi OT, et al. The social and economic impacts of epilepsy on women in Nigeria. Epilepsy Behav. 2012;24:97–101. doi: 10.1016/j.yebeh.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Isojärvi JI, Taubøll E, Herzog AG. Effect of antiepileptic drugs on reproductive endocrine function in individuals with epilepsy. CNS Drugs. 2005;19:207–223. doi: 10.2165/00023210-200519030-00003. [DOI] [PubMed] [Google Scholar]
- Lee KC, Finley PR, Alldredge BK. Risk of seizures associated with psychotropic medications: emphasis on new drugs and new findings. Expert Opin Drug Saf. 2003;2:233–247. doi: 10.1517/14740338.2.3.233. [DOI] [PubMed] [Google Scholar]
- Lobo RA. A disorder without identity: “HCA”, “PCO”, “PCOD”, “PCOS”, “SLS”. What are we to call it?! Fertil Steril. 1995;63:1158–1160. doi: 10.1016/s0015-0282(16)57589-x. [DOI] [PubMed] [Google Scholar]
- Maguire MJ, Weston J, Singh J, et al. Antidepressants for people with epilepsy and depression. Cochrane Database Syst Rev. 2014;(12):CD010682. doi: 10.1002/14651858.CD010682.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkan A, Beran RG. An appraisal of the new operational definition of epilepsy – then and now. Epilepsy Behav. 2014;41:217–220. doi: 10.1016/j.yebeh.2014.09.084. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Baker GA, Browning N, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360:1597–1605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkonen K, Vainionpää LK, Pakarinen AJ, et al. Long-term reproductive endocrine health in young women with epilepsy during puberty. Neurology. 2004;62:445–450. doi: 10.1212/01.wnl.0000106942.35533.62. [DOI] [PubMed] [Google Scholar]
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200–211. doi: 10.1002/ana.21411. [DOI] [PubMed] [Google Scholar]
- Morrell MJ. Epilepsy in women: the science of why it is special. Neurology. 1999;53(Suppl 1):S42–48. [PubMed] [Google Scholar]
- Mula M. Anticonvulsants - antidepressants pharmacokinetic drug interactions: the role of the CYP450 system in psychopharmacology. Curr Drug Metab. 2008;9:730–737. doi: 10.2174/138920008786049311. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. CG137. London: National Institute for Health and Care Excellence; 2012. The epilepsies: The diagnosis and management of the epilepsies in adults and children in primary and secondary care. [Google Scholar]
- Nei M, Daly S, Liporace J. A maternal partial complex seizure in labor can affect fetal heart rate. Neurology. 1998;51:904–906. doi: 10.1212/wnl.51.3.904. [DOI] [PubMed] [Google Scholar]
- O’Brien MD, Guillebaud J. Contraception for women taking antiepileptic drugs. J Fam Plann Reprod Health Care. 2010;36:239–242. doi: 10.1783/147118910793048764. [DOI] [PubMed] [Google Scholar]
- Okazaki M, Adachi N, Akanuma N, et al. Do antipsychotic drugs increase seizure frequency in epilepsy patients? Eur Neuropsychopharmacol. 2014;24:1738–1744. doi: 10.1016/j.euroneuro.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Ozkara C, Ozdemir S, Yilmaz A, et al. Orgasm-induced seizures: a study of six patients. Epilepsia. 2006;47:2193–2197. doi: 10.1111/j.1528-1167.2006.00648.x. [DOI] [PubMed] [Google Scholar]
- Pack AM, Morrell MJ, Randall A, et al. Bone health in young women with epilepsy after one year of antiepileptic drug monotherapy. Neurology. 2008;70:1586–1593. doi: 10.1212/01.wnl.0000310981.44676.de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman MD, Tintinalli JE, Lorenz RP. Blunt trauma during pregnancy. N Engl J Med. 1990;323:1609–1613. doi: 10.1056/NEJM199012063232307. [DOI] [PubMed] [Google Scholar]
- Piedad J, Rickards H, Besag FM, et al. Beneficial and adverse psychotropic effects of antiepileptic drugs in patients with epilepsy: a summary of prevalence, underlying mechanisms and data limitations. CNS Drugs. 2012;26:319–335. doi: 10.2165/11599780-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Neuroendocrine aspects of catamenial epilepsy. Horm Behav. 2013;63:254–266. doi: 10.1016/j.yhbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter SF, Veiby G, Daltveit AK, et al. Psychiatric comorbidity and social aspects in pregnant women with epilepsy: the Norwegian Mother and Child Cohort Study. Epilepsy Behav. 2013;29:379–385. doi: 10.1016/j.yebeh.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Rugg-Gunn FJ, Sander JW. Management of chronic epilepsy. BMJ. 2012;345:e4576. doi: 10.1136/bmj.e4576. [DOI] [PubMed] [Google Scholar]
- Sabers A, Ohman I, Christensen J, et al. Oral contraceptives reduce lamotrigine plasma levels. Neurology. 2003;61:570–571. doi: 10.1212/01.wnl.0000076485.09353.7a. [DOI] [PubMed] [Google Scholar]
- Sahoo S, Klein P. Maternal complex partial seizure associated with fetal distress. Arch Neurol. 2005;62:1304–1305. doi: 10.1001/archneur.62.8.1304. [DOI] [PubMed] [Google Scholar]
- Santosh D, Kumar TS, Sarma PS, et al. Women with onset of epilepsy prior to marriage: disclose or conceal? Epilepsia. 2007;48:1007–1010. doi: 10.1111/j.1528-1167.2007.01040.x. [DOI] [PubMed] [Google Scholar]
- Savic I. Sex differences in human epilepsy. Exp Neurol. 2014;259:38–43. doi: 10.1016/j.expneurol.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Shannon GD, Alberg C, Nacul L, et al. Preconception healthcare and congenital disorders: systematic review of the effectiveness of preconception care programs in the prevention of congenital disorders. Matern Child Health J. 2014;18:1354–1379. doi: 10.1007/s10995-013-1370-2. [DOI] [PubMed] [Google Scholar]
- Svalheim S, Taubøll E, Bjørnenak T, et al. Do women with epilepsy have increased frequency of menstrual disturbances? Seizure. 2003;12:529–533. doi: 10.1016/s1059-1311(03)00195-x. [DOI] [PubMed] [Google Scholar]
- Taylor D, Paton C, Kapur S. The Maudsley Prescribing Guidelines in Psychiatry, Oxleas NHS Foundation. 11th Edition. London: Wiley Blackwell; 2012. [Google Scholar]
- Vajda FJ, O’Brien TJ, Lander CM, et al. The teratogenicity of the newer antiepileptic drugs – an update. Acta Neurol Scand. 2014;130:234–238. doi: 10.1111/ane.12280. [DOI] [PubMed] [Google Scholar]
- Vajda FJ, Hitchcock A, Graham J, et al. Foetal malformations and seizure control: 52 months data of the Australian Pregnancy Registry. Eur J Neurol. 2006;13:645–654. doi: 10.1111/j.1468-1331.2006.01359.x. [DOI] [PubMed] [Google Scholar]
- Viale L, Allotey J, Cheong-See F, et al. Epilepsy in pregnancy and reproductive outcomes: a systematic review and meta-analysis. Lancet. 2015;386:1845–1852. doi: 10.1016/S0140-6736(15)00045-8. [DOI] [PubMed] [Google Scholar]
- Voinescu PE, Pennell PB. Management of epilepsy during pregnancy. Expert Rev Neurother. 2015;15:1171–1187. doi: 10.1586/14737175.2015.1083422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelená V, Kuba R, Soška V, et al. Depression as a prominent cause of sexual dysfunction in women with epilepsy. Epilepsy Behav. 2011;20:539–544. doi: 10.1016/j.yebeh.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Zhou JQ, Zhou LM, Chen LJ, et al. Polycystic ovary syndrome in patients with epilepsy: a study in 102 Chinese women. Seizure. 2012;21:729–733. doi: 10.1016/j.seizure.2012.08.001. [DOI] [PubMed] [Google Scholar]