Summary
Background
To assess the safety and potential efficacy of a standardized technique consisting of intra-articular injection of 10 cc of a homogeneous mixed product using autologous micro-fat and platelet rich plasma (PRP) (ratio 1:1) in the carpus or the fetlock joint of sport horses presenting degenerative joint disease (DJD).
Methods
Eight sport horses with DJD confirmed by radiography and ultrasonography and causing lameness and the impossibility to compete were treated. PRP was prepared after a double centrifugation whereas micro-fat was harvested and purified using a closed system. The two products were connected and mixed by gentle back and forth shaking of the syringes to finally obtain 10 ml of an homogeneous mixed product. Follow up was performed from 5 to 10 months with assessment of AAEP lameness score and return to training and competition.
Results
Nine joints were treated with significant improvement of the AAEP lameness score three months after the procedure (p = 0.021). Four horses returned to official competition between 5 to 10 months after the procedure (7.0±2.5) and three of them resumed intensive training between 5 to 9 months (6.3±2.3). No adverse event occurred.
Conclusion
This study is a first step in the development of innovative therapy for DJD which combines the potential chondrogenic differentiation of MSCs inside equine adipose tissue with the proliferative effect of growth factors present in PRP.
Keywords: knee, mesenchymal stem cells, PRP, stem cells, stromal vascular fraction
Introduction
Degenerative Joint Disease (DJD) is one of the main causes of reduced athletic function and retirement1–3 for race horses. DJD is a degenerative process that affects the synovial joints and can involve all of the components of the joint: cartilage, subchondral bone, articular capsule, ligament and pre-joint muscle. The late stages of DJD can also reveal cartilage cracks, subchondral bone exposure on extended surface, subchondral sclerosis and formation of marginal osteophytes. Clinically, DJD is marked by lameness as classified by the American Association of Equine Practitioners (AAEP) lameness scale (Tab. I). In order to reduce inflammation, anti-inflammatory and analgesic drugs represent standard treatments. Disease-modifying drugs such as glucosamine, chondroitin sulphate or hayluronic acid can also be used4–6. In cases of severe cartilage and bone degeneration, the use of articular cartilage curettage, osteophyte removal or surgical arthroscopy and arthrodesis can be prescribed4,7. However, these therapies are simply meant to reduce symptoms or enhance clinical recovery without healing or regenerating affected joints.
Table I.
American Association of Equine Practitioners (AAEP) lameness scale.
| 0 | No lameness not perceptible under any circumstances |
| 1 | Lameness is difficult to observe and is not consistently apparent, regardless of circumstances (e.g. under saddle, circling, inclines, hard surface, etc.) |
| 2 | Lameness is difficult to observe at a walk or when trotting in a straight line but consistently apparent under certain circumstances (e.g. weight-carrying, circling, inclines, hard surface, etc.) |
| 3 | Lameness is consistently observable at a trot under all circumstances |
| 4 | Lameness is obvious at a walk |
| 5 | Lameness produces minimal weight bearing in motion and/or at rest or a complete inability to move |
Regenerative therapy for racehorses is a rapidly growing field of research driven by the potentially significant economic impact on the horse industry. In fact, healing is of crucial relevance in DJD and has become a priority in veterinarian research both for the quality of life of horses and for a rapid return to competition. The few available placebo controlled studies on horses consist of experimentally-induced cartilage lesions8–10 which are very different from natural joint diseases. However, race horses can serve as a valuable large animal model for the evaluation of new human therapies for in vivo efficacy and safety owing to interspecies similarities in the thickness of the non-calcified cartilage of the stifle joint11. Evaluation of new treatments for musculoskeletal injuries in horses could therefore benefit both equine and human medicine. Among them, the association of platelet rich plasma (PRP) obtained from whole blood centrifugation combined with micro-fat offers a potential new regenerative product available in a one step surgical procedure. Micro-fat differs from fat obtained with classical harvesting procedure (3 mm cannula) by the use of a multiperforated cannula with holes of 1 mm allowing to harvest smaller lobulas of fat (around 600 μm) in which regenerative cells are contained in the vascular wall. In this study, we assessed the tolerance and safety of a technique that consisted of intra-articular injection of a mixed component of autologous micro-fat and platelet rich plasma (PRP) in the carpus or the fetlock joint of 8 race horses with DJD causing lameness and the impossibility to compete. Minimum follow-up was 3 months. Our secondary objectives were to evaluate the clinical improvement of the lameness score and its correlation with the recovery of training and/or competitive activities.
Materials and methods
Inclusion criteria
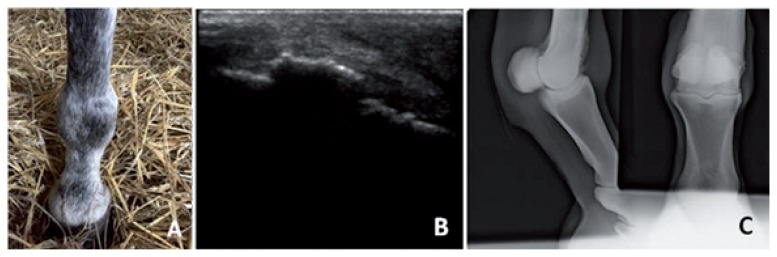
Inclusion criteria were the presence of severe DJD confirmed by radiography and ultrasonography (including loss of cartilage thickness, entesiophytes, chip fracture, bone edema) (Fig. 1A, B, C) and an AAEP lameness scale superior or equal to 1/5 before treatment.
Figure 1.
Clinical, ultrasound scan and X-ray of case # 1 (right fetlock DJD)
A) Clinical aspect reveals synovial effusion in the right fetlock. B) Longitudinal ultrasound scan with right fetlock DJD, calcification of the synovial capsule and severe synovitis. C) Antero-posterior and latero-medial X-ray of the right fetlock. Loss of cartilage and calcification of the synovial capsule.
Horses
Eight horses (6 geldings, 1 stallion, 1 mare) aged from 2 to 13 years (6.5±3.9) were treated. The following breeds were included: 2 French trotters, 1 Percheron, 3 Thoroughbreds, 1 Arabian and 1 French saddle horse. Their competitions were trotter races (n = 2), jockey-ridden races (n = 3), hunter-class shows (n = 1), endurance shows (n = 1) and dressage (n = 1). They presented DJD (n = 8), chip fracture joint disease (n = 2), chronic inflammatory joint disease (n = 8), chronic synovitis (n = 8), and subchondral cyst (n = 1). In two cases, DJD was associated with osteophytes and subchondral bone sclerosis. Three had previously been treated with intra-articular injection of corticosteroids more than 4 months beforehand. The AAEP lameness scale varied from 1 to 4 with a mean ± SD of 2.33±1.22. Six of them had discontinued competition for a mean ± SD of 4.2±4.1 months but all of them were slated for retirement from competition owing to a lack of performance and had been considered as good performers before joint disabilities. Nine joints were injected since one horse was treated in the two anterior fetlocks. As of November 1, 2015, the horses had been followed up from 7 to 16 months (mean ± SD = 10.6±2.6). The baseline characteristics of the horses are provided in Table I.
Microfat harvesting and purification
The horses were placed in the operating stock and sedated with a combination of detomidine (20 μg/kg IV) and butorphanol (0.02 mg/kg IV). The elective areas for fat harvesting were clipped and corresponded to the lateral zones at 10 cm from each side of the tail insertion. A sterile single-use drape was placed on the tail region and secured by single-use staples after performing a disinfection protocol four times (betadine scrub, rinsing, drying, alcoholic spray). The micro-fat was harvested under a strict aseptic protocol. All of the equipment that was used came from a single-use set.
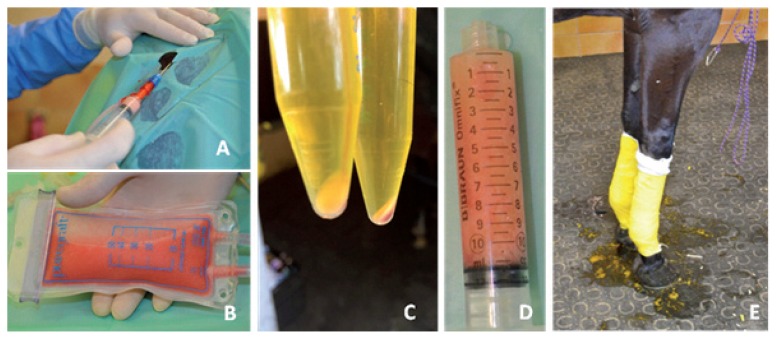
The entry points for the aspiration cannula were anesthetized with pure adrenaline lidocaine 2% with a 23G (0.6 mm) needle. The quantity injected at each entry point was 2 ml. Two incisions were performed 3 cm from each side of the tail with a N°11 sterile surgical blade. Infiltration of the subcutaneous adipose tissue was then carried out in the area with a 14-gauge (2 mm) cannula with a mixture of 60 ml of xylocaine (20 mg/ml) and epinephrine (0.005 mg/ml) with 60 ml of NaCl 0.9%. Liposuction was performed with a 14-gauge (2 mm) cannula (st’RIM cannula, Thiebaud Biomedical Devices, Margencel, France) connected to a 3-way stopcock with an anti-return valve and a 10 ml luer-lock syringe to enable the collection of the fat under gentle manual aspiration (Fig. 2A). The harvested fat was directly injected into a closed 50 ml filtration system pocket (Puregraft, San Diego, CA, USA). The PureGraftTM system allows the purification of adipose tissue by elimination of fluid excess, lipid phase, blood cells and fragments through filtration by membrane in a sterile environment (Fig. 2B). Approximately 30 ml of fat was sampled per horse. This made it possible to obtain a final volume of pure fat ranging from 5 to 10 ml. Five milliliters of the cleaned tissue was directly retrieved by connecting the Pure-GraftTM system to a 10 ml syringe.
Figure 2.
Procedure of microfat harvesting and prp preparation.
A) Microfat harvesting under sterile conditions and closed system. B) Macroscopic aspect of adipose tissue and purification with PG 50. C) Macroscopic aspect of platelet pellet after the second centrifuge. D) Macroscopic aspect of mixed microfat and PRP. E) Cohesive bandage on the joint to maintain sterile gauze.
After collection, incisions were cleaned and left open (second intention healing). It was recommended to the owner to clean the wounds until recovery.
Preparation of Platelet Rich Plasma (PRP)
PRP preparation was adapted from a previously published method12. Briefly, 64 ml of blood was collected by venipuncture of the jugular vein using 8 anticoagulated 9-ml tubes with 10% ACD-A (Vacuette ®, Greiner Bio-One, Frickenhausen, Germany). The collected whole blood was centrifuged in a Hettich EBA 20 (Sigma Aldrich, St Louis, MO, USA) at 78G for 10 minutes. Approximately 30 ml of plasma were collected and placed in two 15-ml sterile conic tubes (Nunc, Thermo Fisher Scientific, Waltham, MA, USA). In order to reduce the final volume and concentrate the platelets, we performed a second spin at 509G for 10 minutes (Fig. 2C). Platelets were then pelleted at the bottom of each tube and 2.5 ml of PRP was collected in each tube to obtain a final volume of 5 ml in a 10-ml syringe. Both whole blood and PRP samples were collected to perform a complete cell count on a dedicated device for horse cells (Pentra 60®, Horiba, Montpellier, France).
Preparation of micro-fat and PRP syringe
The two 10 cc syringes were connected and mixed by gentle back and forth shaking of the syringes two times to finally obtain a final volume of 10 ml of a homogeneous mixed product (Fig. 2D).
Intra-articular injection
The joints (1 carpus and 8 fetlocks) were prepared for injection following a disinfection protocol four times. Synovial fluid was then aspirated from the joints with a 21-gauge needle (0.8 mm × 50 mm). This provided the correct placement in the articular area and avoided any excess of pressure after injection. The same needle was used to connect the luer-lock syringe of the mixed product via intra-articular injection.
After injection, the joints were wrapped in cohesive bandages with sterile gauze for 12 hours (Fig. 2E). Their temperature was monitored for 48 hours and the horses were confined in a small paddock (10 × 5 meters) for 2 weeks. The horses were then transferred to a larger paddock of 1002 meters. Exercise was gradually resumed after 3 months to achieve normal level at month 4. The AAEP lameness scale was re-assessed at 3 months.
Statistical analysis
Statistical analyses were performed with SPSS statistical software, version 16.0 (SPSS, Chicago, IL). The 5% level of significance was used for all statistical tests. Data are presented as mean ± standard deviation. The difference in AAEP scale was performed using a non parametric Wilcoxon test.
Results
All results are detailed in Tables II and III.
Table II.
Baseline characteristics of horses and results.
| Breed | Age (years) | Activity | Activity stop (months) | Joint disease | AAEP score | Follow up (months) | Return to activity | ||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Initial | 3 months | ||||||||
| 1 | Thoroughbred | 7 | Galloping race | 5 |
Anterior right fetlock Loss of cartilage thickness, entesiophytes, chip fracture |
4 | 0 | 16 | Racing 5 months |
|
| |||||||||
| 2 | Percheron | 9 | Show | 12 |
Anterior left fetlock Loss of cartilage thickness entesiophytes, chip fracture, bone edema medial condyle |
3 | 0 | 12 | Competition 10 months |
|
| |||||||||
| 3 | French trotter | 4 | Trotting race | 3 |
Anterior right fetlock Loss of cartilage thickness |
1 | 0 | 7 | Training 5 months |
|
| |||||||||
| 4 | Thoroughbred | 2 | Galloping race | 1 |
Anterior right fetlock Bone edema medial condyle |
3 | 0 | 11 | Racing 8 months |
|
| |||||||||
| 5 | French saddle | 13 | Jumping | Not stopped | Anterior left fetlock Loss of cartilage thickness, chip fracture, osseous cyst of medial condyle |
2 | 2 | 10 | Competition 8 months and discharged |
|
| |||||||||
| 6 | Arabian | 10 | Endurance | 3 | Anterior left fetlock Loss of cartilage thickness, entesiophytes, chip fracture, mineralization synovial capsule |
2 | 1 | 10 | Training 5 months |
|
| |||||||||
| 7 | Thoroughbred | 3 | Galloping race | 1 |
Carpus Chip fracture |
4 | 0 | 10 | Racing 5 months |
|
| |||||||||
| 8 | French trotter | 4 | Trotting race | Not stopped |
Anterior right fetlock Chronic synovitis |
Training 9 months | |||
|
Anterior left fetlock Chronic synovitis |
1 | 0 | 9 | ||||||
Table III.
Blood vs PRP biological parameters (mean ± SD) from horses number 4, 6, 7 and 8.
| Whole Blood | Platelet Rich Plasma | |
|---|---|---|
| Volume (ml) | 64 | 5 |
| Platelet concentration (×109/L) | 179,75 ± 66,90 | 868,25 ± 227,96 |
| RBC concentration (×1012/L) | 7,53 ± 2,32 | 0,05 ± 0,02 |
| Leukocyte concentration (×109/L) | 6,28 ± 1,95 | 3,08 ± 1,22 |
| Platelet dose (billions) | 11,50 ± 4,28 (2,33%) | 4,34 ± 1,14 (94,75%) |
| RBC dose (billions) | 481,92 ± 148,29 (97,59%) | 0,23 ± 0,12 (4,91%) |
| Leukocyte dose (millions) | 401,60 ± 124,69 (0,08%) | 15,38 ± 6,09 (0,34%) |
| Increase factor in platelets | 4,83 | |
| Increase factor in leukocytes | 0,49 |
PRP characteristics
Complete cell count on blood and PRP was performed on 4 horses. Differences in initial blood composition were not related to age, breed or horse activity. Platelet concentration was 179.8±66.9 × 109/L, leukocyte concentration was 6.3±1.9 × 109/L and red blood cell concentration was 7.5±2.3 × 1012/L. Preparation of PRP resulted in an increase in platelet and leukocyte concentrations of 5.2±1.7 -fold and 0.7±0.5 -fold respectively. The mean ± SD composition of the PRP was therefore 4.3±1.1 (94.6±1.0%) billion platelets, 238±111 (5.0±1.0%) million red blood cells and 20±9 million (0.4±0.2%) leukocytes.
Feasibility and safety
Autologous micro-fat was harvested from 8 race horses even if they were fit. Concerning safety, no postoperative adverse reaction was observed in our series.
AAEP Lameness scale
Significant differences were noted regarding the AAEP lameness scale with a decrease from 2.33±1.22 to 0.33±0.66 three months after the procedure (p = 0.21).
Return to competition and training
Seven of the eight horses resumed their previous activities. Four of them returned to official competition between 5 to 10 months after the procedure (mean ± SD = 7.0±2.5) whereas three of them resumed intensive training between 5 to 9 months (mean ± SD = 6.3±2.3). Only one horse returned to competition 8 months after the procedure but was finally retired for breeding purposes because of persistent lameness (2/5 on the AAEEP lameness score). All horses survived.
Discussion
Degenerative joint disease, also commonly referred to as osteoarthritis (OA), can be considered as a group of disorders characterized by a common end stage owing to progressive deterioration of the articular cartilage, accompanied by changes in the bone and the soft tissues surrounding the joint. Current therapies are aimed at alleviating the symptoms or enhancing clinical recovery without inducing healing or regeneration of the affected joint. Horses are recognized as the ideal large animal model for the pre-clinical study of cellular therapy in joints. One of the major advantages of this species is the large size of the joints. This makes surgical procedures easy to equate with human surgery when compared with other animal models. Moreover, the horse is the only model species with a cartilage thickness in the knee joint comparable to that of humans. Adipose tissue is of specific interest as it is a source of different regenerative cells. Among them, MSCs able to differentiate in vitro towards cells with hyaline-like cartilage morphology and can produce cartilage-specific components such as collagen type II and glycosaminoglycans13–16. Positive clinical outcome was reported from a case report using MSC therapy occurring in a natural DJD17. In 2014, Broeckx et al. reported significant improvement in the functionality and sustainability of damaged joints from 6 weeks to 12 months after treatment18 with the combined use of MSCs and PRP. However, cartilage regeneration in preclinical and clinical studies have to be confirmed through magnetic resonance imaging. Our hypothesis was that a single injection of combined micro-fat and PRP in joint of sport horses is well tolerated, safe and potentially efficient. We performed injections in 8 clinical cases (9 joints treated) in the carpus or the fetlock joint. Minimum follow-up was 3 months. Our secondary objectives were to evaluate the clinical outcome of lameness and the return to training or competitive activities. This therapy was proposed to sport-horse owners whose horses presented severe DJD with radiographic and ultrasonographic changes and AAEP lameness scores superior or equal to 1/5 were included. This study suggests that injection of autologous adipose tissue mixed with PRP in horses can be performed safely and is well tolerated. The only tolerance event was mild synovitis for 4 days which resolved without treatment. We did not observe any pain or local reaction, including the harvesting rump area, in any horse. Seven of the eight horses resumed their previous activity without supplementary treatment (4 in competition and 3 in training). The AAEP lameness scale significantly decreased from 2.33±1.22 to 0.37±0.74 (p = 0.021).
The role of adipocytes in joint healing remains to be assessed but trophic properties of micro-fat can be attributed to the undifferentiated cells which are isolated from adult harvested adipose tissue. These cells show many key properties of mesenchymal stem cells from bone marrow19. They are contained in the stromal vascular fraction (SVF) which is a heterogeneous mix of cells including MSC. Furthermore, MSCs from micro-fat has been characterized in vitro for its mechanical and trophic properties and has been shown to provide better adhesion and migration of isolated stem cells compared with adipose tissue collected in a conventional manner20.
On the other hand, platelets contain many growth factors such as platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF) and Transforming Growth Factor β1 (TGF-β1), which are known to participate in tissue healing. Use of PRP was then anticipated to improve clinical outcome since it has been reported that PRP enhances MSC proliferation and chondrogenic differentiation21–23 and could further improve cartilage regeneration.
It is important to note that these results were obtained with a complete standardization of both standard operating procedures to harvest adipose tissue from horses and to prepare PRP. The difficulty to retrieve adipose tissue from sport horses was circumvented by the use of single use cannula allowing the harvesting of small fat lobules24 and the risk of septic arthritis was limited by the use of a totally closed system. Given the high biological variability in available systems for the preparation of equine PRP25, and the complexity in PRP classification26, 27, a human homemade preparation was adapted to horse whole blood characteristics from a published method12 with cell count control from blood and PRP for 4 horses. It consisted of two serial centrifugations of anticoagulated whole blood showing an appropriate enrichment of platelets with preservation of platelet functionality and growth factor release capacity. This accurate preparation method provides a pure PRP (more than 90% of platelets) and a total injected dose of platelets of 4.3±1.1 billion. We previously extracted SVF from horses microfat (n=2) after enzymatic digestion and adipocytes removal showing a number of 87,000±14,000 (mean ± SD) viable nucleated cells by cc of micro-fat and a viability of 86.05±3.46% (mean ± SD). Unfortunately, characterization of subpopulations was not possible due to the lack of appropriate antibodies for horses.
This technique provides the following advantages: i) it is safe and easy to manage in the daily work of equine practitioners; ii) it is affordable; iii) it takes less than an hour to execute all of the stages. It also differs from strategies using MSCs given that no culture step is necessary and that it can be considered as a one-step surgical procedure that can be conducted as point-of-care regenerative therapy.
This study presents some limitations such as the limited number of horses treated, the variability in their breed, age and lameness degree as well as the lack of a control group and the short follow-up. However, it does represent a first step in the development of an innovative therapy for DJD which combines the potential chondrogenic differentiation of MSCs inside equine adipose tissue with the proliferative effect of GF present in PRP. The results demonstrate the safety and potential efficacy of the technique. Further standardized and randomized studies with histology are necessary to confirm this preliminary study28.
Footnotes
Conflicts of interests
The Authors declare that they have no competing interests.
References
- 1.Jeffcott LB, Rossdale PD, Freestone J, Frank CJ, Towers Clark PF. An assessment of wastage in thouroughbred racing from conception to 4 years of age. Equine Vet J. 1982;14:185–198. doi: 10.1111/j.2042-3306.1982.tb02389.x. [DOI] [PubMed] [Google Scholar]
- 2.McIlwraith CW. Current concepts in equine degenerative joint disease. J Am Vet Med Assoc. 1982;180:239–250. [PubMed] [Google Scholar]
- 3.Frisbie DD. Future directions in treatment of joint disease in horses. Vet Clin North Am Equine Pract. 2005;21:713–724. doi: 10.1016/j.cveq.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Malone ED. Managing chronic arthritis. Vet Clin North Am Equine Pract. 2002;18:411–437. doi: 10.1016/s0749-0739(02)00024-x. [DOI] [PubMed] [Google Scholar]
- 5.Nizolek DJ, White KK. Corticosteroid and hyaluronic acid treatments in equine degenerative joint disease. A review. The Cornell veterinarian. 1981;71:355–375. [PubMed] [Google Scholar]
- 6.Goodrich LR, Nixon AJ. Medical treatment of osteoarthritis in the horse – a review. Vet J. 2006;171:51–69. doi: 10.1016/j.tvjl.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Zubrod CJ, Schneider RK. Arthrodesis techniques in horses. Vet Clin North Am Equine Pract. 2005;21:691–711. doi: 10.1016/j.cveq.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, Mc Ilwraith CW. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res. 2009;27:1675–1680. doi: 10.1002/jor.20933. [DOI] [PubMed] [Google Scholar]
- 9.Mc Ilwraith CW, Frisbie DD, Rodkey WG, Kisiday JD, Werpy NM, et al. Evaluation of intra articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy. 2011;27:1552–1561. doi: 10.1016/j.arthro.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Wike MM, Nydam DV, Nixon AJ. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in equine model. J Orthop Res. 2007;25:913–925. doi: 10.1002/jor.20382. [DOI] [PubMed] [Google Scholar]
- 11.Frisbie DD, Cross MW, Mc Ilwraith CW. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet Comp Orthop Traumatol. 2006;19:142–146. [PubMed] [Google Scholar]
- 12.Bausset O, Giraudo L, Veran J, et al. Formulation and storage of platelet-rich plasma homemade product. Biores Open Access. 2012;1(3):115–123. doi: 10.1089/biores.2012.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch TG, Betts DH. Stem cell therapy for joint problems using the horse as clinically relevant model. Expert Opin Bio Ther. 2007;7:1621–1626. doi: 10.1517/14712598.7.11.1621. [DOI] [PubMed] [Google Scholar]
- 14.Berg L, Koch T, Heerkens T, Bessonov K, Thomsen P, et al. Chondrogenic potential of mesenchymal stromal cells derived from equine bone marrow and umbilical cord blood. Vet Comp Orthop Traumatol. 2009;22:363–370. doi: 10.3415/VCOT-08-10-0107. [DOI] [PubMed] [Google Scholar]
- 15.Spaas JH, Schauwer CD, Cornillie P, Meyer E, Soom AV, et al. Culture and characterization of equine peripheral blood mesenchymal stromal cells. Vet J. 2013;295:107–113. doi: 10.1016/j.tvjl.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Giai Via A, Frizziero A, Oliva F. Biological properties of mesenchymal stem cells from different sources. Muscles, Ligaments and Tendons Journal. 2012;2(3):154–162. [PMC free article] [PubMed] [Google Scholar]
- 17.Spaas JH, Oosterlinck M, Broeckx S, Dumoulin M, Saunders J, et al. Treatment of equine degenerative joint disease with autologous peripheral blood-derived mesenchymal stem cells: a case report. Vlaams Dierg Tijdschrift. 2012;81:11–15. [Google Scholar]
- 18.Broeckx S, Zimmerman M, Crocetti S, Suls M, Mariën T, et al. Regenerative therapies for equine degenerative joint disease: a preliminary study. PLoS ONE. 2014;9(1):e85917. doi: 10.1371/journal.pone.0085917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuk PA. ISRN Stem Cells Review Article. Adipose-Derived Stem Cells in Tissue Regeneration: A Review-Hindawi Publishing Corporation. ISRN Stem Cells. 2013 [Google Scholar]
- 20.Alharbi Z, Opländer C, Almakadi S, Fritz A, Vogt M, Pallua N. Conventional vs. micro-fat harvesting: how fat harvesting technique affects tissue-engineering approaches using adipose tissue-derived stem/stromal cells. J Plast Reconstr Aesthet Surg. 2013;66(9):1271–1278. doi: 10.1016/j.bjps.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Mishra A, Tummala P, King A, Lee B, Kraus M, et al. Buffered platelet rich plasma enchances mesenchymal stem cells proliferation and chondrogenic differentiation. Tissue Eng Part C Methods. 2009;15:431–435. doi: 10.1089/ten.tec.2008.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atashi F, Jaconi ME, Pittet-Cuénod B, Modarressi A. Autologous platelet-rich plasma: a biological supplement to enhance adipose-derived mesenchymal stem cell expansion. Tissue Eng Part C Methods. 2015;21(3):253–262. doi: 10.1089/ten.tec.2014.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubio-Azpeitia E, Andia I. Partnership between platelet-rich plasma and mesenchymal stem cells: in vitro experience. Muscles, Ligaments and Tendons Journal. 2014;4(1):52–62. [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen PS, Desouches C, Gay AM, Hautier A, Magalon G. Development of micro-injection as an innovative autologous fat graft technique: the use of adipose tissue as dermal filler. J Plast Reconstr Aesthet Surg. 2012;65(12):1692–1699. doi: 10.1016/j.bjps.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Hessel LN, Bosch G, Van Weeren PR, Ionita JC. Equine autologous platelet concentrates: a comparative study between different available systems. Equine Vet J. 2013;47(3):319–325. doi: 10.1111/evj.12288. [DOI] [PubMed] [Google Scholar]
- 26.Anitua E, Sanchez M, Prado R, Orive G, Padilla S. You are not walking alone in the PRP consensus road. Muscles, Ligaments and Tendons Journal. 2014;4(4):471–472. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Dohan Ehrenfest DM, Andia I, Zumstein MA, Zhang CQ, Pinto NR, Bielecki T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles, Ligaments and Tendons Journal. 2014;4(1):3–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Padulo J, Oliva F, Frizziero A, Maffulli N. Muscles, Ligaments and Tendons Journal. Basic principles and recommendations in clinical and field science research:2016 update. MLTJ. 2016;6( 1):1–5. doi: 10.11138/mltj/2016.6.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]