Abstract
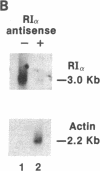
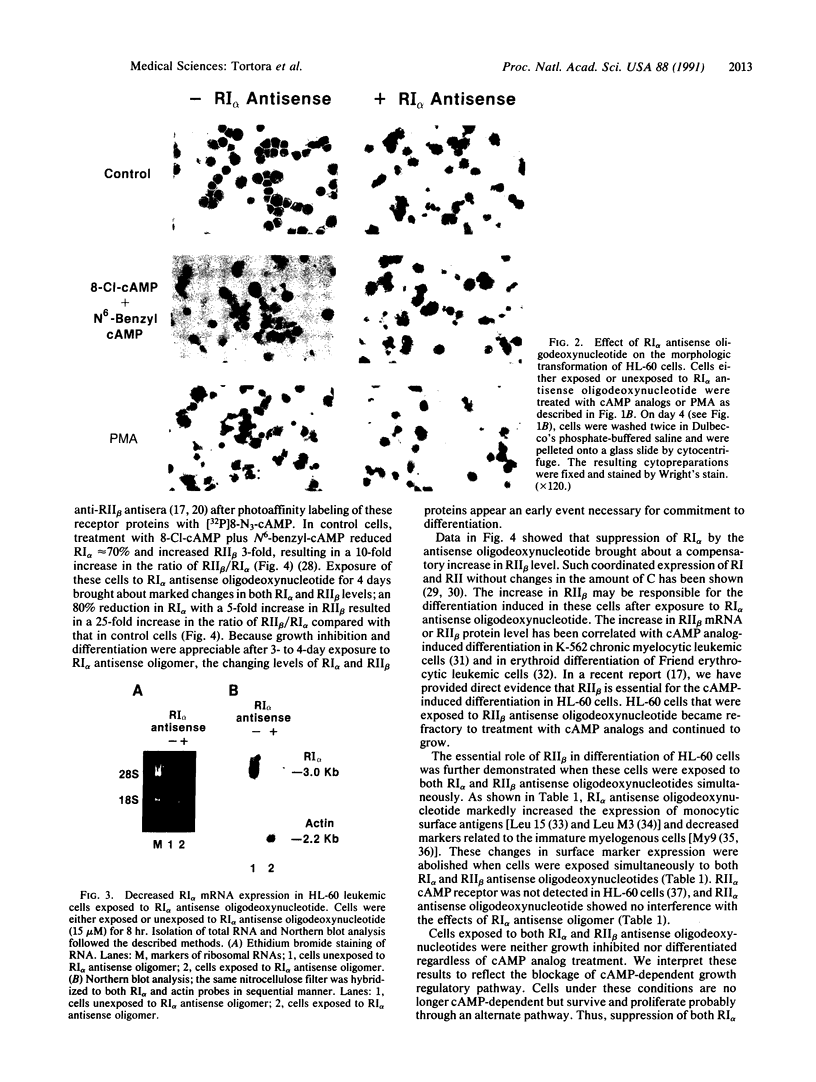
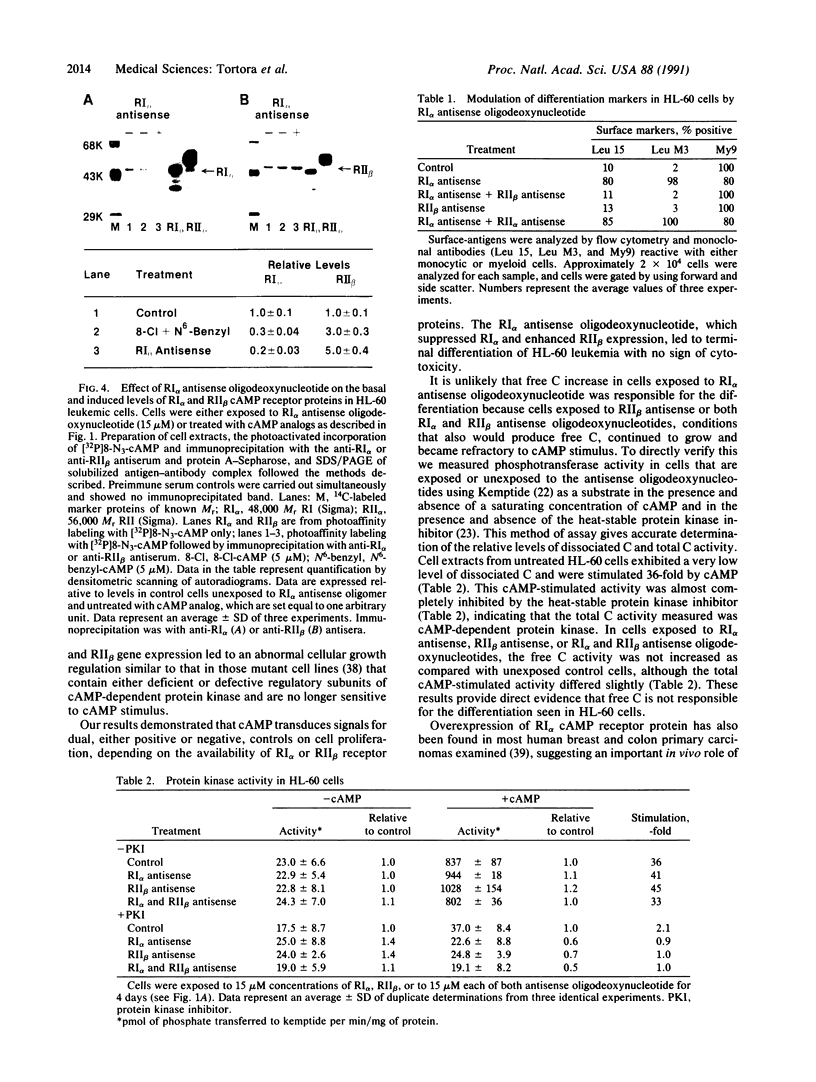
A marked decrease in the type I cAMP-dependent protein kinase regulatory subunit (RI alpha) and an increase in the type II protein kinase regulatory subunit (RII beta) correlate with growth inhibition and differentiation induced in a variety of types of human cancer cells, in vitro and in vivo, by site-selective cAMP analogs. To directly determine whether RI alpha is a growth-inducing protein essential for neoplastic cell growth, human HL-60 promyelocytic leukemia cells were exposed to 21-mer RI alpha antisense oligodeoxynucleotide, and the effects on cell replication and differentiation were examined. The RI alpha antisense oligomer brought about growth inhibition and monocytic differentiation, bypassing the effects of an exogenous cAMP analog. These effects of RI alpha antisense oligodeoxynucleotide correlated with a decrease in RI alpha receptor and an increase in RII beta receptor level. The growth inhibition and differentiation were abolished, however, when these cells were exposed simultaneously to both RI alpha and RII beta antisense oligodeoxynucleotides. The RII beta antisense oligodeoxynucleotide alone has been previously shown to specifically block the differentiation inducible by cAMP analogs. These results provide direct evidence that RI alpha cAMP receptor plays a critical role in neoplastic cell growth and that cAMP receptor isoforms display specific roles in cAMP regulation of cell growth and differentiation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bramson H. N., Kaiser E. T., Mildvan A. S. Mechanistic studies of cAMP-dependent protein kinase action. CRC Crit Rev Biochem. 1984;15(2):93–124. doi: 10.3109/10409238409102298. [DOI] [PubMed] [Google Scholar]
- Cheng H. C., van Patten S. M., Smith A. J., Walsh D. A. An active twenty-amino-acid-residue peptide derived from the inhibitor protein of the cyclic AMP-dependent protein kinase. Biochem J. 1985 Nov 1;231(3):655–661. doi: 10.1042/bj2310655. [DOI] [PMC free article] [PubMed] [Google Scholar]
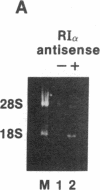
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cho-Chung Y. S., Clair T., Tagliaferri P., Ally S., Katsaros D., Tortora G., Neckers L., Avery T. L., Crabtree G. W., Robins R. K. Site-selective cyclic AMP analogs as new biological tools in growth control, differentiation, and proto-oncogene regulation. Cancer Invest. 1989;7(2):161–177. doi: 10.3109/07357908909038282. [DOI] [PubMed] [Google Scholar]
- Cho-Chung Y. S. Site-selective 8-chloro-cyclic adenosine 3',5'-monophosphate as a biologic modulator of cancer: restoration of normal control mechanisms. J Natl Cancer Inst. 1989 Jul 5;81(13):982–987. doi: 10.1093/jnci/81.13.982. [DOI] [PubMed] [Google Scholar]
- Clegg C. H., Cadd G. G., McKnight G. S. Genetic characterization of a brain-specific form of the type I regulatory subunit of cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3703–3707. doi: 10.1073/pnas.85.11.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin J. D., Keely S. L., Park C. R. The distribution and dissociation of cyclic adenosine 3':5'-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem. 1975 Jan 10;250(1):218–225. [PubMed] [Google Scholar]
- Dimitriu-Bona A., Burmester G. R., Waters S. J., Winchester R. J. Human mononuclear phagocyte differentiation antigens. I. Patterns of antigenic expression on the surface of human monocytes and macrophages defined by monoclonal antibodies. J Immunol. 1983 Jan;130(1):145–152. [PubMed] [Google Scholar]
- Ekanger R., Sand T. E., Ogreid D., Christoffersen T., Døskeland S. O. The separate estimation of cAMP intracellularly bound to the regulatory subunits of protein kinase I and II in glucagon-stimulated rat hepatocytes. J Biol Chem. 1985 Mar 25;260(6):3393–3401. [PubMed] [Google Scholar]
- Gottesman M. M. Genetic approaches to cyclic AMP effects in cultured mammalian cells. Cell. 1980 Nov;22(2 Pt 2):329–330. doi: 10.1016/0092-8674(80)90342-6. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Beavo J. A., Bechtel P. J., Krebs E. G. Comparison of adenosine 3':5'-monophosphate-dependent protein kinases from rabbit skeletal and bovine heart muscle. J Biol Chem. 1975 Oct 10;250(19):7795–7801. [PubMed] [Google Scholar]
- Hofmann F., Bechtel P. J., Krebs E. G. Concentrations of cyclic AMP-dependent protein kinase subunits in various tissues. J Biol Chem. 1977 Feb 25;252(4):1441–1447. [PubMed] [Google Scholar]
- Jahnsen T., Hedin L., Kidd V. J., Beattie W. G., Lohmann S. M., Walter U., Durica J., Schulz T. Z., Schiltz E., Browner M. Molecular cloning, cDNA structure, and regulation of the regulatory subunit of type II cAMP-dependent protein kinase from rat ovarian granulosa cells. J Biol Chem. 1986 Sep 15;261(26):12352–12361. [PubMed] [Google Scholar]
- Kemp B. E., Graves D. J., Benjamini E., Krebs E. G. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinase. J Biol Chem. 1977 Jul 25;252(14):4888–4894. [PubMed] [Google Scholar]
- Landay A., Gartland G. L., Clement L. T. Characterization of a phenotypically distinct subpopulation of Leu-2+ cells that suppresses T cell proliferative responses. J Immunol. 1983 Dec;131(6):2757–2761. [PubMed] [Google Scholar]
- Lee D. C., Carmichael D. F., Krebs E. G., McKnight G. S. Isolation of a cDNA clone for the type I regulatory subunit of bovine cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3608–3612. doi: 10.1073/pnas.80.12.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy F. O., Oyen O., Sandberg M., Taskén K., Eskild W., Hansson V., Jahnsen T. Molecular cloning, complementary deoxyribonucleic acid structure and predicted full-length amino acid sequence of the hormone-inducible regulatory subunit of 3'-5'-cyclic adenosine monophosphate-dependent protein kinase from human testis. Mol Endocrinol. 1988 Dec;2(12):1364–1373. doi: 10.1210/mend-2-12-1364. [DOI] [PubMed] [Google Scholar]
- Lohmann S. M., Walter U. Regulation of the cellular and subcellular concentrations and distribution of cyclic nucleotide-dependent protein kinases. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:63–117. [PubMed] [Google Scholar]
- Marcus-Sekura C. J. Techniques for using antisense oligodeoxyribonucleotides to study gene expression. Anal Biochem. 1988 Aug 1;172(2):289–295. doi: 10.1016/0003-2697(88)90447-2. [DOI] [PubMed] [Google Scholar]
- Otten A. D., McKnight G. S. Overexpression of the type II regulatory subunit of the cAMP-dependent protein kinase eliminates the type I holoenzyme in mouse cells. J Biol Chem. 1989 Dec 5;264(34):20255–20260. [PubMed] [Google Scholar]
- Oyen O., Myklebust F., Scott J. D., Hansson V., Jahnsen T. Human testis cDNA for the regulatory subunit RII alpha of cAMP-dependent protein kinase encodes an alternate amino-terminal region. FEBS Lett. 1989 Mar 27;246(1-2):57–64. doi: 10.1016/0014-5793(89)80253-4. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr Assays of protein kinase. Methods Enzymol. 1983;99:3–6. doi: 10.1016/0076-6879(83)99034-1. [DOI] [PubMed] [Google Scholar]
- Sandberg M., Taskén K., Oyen O., Hansson V., Jahnsen T. Molecular cloning, cDNA structure and deduced amino acid sequence for a type I regulatory subunit of cAMP-dependent protein kinase from human testis. Biochem Biophys Res Commun. 1987 Dec 31;149(3):939–945. doi: 10.1016/0006-291x(87)90499-2. [DOI] [PubMed] [Google Scholar]
- Schwartz D. A., Rubin C. S. Identification and differential expression of two forms of regulatory subunits (RII) of cAMP-dependent protein kinase II in Friend erythroleukemic cells. Differentiation and 8-bromo-cAMP elicit a large and selective increase in the rate of biosynthesis of only one type of RII. J Biol Chem. 1985 May 25;260(10):6296–6303. [PubMed] [Google Scholar]
- Scott J. D., Glaccum M. B., Zoller M. J., Uhler M. D., Helfman D. M., McKnight G. S., Krebs E. G. The molecular cloning of a type II regulatory subunit of the cAMP-dependent protein kinase from rat skeletal muscle and mouse brain. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5192–5196. doi: 10.1073/pnas.84.15.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showers M. O., Maurer R. A. A cloned bovine cDNA encodes an alternate form of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1986 Dec 15;261(35):16288–16291. [PubMed] [Google Scholar]
- Stein C. A., Cohen J. S. Oligodeoxynucleotides as inhibitors of gene expression: a review. Cancer Res. 1988 May 15;48(10):2659–2668. [PubMed] [Google Scholar]
- Talle M. A., Rao P. E., Westberg E., Allegar N., Makowski M., Mittler R. S., Goldstein G. Patterns of antigenic expression on human monocytes as defined by monoclonal antibodies. Cell Immunol. 1983 May;78(1):83–99. doi: 10.1016/0008-8749(83)90262-9. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Griffin J. D., Ritz J., Nadler L. M., Abrams T., Schlossman S. F. Expression of normal monocyte-macrophage differentiation antigens on HL60 promyelocytes undergoing differentiation induced by leukocyte-conditioned medium or phorbol diester. Leuk Res. 1981;5(6):491–495. doi: 10.1016/0145-2126(81)90119-3. [DOI] [PubMed] [Google Scholar]
- Tortora G., Clair T., Cho-Chung Y. S. An antisense oligodeoxynucleotide targeted against the type II beta regulatory subunit mRNA of protein kinase inhibits cAMP-induced differentiation in HL-60 leukemia cells without affecting phorbol ester effects. Proc Natl Acad Sci U S A. 1990 Jan;87(2):705–708. doi: 10.1073/pnas.87.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortora G., Clair T., Katsaros D., Ally S., Colamonici O., Neckers L. M., Tagliaferri P., Jahnsen T., Robins R. K., Cho-Chung Y. S. Induction of megakaryocytic differentiation and modulation of protein kinase gene expression by site-selective cAMP analogs in K-562 human leukemic cells. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2849–2852. doi: 10.1073/pnas.86.8.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortora G., Tagliaferri P., Clair T., Colamonici O., Neckers L. M., Robins R. K., Cho-Chung Y. S. Site-selective cAMP analogs at micromolar concentrations induce growth arrest and differentiation of acute promyelocytic, chronic myelocytic, and acute lymphocytic human leukemia cell lines. Blood. 1988 Jan;71(1):230–233. [PubMed] [Google Scholar]
- Uhler M. D., Carmichael D. F., Lee D. C., Chrivia J. C., Krebs E. G., McKnight G. S. Isolation of cDNA clones coding for the catalytic subunit of mouse cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1300–1304. doi: 10.1073/pnas.83.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhler M. D., Chrivia J. C., McKnight G. S. Evidence for a second isoform of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1986 Nov 25;261(33):15360–15363. [PubMed] [Google Scholar]
- Uhler M. D., McKnight G. S. Expression of cDNAs for two isoforms of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1987 Nov 5;262(31):15202–15207. [PubMed] [Google Scholar]