Abstract
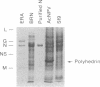
A cDNA copy of the RNA gene that encodes the nucleoprotein N of rabies virus Evelyn-Rokitnicki-Abelseth strain was cloned into baculovirus. The recombinant baculovirus expressed the N protein abundantly in Spodoptera frugiperda cells. The N protein was extracted from infected Spodoptera frugiperda cells and purified to near homogeneity by affinity chromatography. The purified N protein reacted with 31 of 32 monoclonal antibodies that recognize native rabies virus ribonucleoprotein. Like the ribonucleoprotein, the purified N protein was a major antigen capable of inducing virus-specific helper T cells. Priming of mice with the purified N protein prior to a booster inoculation with inactivated Evelyn-Rokitnicki-Abelseth virus vaccine resulted in a 20-fold increase in the production of virus-neutralizing antibodies. After immunization with the purified N protein, mice developed a strong anti-ribonucleoprotein antibody response and were protected against a lethal challenge of rabies virus. These data indicate that the N protein expressed in insect cells is antigenically and immunogenically comparable to the authentic rabies virus ribonucleoprotein and therefore represents a potential source of an effective and economical vaccine for large-scale immunization of humans and animals against rabies.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cox J. H., Dietzschold B., Schneider L. G. Rabies virus glycoprotein. II. Biological and serological characterization. Infect Immun. 1977 Jun;16(3):754–759. doi: 10.1128/iai.16.3.754-759.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick J., Brown F. Viral subunits for rabies vaccination. Nature. 1969 Apr 5;222(5188):92–92. doi: 10.1038/222092a0. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Cox J. H., Schneider L. G., Wiktor T. J., Koprowski H. Isolation and purification of a polymeric form of the glycoprotein of rabies virus. J Gen Virol. 1978 Jul;40(1):131–139. doi: 10.1099/0022-1317-40-1-131. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Lafon M., Wang H., Otvos L., Jr, Celis E., Wunner W. H., Koprowski H. Localization and immunological characterization of antigenic domains of the rabies virus internal N and NS proteins. Virus Res. 1987 Aug;8(2):103–125. doi: 10.1016/0168-1702(87)90023-2. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Rupprecht C. E., Tollis M., Lafon M., Mattei J., Wiktor T. J., Koprowski H. Antigenic diversity of the glycoprotein and nucleocapsid proteins of rabies and rabies-related viruses: implications for epidemiology and control of rabies. Rev Infect Dis. 1988 Nov-Dec;10 (Suppl 4):S785–S798. doi: 10.1093/clinids/10.supplement_4.s785. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Wang H. H., Rupprecht C. E., Celis E., Tollis M., Ertl H., Heber-Katz E., Koprowski H. Induction of protective immunity against rabies by immunization with rabies virus ribonucleoprotein. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9165–9169. doi: 10.1073/pnas.84.24.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl H. C., Dietzschold B., Gore M., Otvos L., Jr, Larson J. K., Wunner W. H., Koprowski H. Induction of rabies virus-specific T-helper cells by synthetic peptides that carry dominant T-helper cell epitopes of the viral ribonucleoprotein. J Virol. 1989 Jul;63(7):2885–2892. doi: 10.1128/jvi.63.7.2885-2892.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung M. C., Chiu K. Y., Weber T., Chang T. W., Chang N. T. Detection and purification of a recombinant human B lymphotropic virus (HHV-6) in the baculovirus expression system by limiting dilution and DNA dot-blot hybridization. J Virol Methods. 1988 Jan;19(1):33–42. doi: 10.1016/0166-0934(88)90005-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Préhaud C., Harris R. D., Fulop V., Koh C. L., Wong J., Flamand A., Bishop D. H. Expression, characterization, and purification of a phosphorylated rabies nucleoprotein synthesized in insect cells by baculovirus vectors. Virology. 1990 Oct;178(2):486–497. doi: 10.1016/0042-6822(90)90346-s. [DOI] [PubMed] [Google Scholar]
- Reid-Sanden F. L., Sumner J. W., Smith J. S., Fekadu M., Shaddock J. H., Bellini W. J. Rabies diagnostic reagents prepared from a rabies N gene recombinant expressed in baculovirus. J Clin Microbiol. 1990 May;28(5):858–863. doi: 10.1128/jcm.28.5.858-863.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L. G., Dietzschold B., Dierks R. E., Matthaeus W., Enzmann P. J., Strohmaier K. Rabies group-specific ribonucleoprotein antigen and a test system for grouping and typing of rhabdoviruses. J Virol. 1973 May;11(5):748–755. doi: 10.1128/jvi.11.5.748-755.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Dietzschold B., Leamnson R. N., Koprowski H. Induction and biological properties of defective interfering particles of rabies virus. J Virol. 1977 Feb;21(2):626–635. doi: 10.1128/jvi.21.2.626-635.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Macfarlan R. I., Foggin C. M., Koprowski H. Antigenic analysis of rabies and Mokola virus from Zimbabwe using monoclonal antibodies. Dev Biol Stand. 1984;57:199–211. [PubMed] [Google Scholar]
- Wunner W. H., Larson J. K., Dietzschold B., Smith C. L. The molecular biology of rabies viruses. Rev Infect Dis. 1988 Nov-Dec;10 (Suppl 4):S771–S784. doi: 10.1093/clinids/10.supplement_4.s771. [DOI] [PubMed] [Google Scholar]