Summary
Background
Proximal Hamstring Tendinopathy-related Sciatic Nerve Entrapment (PHTrSNE) is a neuropathy caused by fibrosis interposed between the semimembranosus tendon and the sciatic nerve, at the level of the ischial tuberosity.
Methods
Ultrasound-guided Intratissue Percutaneous Electrolysis (US-guided EPI) involves galvanic current transfer within the treatment target tissue (fibrosis) via a needle 0.30 to 0.33 mm in diameter. The galvanic current in a saline solution instantly develops the chemical process of electrolysis, which in turn induces electrochemical ablation of fibrosis. In this article, the interventional procedure is presented in detail, and both the strengths and limits of the technique are discussed.
Results
US-guided EPI eliminates the fibrotic accumulation that causes PHTrSNE, without the semimembranosus tendon or the sciatic nerve being directly involved during the procedure. The technique is however of limited use in cases of compression neuropathy.
Conclusion
US-guided EPI is a technique that is quick to perform, minimally invasive and does not force the patient to suspend their activities (work or sports) to make the treatment effective. This, coupled to the fact that the technique is generally well-tolerated by patients, supports use of US-guided EPI in the treatment of PHTrSNE.
Keywords: ablation techniques, entrapment neuropathies, tendon injuries, ultrasonography
Introduction
Proximal hamstring tendinopathy (PHT) is an overuse injury of current interest in orthopaedic and sports medicine. PHT is clinically characterised by pain in the sub-gluteal region, at the proximal insertion of the hamstring muscles onto the ischial tuberosity, with possible radiation to the posterior region of the thigh; sprinting and sitting for long periods are the activities in which the symptoms are typically exacerbated1, 2. The proximal insertion of the semimembranosus (SM) muscle onto the ischial tuberosity is superior-lateral, relative to the insertion of the conjoint tendon of the biceps femoris and semintendinosus.1,3–5. In patients with PHT, the proximal SM tendon is the one that is typically degenerated, appearing thickened at the lateral edge1.
In addition to this, the SM tendon is located in the vicinity of the Sciatic Nerve (SN)6, 7 which runs just lateral to the tendon (Fig. 1). In patients with PHT, fibrotic adhesions between the SM tendon and the SN occasionally form1,3,6,8; this anatomical alteration can cause entrapment syndrome of the SN, overlapping symptoms of tendinopathy, and those typical of an irritation of the nerve (sudden stabbing pain, sciatica, burning sensation or other paraesthesias). Proximal Hamstring Tendinopathy-related Sciatic Nerve Entrapment (PHTrSNE) therefore represents a possible complication of PHT. Rapid movements of hip flexion and extension or maximum hip flexion can worsen the symptoms, being the SN bound to the hamstring tendon complex (anchoring fibrosis)1,3,6,8. However, distinguishing symptoms of tendinopathy from those of neuropathy is fairly complicated.
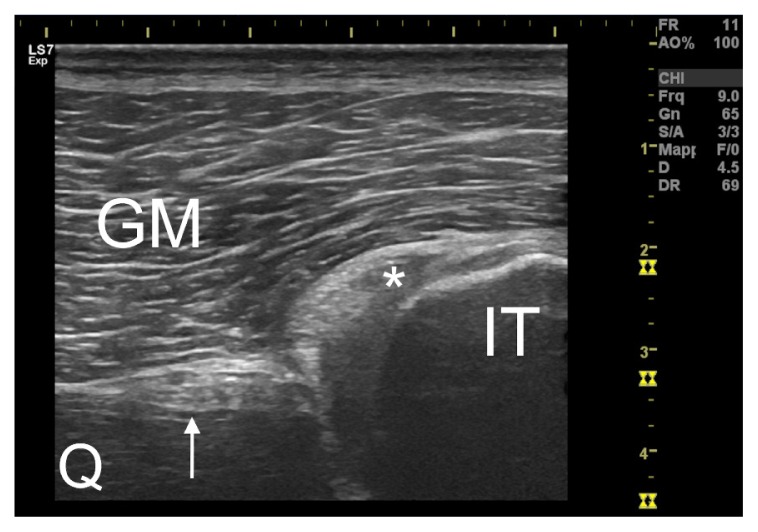
Figure 1.
Transversal ultrasound section of the proximal semimembranosus tendon (asterisked) on the ischial tuberosity (IT). The sciatic nerve (indicated by the arrow) is easily recognizable lateral to the tendon. GM=gluteus maximus. Q=quadratus femoris.
Conservative treatment and infiltrative therapies for the treatment of PHT are generally preferred to surgery, which is considered when these do not lead to satisfactory results. Potentially applicable treatments are numerous: shockwaves, injections of PRP or corticosteroids, eccentric exercises and others (review2). In addition to the dearth of scientific evidence about their effectiveness, the limitation of these therapies is that they are unspecific and most likely ineffective for the treatment of PHTrSNE, in case the latter has been detected and taken into consideration. One scenario that can occur is that symptoms related to tendinopathy recede, while those derived from irritation of the SN persist.
Surgical treatment for managing PHT involves tenotomy of the SM tendon, which is sutured to the tendon of the biceps femoris1–2. The positive outcome of SM tenotomy is that the SN is anatomically unbridled from the tendon; the intervention is therefore also valid for the treatment of PHTrSNE. The only significant disadvantage of the intervention is the long post-surgical recovery time (from 1 to 12 months)2,9; this point certainly has a significant impact, especially for professional athletes, who may decide to postpone excessively or not to undergo surgery at all.
Finding a technique that can eliminate fibrosis between the SM tendon and the SN without requiring long periods of post-interventional recovery is therefore challenging. Ultrasound-guided Intratissue Percutaneous Electrolysis fulfils these requirements. The aims of this article are: i) to present the rationale for using this technique in the treatment of PHTrSNE; ii) to present the method of application and iii) to discuss both the strengths and limitations of the technique. The hypothesis of the Authors is that Ultrasound-guided Intratissue Percutaneous Electrolysis may be a useful complement to non-surgical proposals in the treatment of PHT with concomitant PH-TrSNE.
Materials and methods
This study was conducted in accordance with the Declaration of Helsinki and complied with the ethical standards of the Muscles, Ligaments and Tendons Journal10.
Ultrasound-guided Intratissue Percutaneous Electrolysis
Intratissue Percutaneous Electrolysis (also known as Electrólisis Percutánea Intratisular or EPI) is a minimally invasive technique that involves galvanic current transfer within the treatment target tissue via a needle 0.30 to 0.33 mm in diameter. The galvanic current in a saline solution rapidly develops the chemical process of electrolysis, which in turn induces tissue ablation11. The EPI technique finds indications in the treatment of tendinopathies and fibrosis12–16.
The rationale for using EPI in the treatment of PH-TrSNE is the ability of the technique to specifically degrade fibrotic adhesions that bind the SN and SM tendon. Use of the technique is indicated only when le evident ultrasound signs of fibrosis between the SN and SM tendon are identifiable (Fig. 2) concomitant to the presence of the clinical pattern of PHTrSNE. Clinical assessment is needed, but is not sufficient to diagnose PHTrSNE, as the SN can be trapped in many other areas in the sub-gluteal region6.
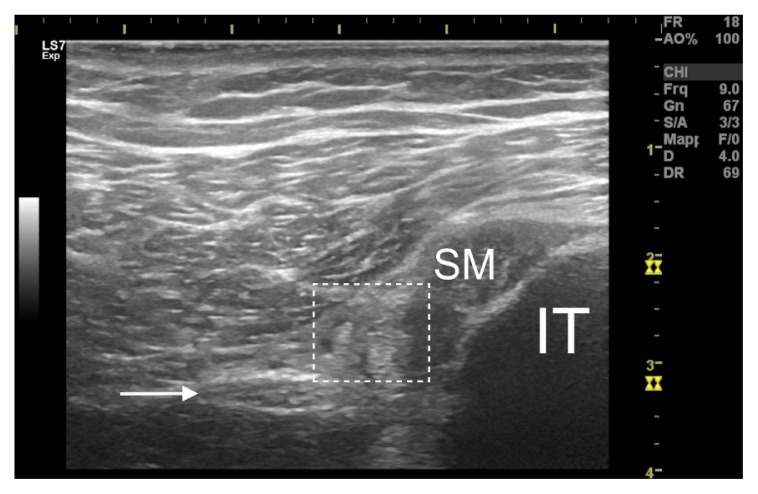
Figure 2.
Proximal hamstring tendinopathy-related sciatic nerve entrapment. Between the sciatic nerve (indicated by arrow) and the tendon of the semimembranosus (SM) a fibrotic accumulation is interposed (hyper-echoic area visible within the dashed box) which makes it difficult to distinguish the anatomical limits of the structures. IT=ischial tuberosity.
We apply the EPI technique using a specifically developed and medically certified (Directive 93/42/EEC) device (EPI Advanced Medicine® Barcelona, Spain). The main feature of the device is that the cathodic flow is the only one usable. To ensure maximum precision, the technique must be performed in an ultrasound-guided manner (US-guided EPI). We use the GE Healthcare Logiq S7 Expert® ultrasound with a linear probe (6–15 MHz) to guide the insertion of the needle. The operator must be well-trained in the use of the technique, and must have experience in the ultrasound examination of the lower limb.
Procedure for the US-guided EPI intervention
The patient lies in a prone position. The gluteal, subgluteal and trochanteric regions are disinfected by applying an appropriate protocol. The proximal tendon insertions of the hamstring muscles on the ischial tuberosity are identified by the operator guiding the ultrasound probe using their non-dominant hand; a transversal section is required to be able to simultaneously view the tendon complex and the SN7.
The needle is inserted by the operator with the dominant hand between the two structures through the gluteus maximus muscle, with an inclination of 0+30° around the vertical axis, in the medial-lateral direction (Figs. 3, 4); the SN, the SM tendon, the inferior gluteal artery and the inferior gluteal vein must be avoided during insertion. Depending on the physical characteristics of the patient, the needle can have a variable length, starting at 50 millimetres.
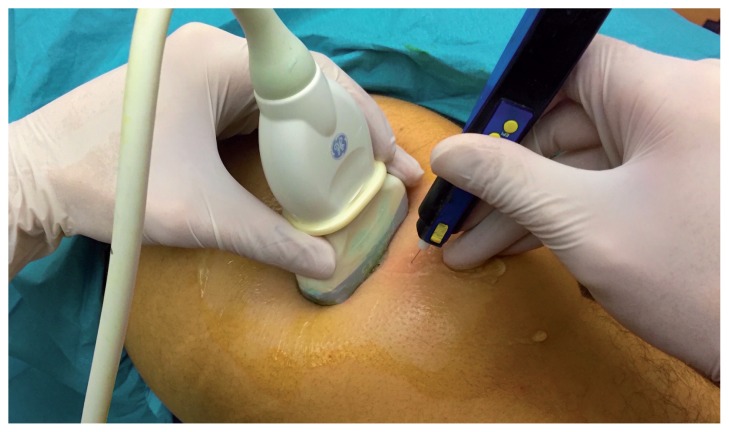
Figure 3.
Ultrasound-guided insertion of the needle. Being the needle inserted perpendicular to the skin, it is displayed through movement of surrounding tissues.
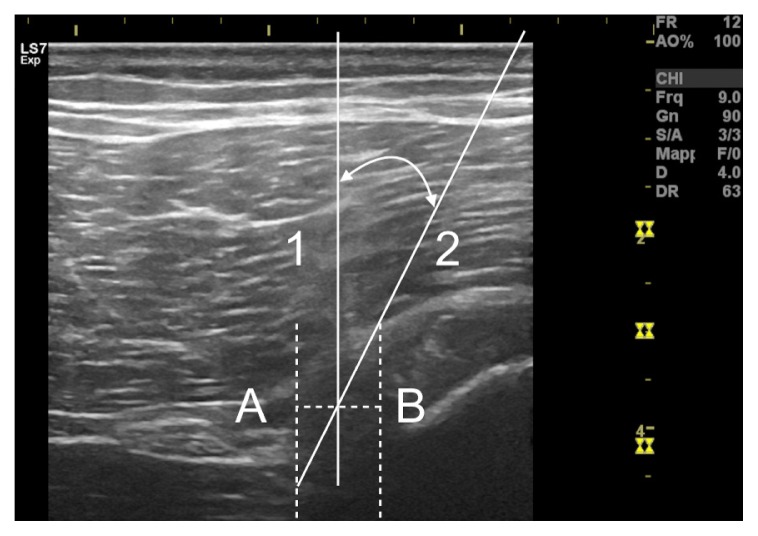
Figure 4.
Schematic diagram of the Ultrasound-guided Intratissue Percutaneous Electrolysis intervention. The medial edge of the sciatic nerve and associated vessels is represented by the vertical dashed line A. The lateral limit of the semimembranosus tendon is represented by the vertical dashed line B. The needle is inserted into the area bounded by line 1 (perpendicular to the axis joining lines A and B) and by line 2 (tangent to the superior-lateral point of the semimembranosus tendon). In this way, the technique can be performed safely, without structures other than the fibrosis being involved in the intervention.
When the needle tip is positioned inside the fibrosis, the galvanic current can be transferred; the intensity of the current is pre-set to 4 mA. The single application can have a variable duration between 2–10 seconds, depending on the tolerability of the technique to the patient (later) and the mechanical resistance offered by the tissue: where it is possible to penetrate the fibrosis with the needle by applying minimum pressure, the single application can be interrupted. At this point the needle is partially withdrawn (without exiting from the skin) and inserted again with a slight deviation to treat a different portion of the fibrotic accumulation.
One session can consist of 2–15 individual applications depending on the extent and hardness of the fibrosis. Consequently, administration of the galvanic current does not exceed 150 seconds in any one session; this should be taken into consideration as local anaesthetic drugs are not used. The session, including disinfection and dressing processes, lasts a maximum of 20 minutes in total.
Tolerability of pain
US-guided EPI is generally well-tolerated by patients. Some may have minor discomfort during the insertion of the needle. Application of the technique can cause mild to moderately strong pain. In addition to this, it is possible that the posterior femoris cutaneous nerve will be indirectly stimulated, causing tingling in the back of the thigh. Vagal reactions (nausea, headache, fainting) are rare but possible17; in this sense it is advisable to remind the patient before each session that he or she can ask for the session to be interrupted or stopped at any time. Bleeding in the area of needle insertion may occasionally occur. Patients sometimes report mild tenderness in the treated area, which generally does not last longer than 12–48 hours. The Authors do not advise conducting more than one session per week.
Discussion
The aims of this article are: i) to present the rationale for using US-guided EPI in the treatment of PH-TrSNE; ii) to present the method of application and iii) to discuss both the strengths and limitations of the technique. The hypothesis of the Authors is that Ultrasound-guided EPI may be an useful complement to conservative proposals when treating PHT with concomitant PHTrSNE. By using the US-guided EPI technique it is possible to eliminate the fibrosis interposed between the SN and SM tendon, degrading it by electrolytic ablation, without expecting the patient to suspend sporting or work activities for more than 48 hours after the intervention.
Strengths and limitations of the technique
US-guided EPI is a technique that allows specific treatment of the fibrotic adhesions that cause PH-TrSNE. The minimal invasivity and lack of necessity of suspending activities (work or sports) after treatment are the main strengths of this technique. The main limitation of the technique is the impossibility of completely resolving the symptoms if nerve irritation is caused by SN compression and not by its entrapment by the SM tendon.
EPI is an ultrasound-guided minimally invasive technique in which the needle is inserted with maximum precision into the structure to be treated, with minimal structural damage to any of the other tissues. In this way, one can eliminate a biomechanical cause of neuropathic symptoms, or the fibrosis that binds the SN to the SM tendon, without the nerve and the tendon being directly involved in the intervention. A further advantage of the mini-invasivity is the low chance of experiencing adverse events (in particular damage of the peri-nervous blood vessels).
One point that is certainly an advantage is that the patient does not need to undergo a specific rehabilitative intervention post US-guided EPI to render the treatment effective; this is particularly important for athletes suffering from PHTrSNE who will be required to suspend activities for 24–48 hours at most. US-guided EPI intervention does not therefore have a significant impact on the scheduling of sporting activities for an athlete.
The main limitation of the technique depends upon the type of anatomical alteration that has caused the neuropathy. In this article, SN entrapment neuropathy has been presented. It is however possible that the neuropathy is not derived from SN entrapment, but from compression of the nerve due to SM tendon hypertrophy1,3. The two conditions can be concomitant. Using the US-guided EPI intervention, it is not possible to reduce SM tendon hypertrophy in a consistent way; furthermore, on debriding the SN, it is possible that symptoms will persist because of compressive trauma suffered by the nerve. In such case, surgery remains the only therapeutic solution that can be considered.
Another possible scenario is the one in which the symptomatology persists due to irritation of the nerve at another location despite resolution of the PH-TrSNE. The anatomy of the sub-gluteal region is in fact highly complex and many different conditions (e.g. piriformis syndrome, gemelli-obturator internus syndrome, quadratus femoris pathology and gluteal disorders) provoking sciatica fall within differential diagnosis1,6. As a consequence of this, identification of the primary etiological cause determining the symptoms can be challenging. Instrumental investigations have an important role in detecting anatomical alterations1; to date, Magnetic Resonance Imaging (MRI) is typically the procedure of choice1–3,6,9,18, especially when lower back pain is concomitant (the exam is useful to exclude other pathologies). However, MRI findings can be not associated with symptoms. Medical history and clinical examination1, 2 can be helpful but, considering that different conditions present similar symptoms, only few tests have shown to have good sensibility and specificity in the diagnosis of SN entrapment6,19. For all these reasons, PHTrSNE remains fundamentally a diagnosis of exclusion.
Consequently, in order to evaluate the benefit of the US-guided EPI technique in the treatment of PH-TrSNE, the operator shall continuously monitor the evolution of the symptoms related to the disease. The Visual Analogic Scale (VAS)9,18 and the Victorian Institute of Sport Assessment-Proximal Hamstring Tendons (VISA-H) questionnaire20 are useful for this purpose. In particular, VISA-H has proven to have high degree of internal consistency and high test-retest reliability20.
Ultrasound-guided EPI in the treatment of tendinopathies
US-guided EPI finds indications in the treatment of tendinopathies and fibrosis. To date however, few studies have tested the effectiveness of the technique. The therapeutic utility of EPI (by some Authors replaced with the acronym PNE, Percutaneous Needle Electrolysis) was tested for the treatment of patellar tendinopathy12,13, sub-acromial syndrome14, chronic lateral epicondylitis15 and rectus abdominis-related groin pain16. The clinical results presented in this work are somewhat in conflict, especially given the different methodological choices of the Authors, in particular relative to the intensity and duration of administration of the galvanic current (which varied from 0.3 mA for 1.2 minutes to 3 mA for a few seconds) and suggested physiotherapy administered in combination with the technique.
The desired effects with the US-guided EPI are, on the one hand, elimination of the degenerated portion of the tendon and, on the other, development of an extremely localized and controlled inflammatory process, that may promote the tendon healing process. The histological effects of administering galvanic current within the tendon tissue are, however, only partially understood11,21–24. The method of applying the US-guided EPI technique presented in this article however differs to the one described in the articles listed above. In those articles, the technique is performed by inserting the needle within the degenerated portion of the tendon; the tendon is therefore the target of the therapy. In this work, the purpose was the electrolytic elimination of peri-tendinous fibrotic tissue, without treating the tendon. The ablative action is therefore the only one that has been fundamentally investigated. This also means there is no need to propose a specific rehabilitation protocol after the intervention.
Conclusions
US-guided EPI is a technique that allows specific treatment of the anatomical alterations that cause PHTrSNE, eliminating the fibrosis that binds the SN to the SM tendon. US-guided EPI is a technique that is quick to carry out, minimally invasive and does not force the patient to suspend their activities (work or sporting) to make the treatment effective. This, coupled to the fact that the technique is generally well-tolerated by patients, supports use of US-guided EPI in the treatment of PHTrSNE. Future studies with high quality designs are needed to test the efficacy of US-guided EPI in the treatment of PHTrSNE.
Footnotes
Conflicts of interest
The Authors declare no conflicts of interest concerning this article.
References
- 1.Lempainen L, Sarimo J, Mattila K, Vaittinen S, Orava S. Proximal Hamstring Tendinopathy. Am J Sports Med. 2009;37:727. doi: 10.1177/0363546508330129. [DOI] [PubMed] [Google Scholar]
- 2.Lempainen L, Johansson K, Banke IJ, et al. Expert opinion: diagnosis and treatment of proximal hamstring tendinopathy. Muscles Ligaments Tendons J. 2015;5(1):23–28. [PMC free article] [PubMed] [Google Scholar]
- 3.Beltran L, Ghazikhanian V, Padron M, Beltran J. The proximal hamstring muscle-tendon-bone unit: A review of the normal anatomy, biomechanics and pathophysiology. Eur J Radiol. 2012;81:3772–3779. doi: 10.1016/j.ejrad.2011.03.099. [DOI] [PubMed] [Google Scholar]
- 4.Obey MR, Broski SM, Spinner RJ, Collins MS, Krych AJ. Anatomy of the Adductor Magnus Origin. Implication for Proximal Hamstring Injuries. Orthop J Sports Med. 2016;4(1):1–6. doi: 10.1177/2325967115625055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feucht MJ, Plath JE, Seppel G, Hinterwimmer S, Imhoff AB, Brucker PU. Gross anatomical and dimensional characteristics of the proximal hamstring origin. Knee Surg Sports Traumatol Arthrosc. 2015;23:2576–2582. doi: 10.1007/s00167-014-3124-0. [DOI] [PubMed] [Google Scholar]
- 6.Hernando MF, Cerezal L, Perez-Carro L, Abascal F, Canga A. Deep gluteal syndrome: anatomy, imaging, and management of sciatic nerve entrapments in the subgluteal space. Skeletal Radiol. 2015;44:919–934. doi: 10.1007/s00256-015-2124-6. [DOI] [PubMed] [Google Scholar]
- 7.Silvestri E, Muda A, Orlandi D. Ultrasound Anatomy of Lower Limb Muscles. A practical Guide. Foreword by Nicola Maffulli. Springer International Publishing; Switzerland: 2015. Hamstrings; pp. 101–113. [Google Scholar]
- 8.McGregor C, Ghosh S, Young DA, Maffulli N. Traumatic and overuse injuries of the ischial origin of the hamstrings. Disabil Rehabil. 2008;30(20–22):1597–1601. doi: 10.1080/09638280701786138. [DOI] [PubMed] [Google Scholar]
- 9.Benazzo F, Marullo M, Zanon G, Indino C, Pelillo F. Surgical management of chronic proximal hamstring tendinopathy in athletes: a 2 to 11 years of follow-up. J Orthopaed Traumatol. 2013;14:83–89. doi: 10.1007/s10195-013-0226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padulo J, Oliva F, Frizziero A, Maffulli N. Muscles, Ligaments and Tendons Journal. Basic principles and recommendations in clinical and field science research: 2016 update. MLTJ. 2016;6(1):1–5. doi: 10.11138/mltj/2016.6.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez-Ibañez JM, Colmena C, Benabent J, Garcia-Herreros S, Valles SL. New Technique in Tendon Sport Recovery. Percutaneous Electrolysis Intratissue (EPI®) Int J Phys Med Rehabil. 2013;1(2):1000113. [Google Scholar]
- 12.Abat F, Gelber PE, Polidori F, Monllau JC, Sanchez-Ibañez JM. Clinical results after ultrasound-guided intratissue percutaneous electrolysis (EPI®) and eccentric exercise in the treatment of patellar tendinopathy. Knee Surg Sports Traumatol Arthrosc. 2014 doi: 10.1007/s00167-014-2855-2. [DOI] [PubMed] [Google Scholar]
- 13.Abat F, Diesel WJ, Gelber PE, Polidori F, Monllau JC, Sanchez-Ibañez JM. Effectiveness of the Intratissue Percutaneous Electrolysis (EPI®) technique and isoinertial eccentric exercise in the treatment of patellar tendinopathy at two years follow-up. Muscles Ligaments Tendons J. 2014;4(2):188–193. [PMC free article] [PubMed] [Google Scholar]
- 14.Arias-Buria JL, Truyols-Dominguez S, Valero-Alcaide R, Salom-Moreno J, Atin-Arratibel MA, Fernandes delas-Peñas C. Ultrasound-Guided Percutaneous Electrolysis and Eccentric Exercises for Subacromial Pain Syndrome: A Randomized Clinical Trial. Evid Based Complement Alternat Med. 2015 doi: 10.1155/2015/315219. 315219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valera-Garrido F, Minaya-Muñoz F, Medina-Mirapeix F. Ultrasound-guided percutaneous needle electrolysis in chronic lateral epicondylitis: short-term and long-term results. Acupunct Med. 2014;32:446–454. doi: 10.1136/acupmed-2014-010619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreno C, Mattiussi G, Nuñez FJ. Therapeutic results after ultrasound-guided Intratissue Percutaneous Electrolysis (EPI®) in the treatment of rectus abdominis-related groin pain in professional footballers: a pilot study. J Sport Med Phys Fitness. 2016 [PubMed] [Google Scholar]
- 17.de la Cruz Torres B, Albornoz Cabello M, Garcia Bermejo P, Naranjo Orellana J. Autonomic responses to ultrasound-guided percutaneous needle electrolysis of the patellar tendon in healthy male footballers. Acupunct Med. 2016 doi: 10.1136/acupmed-2015-010993. [DOI] [PubMed] [Google Scholar]
- 18.Fader RR, Mitchell JJ, Traub S, et al. Platelet-rich plasma treatment improves outcomes for chronic proximal hamstring injuries in an athletic population. Muscles Ligaments Tendons J. 2015;4(4):461–466. [PMC free article] [PubMed] [Google Scholar]
- 19.Martin HD, Kivlan BR, Palmer IJ, Martin RL. Diagnostic accuracy of clinical tests for sciatic nerve entrapment in the gluteal region. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):882–888. doi: 10.1007/s00167-013-2758-7. [DOI] [PubMed] [Google Scholar]
- 20.Cacchio A, De Paulis F, Maffulli N. Development and validation of a new visa questionnaire (VISA-H) for patients with proximal hamstring tendinopathy. Br J Sports Med. 2014;48( 6):448–452. doi: 10.1136/bjsports-2012-091552. [DOI] [PubMed] [Google Scholar]
- 21.Abat F, Valles SL, Monllau JC, Sanchez-Ibañez JM. Molecular repair mechanisms using the Intratissue Percutaneous Electrolysis technique in patellar tendonitis. Rev Esp Cir Ortop Traumatol. 2014;58(4):201–205. doi: 10.1016/j.recot.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Almeida Mdos S, Oliveira LP, Vieira CP, Guerra Fda R, Pimentel ER. Birefringence of collagen fibres in rat calcaneal tendons treated with acupuncture during three phases of healing. Acupunct Med. 2016;34(1):27–32. doi: 10.1136/acupmed-2015-010845. [DOI] [PubMed] [Google Scholar]
- 23.Inoue M, Nakajima M, Oi Y, Hojo T, Itoi M, Kitakoji H. The effect of electroacupuncture on tendon repair in a rat Achilles tendon rupture model. Acupunct Med. 2015;33(1):58–64. doi: 10.1136/acupmed-2014-010611. [DOI] [PubMed] [Google Scholar]
- 24.de Almeida Mdos S, de Aro AA, Guerra Fda R, Vieira CP, de Campos Vidal B, Rosa Pimentel E. Electroacupuncture increases the concentration and organization of collagen in a tendon healing model in rats. Connect Tissue Res. 2012;53( 6):542–547. doi: 10.3109/03008207.2012.710671. [DOI] [PubMed] [Google Scholar]