Abstract
Experimental evidence suggests that the let-7 family of noncoding RNAs suppresses adaptive immune responses, contributing to immune evasion by the tumor. We hypothesized that the amount of let-7a and let-7b expression in colorectal carcinoma might be associated with limited T-lymphocyte infiltrates in the tumor microenvironment and worse clinical outcome. Utilizing the molecular pathological epidemiology resources of 795 rectal and colon cancers in two U.S.-nationwide prospective cohort studies, we measured tumor-associated let-7a and let-7b expression levels by quantitative reverse-transcription PCR, and CD3+, CD8+, CD45RO (PTPRC)+, and FOXP3+ cell densities by tumor tissue microarray immunohistochemistry and computer-assisted image analysis. Logistic regression analysis and Cox proportional hazards regression were used to assess associations of let-7a (and let-7b) expression (quartile predictor variables) with T-cell densities (binary outcome variables) and mortality, respectively, controlling for tumor molecular features, including microsatellite instability, CpG island methylator phenotype, LINE-1 methylation, and KRAS, BRAF, and PIK3CA mutations. Compared with cases in the lowest quartile of let-7a expression, those in the highest quartile were associated with lower densities of CD3+ [multivariate odds ratio (OR), 0.40; 95% confidence interval (CI), 0.23 to 0.67; Ptrend = 0.003] and CD45RO+ cells (multivariate OR, 0.31; 95% CI, 0.17 to 0.58; Ptrend = 0.0004), and higher colorectal cancer-specific mortality (multivariate hazard ratio, 1.82; 95% CI, 1.42 to 3.13; Ptrend = 0.001). In contrast, let-7b expression was not significantly associated with T-cell density or colorectal cancer prognosis. Our data support the role of let-7a in suppressing antitumor immunity in colorectal cancer, and suggest let-7a as a potential target of immunotherapy.
Keywords: colorectum, epigenetics, molecular pathological epidemiology, noncoding RNA
INTRODUCTION
Accumulating evidence indicates that tumor molecular pathologic changes elicit immune reactions and that immune cells in the tumor microenvironment influence tumor evolution (1–3). Hence, tumor cells need to evade antitumor immune response. In colorectal cancer, the degree of infiltration of T lymphocytes, such as CD8+ cytotoxic T cells and CD45RO (PTPRC)+ effector memory T cells, has been associated with high-level microsatellite instability (MSI-high) (4,5) and favorable prognosis (5–7). Therapeutic antibodies against immune checkpoint molecules, including CTLA-4, PDCD1 (programmed cell death 1; PD-1), and CD274 (PDCD1 ligand 1; PD-L1), can enhance antitumor T-cell activity and improve clinical outcome in various tumor types (8). Immune checkpoint blockade has been found to be effective in the MSI-high subtype of colorectal cancer (9). MSI-high colorectal cancers generate immunogenic neoantigens that can elicit lymphocytic immune response (10), resulting in upregulation of multiple immune checkpoint pathways (11). Beyond MSI status and neoantigen load, it is of interest to identify other factors that influence immune cell infiltration in colorectal cancer.
MicroRNAs are small noncoding RNAs that epigenetically regulate diverse biological and pathological processes, including immune response and tumor progression (12). It has been shown that synthetic oligonucleotides can be delivered into cells or tissues to modulate the function of endogenous microRNAs, and small-molecules may modify the expression or function of microRNAs (13). Through a novel delivery system that can target the tumor microenvironment, antisense oligomers have been shown to reduce tumor volumes of miR155-addicted lymphoma in mice, demonstrating the therapeutic potential of microRNA targeting (14). Our group has shown that tumor expression of microRNA MIR21 is inversely associated with densities of CD3+ and CD45RO+ T cells in colorectal cancer tissue (15). In addition, the let-7 microRNA family has been shown to play an important role in host immunity (16–18). Experimental evidence suggests that let-7 suppresses TLR4-mediated immune activation during infection, and that downregulation of let-7 expression restores immune response against pathogens (16,17). In addition, decreased let-7 expression is associated with the self-renewal of memory T cells (18). Although tumor-associated let-7 expression has been associated with patient survival in lung (19), ovarian (20,21), and other cancers, its prognostic association in colorectal cancer remains unclear. In human, let-7a and let-7b together account for the vast majority of let-7 RNA molecules present in colonic epithelia (22). Therefore, we hypothesized that let-7a or let-7b expression might be associated with a lower density of T cells in colorectal cancer tissue and worse clinical outcome.
To test our hypotheses, we examined let-7a and let-7b expression in colorectal cancer tissues from two U.S.-nationwide prospective cohort studies, the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS), in relation to CD3+, CD8+, CD45RO+, and FOXP3+ T-cell densities in tumor tissue, and colorectal cancer mortality.
METHODS
Study population
We utilized the databases of two U.S.-nationwide prospective cohort studies, the NHS (121,701 women followed since 1976) and the HPFS (51,529 men followed since 1986) (23,24). In both cohorts, follow-up questionnaires were sent at baseline and biennially thereafter to collect and update lifestyle and health-related information, and to identify newly diagnosed diseases. Study physicians reviewed medical records and assigned causes of death. The National Death Index was used to ascertain deaths of study participants and identify unreported fatal colorectal cancer cases. Formalin-fixed paraffin-embedded (FFPE) tissue blocks were collected from hospitals where participants with colorectal cancer had undergone tumor resection. Hematoxylin and eosin-stained tissue sections of all colorectal cancer cases were reviewed by a pathologist (S.O.), who was unaware of other data. Tumor differentiation was categorized as well, as moderate vs. poor (>50% vs. ≤50% glandular area). Based on the availability of follow-up data and adequate tissue specimens for analysis, 795 colorectal cancer cases (diagnosed up to 2008) were included. Written informed consent was obtained from all study participants. Tumor tissue was macro-dissected from FFPE specimens in all cases. Tissue collection and analyses were approved by the human subjects committee at the Harvard T.H. Chan School of Public Health and the Brigham and Women’s Hospital (Boston, MA, USA).
Quantitative reverse-transcription PCR for let-7a and let-7b
RNA was extracted with RecoverAll Total Nucleic Acid Isolation Kit (Ambion, Austin, TX), and cDNA was synthesized with miScript II RT Kit (Qiagen, Valencia, CA). Quantitative PCR was performed using the miScript PCR System (Qiagen) with specific miScript Primer Assays for let-7a (catalog number, MS00031220), let-7b (catalog number, MS00003122), and RNU6-2 (catalog number MS00033740). Amplification was performed in duplicate with the StepOnePlus Real-Time PCR Systems (Applied Biosystems) under the following conditions: 15 minutes at 95°C and 40 cycles of 15 seconds at 94°C, 30 seconds at 55°C, and 30 seconds at 70°C. Spearman’s rank-correlation coefficient between the duplicate cycle threshold (Ct) values was 0.99 for let-7a, 0.97 for let-7b, and 0.98 for RNU6-2.
In our validation study, the Ct values for let-7a, let-7b, and RNU6-2 increased linearly with serial 10-fold dilution of cDNA from the same tumor specimens (R2 ≥ 0.98; Supplementary Fig. S1). The interassay coefficient of variation of Ct values from the same tumor specimens in four different batches was 1.2% for let-7a, 1.4% for let-7b, and 1.0% for RNU6-2 (Supplementary Table S1). let-7a and let-7b expression was normalized with RNU6-2 using the 2−ΔCt method (where ΔCt = “the average Ct value of let-7a or let-7b” – “the average Ct value of RNU6-2”) (25).
Analysis of T-cell densities in tumors
Tissue microarray was constructed to assess the densities of CD3+, CD8+, CD45RO (PTPRC)+, and FOXP3+ T cells in tumors with immunohistochemistry techniques (5). We used an automated scanning microscope and the Ariol image analysis system (Genetix, San Jose, CA) to quantify T-cell density in tissue microarray cores as previously described (5). The median value was used to dichotomize the density of CD3+, CD8+, CD45RO+, or FOXP3+ T cells.
Analyses of BRAF, KRAS, and PIK3CA mutations and other molecular features
DNA was extracted from archival FFPE cancer tissue blocks. PCR and pyrosequencing for BRAF (codon 600) (26), KRAS (codons 12, 13, 61 and 146) (27,28), and PIK3CA (exons 9 and 20) (29) were performed as previously described. Microsatellite instability (MSI) status was assessed using 10 markers (D2S123, D5S346, D17S250, BAT25, BAT26, BAT40, D18S55, D18S56, D18S67, and D18S487) as previously (30). We defined MSI-high as the presence of instability in ≥30% of the markers, and MSI-low/microsatellite stable (MSS) as instability in <30% of the markers. Analyses of long interspersed nucleotide element-1 (LINE-1) methylation (31,32) and eight CpG island methylator phenotype (CIMP)-specific loci (CACNA1G, CDKN2A, CRABP1, IGF2, MLH1, NEUROG1, RUNX3, and SOCS1) (30) were performed.
Laser capture microdissection
To investigate let-7 expression in tumor cells separately from stromal cells, 10 cases were selected, and paired tumor cells and tumor stromal tissues were obtained, using the Arcturus XT LCM system (Applied Biosystems, San Diego, CA). Sections were cut to 8 μm thickness, and placed on Arcturus polyethylene naphthalate membrane frame slides (catalog number, LCM0521) and allowed to dry. Based on histopathological review, tumor cells and adjacent stromal areas were separately micro-dissected and captured onto CapSure Macro LCM Caps (catalog number, LCM0211).
Statistical analysis
Neither tumor-associated let-7 expression nor log-transformed values of let-7 expression fit a normal distribution in the Kolmogorov-Smirnov test for normality (P ≤ 0.01). Thus, we tested our primary hypotheses using a statistical trend test across the ordinal quartiles of let-7 expression as a continuous variable. For our first primary hypothesis that let-7 expression might be inversely associated with immune response, we used a logistic regression model to test the association of let-7a or let-7b expression (an ordinal quartile predictor variable) with T-cell densities (a binary outcome variable). The densities of CD3+, CD8+, CD45RO (PTPRC)+, and FOXP3+ T cells in tumors were dichotomized at the median value. Considering multiple comparisons (using four T-cell variables for each of let-7a and let-7b), the α level was adjusted to 0.006 (= 0.05/8) by simple Bonferroni correction. To control for confounding, multivariate logistic regression model initially included age (continuous), sex, year of diagnosis (continuous), family history of colorectal cancer, tumor location (proximal colon vs. distal colon vs. rectum), MSI (high vs. MSI-low/MSS), CIMP (high vs. low/negative), KRAS (mutant vs. wild-type), BRAF (mutant vs. wild-type), PIK3CA (mutant vs. wild-type), and LINE-1 methylation level (continuous). A backward stepwise elimination with a threshold of P = 0.05 was used to select covariates in the final models. For cases missing any categorical covariate, such as family history (0.4%), tumor location (0.4%), MSI (3.9%), CIMP (8.6%), KRAS (2.6%), BRAF (2.9%), and PIK3CA (9.7%), we included those cases in a majority category of the covariate.
For the second primary hypothesis that let-7 expression in colorectal cancer tissue might be associated with higher mortality, we used a Cox proportional hazards regression model to assess the association of let-7a or let-7b expression (an ordinal quartile predictor variable) with colorectal cancer mortality. Deaths from causes other than colorectal cancer were censored for cancer-specific mortality. Considering multiple comparisons (cancer-specific mortality and overall mortality for each of let-7a and let-7b), the α level was adjusted to 0.012 (= 0.05/4) by simple Bonferroni correction. To control for confounding, the multivariate Cox proportional hazards regression model initially included the same set of covariates as mentioned above, and we used the same backward stepwise elimination procedure to select covariates for the final model.
All other analyses for clinical, pathological, and molecular associations were secondary exploratory analyses, and we adjusted the two-sided α level to 0.003 (= 0.05/15) by simple Bonferroni correction for multiple comparisons. To assess associations between the ordinal categories (first to fourth quartile) of tumor-associated let-7a and let-7b expression and categorical data, the chi-square test was performed. To compare mean age and mean LINE-1 methylation levels, an analysis of variance assuming equal variances was performed. Because of a potential association between let-7 and disease stage, multivariate logistic regression analysis was performed to control for confounding. We used let-7a or let-7b expression as an ordinal quartile predictor variable and disease stage as a binary outcome variable (stage I-II vs. stage III-IV), with the same set of initial covariates and the same model construction procedure as mentioned above. We used the Wilcoxon signed rank test to compare the expression of let-7a or let-7b in colorectal cancer cells vs. tumor stromal cells, and in tumor tissues vs. adjacent mucosal tissues. We used the SAS program (Version 9.3, SAS Institute, Cary, NC) for all statistical analyses. All P values were two-sided.
RESULTS
Expression levels of let-7a and let-7b in colorectal cancer tissue
We measured tumor-associated let-7a and let-7b expression by quantitative reverse-transcription PCR assay on 795 colorectal cancer cases within the NHS and the HPFS. Table 1 and Supplementary Table S2 show clinical, pathological, and molecular characteristics of the 795 cases according to let-7a and let-7b expression levels, respectively. Levels of let-7a expression might be associated with disease stage, but statistical power was limited when we conducted a 4×4 chi-square test (P = 0.10). To assess a statistical trend of this association and control for confounding, we used logistic regression analysis and assessed the association of let-7a or let-7b expression as a quartile predictor variable with disease stage as a binary outcome variable (stage I–II vs. stage III–IV; Supplementary Table S3). Higher levels of let-7a or let-7b expression might be associated with stage III–IV disease (Ptrend = 0.005 or 0.0005, respectively, with adjusted α level of 0.003).
Table 1.
Clinical, pathological, and molecular characteristics according to let-7a expression in 795 colorectal cancer tissues
|
let-7a expression
|
||||||
|---|---|---|---|---|---|---|
| Characteristica | Total (795) | Q1 (198) | Q2 (199) | Q3 (199) | Q4 (199) | Pb |
| Age | ||||||
| Mean ± SD (year) | 68.6 ± 8.7 | 69.3 ± 8.5 | 69.1 ± 8.9 | 67.7 ± 8.6 | 68.4 ± 8.7 | 0.26 |
| Sex | 0.49 | |||||
| Men | 348 (44%) | 86 (43%) | 94 (47%) | 89 (45%) | 79 (40%) | |
| Women | 447 (56%) | 112 (57%) | 105 (53%) | 110 (55%) | 120 (60%) | |
| BMI (kg/m2) | 0.30 | |||||
| <25 | 317 (40%) | 78 (40%) | 87 (44%) | 83 (42%) | 69 (35%) | |
| 25–30 | 315 (40%) | 75 (38%) | 81 (41%) | 78 (39%) | 81 (41%) | |
| >30 | 161 (20%) | 44 (22%) | 31 (16%) | 37 (19%) | 49 (25%) | |
| Year of diagnosis | 0.01 | |||||
| Prior to 1998 | 374 (47%) | 85 (43%) | 113 (57%) | 91 (46%) | 85 (43%) | |
| 1998 to 2008 | 421 (53%) | 113 (57%) | 86 (43%) | 108 (54%) | 114 (57%) | |
| Family history of colorectal cancer | 0.82 | |||||
| Absent | 631 (80%) | 155 (79%) | 156 (78%) | 157 (80%) | 163 (82%) | |
| Present | 161 (20%) | 42 (21%) | 43 (22%) | 40 (20%) | 36 (18%) | |
| Tumor location | 0.38 | |||||
| Cecum | 139 (17%) | 35 (18%) | 40 (20%) | 33 (17%) | 31 (16%) | |
| Ascending to transverse colon | 251 (32%) | 62 (31%) | 51 (26%) | 72 (36%) | 66 (33%) | |
| Splenic flexure to sigmoid | 223 (28%) | 53 (27%) | 58 (29%) | 48 (24%) | 64 (32%) | |
| Rectosigmoid and rectum | 179 (23%) | 47 (24%) | 50 (25%) | 45 (23%) | 37 (19%) | |
| No. of negative lymph nodes | 0.42 | |||||
| Median (interquartile range) | 7 (0–12) | 7.5 (0–14) | 7 (2–11) | 6 (0–12) | 7 (0–12) | |
| Disease stage | 0.10 | |||||
| I | 183 (24%) | 52 (28%) | 52 (28%) | 41 (22%) | 38 (20%) | |
| II | 242 (32%) | 70 (37%) | 58 (31%) | 60 (32%) | 54 (29%) | |
| III | 221 (30%) | 48 (26%) | 54 (29%) | 61 (33%) | 58 (31%) | |
| IV | 104 (14%) | 18 (10%) | 24 (13%) | 25 (13%) | 37 (20%) | |
| Tumor differentiation | 0.65 | |||||
| Well to moderate | 721 (91%) | 183 (93%) | 179 (90%) | 181 (91%) | 178 (89%) | |
| Poor | 73 (9%) | 14 (7%) | 20 (10%) | 18 (9%) | 21 (11%) | |
| MSI status | 0.37 | |||||
| MSI-low/MSS | 648 (85%) | 162 (86%) | 164 (84%) | 155 (82%) | 167 (88%) | |
| MSI-high | 116 (15%) | 27 (14%) | 31 (16%) | 35 (18%) | 23 (12%) | |
| CIMP status | 0.34 | |||||
| Low/negative | 595 (82%) | 144 (84%) | 154 (81%) | 148 (80%) | 149 (82%) | |
| High | 132 (18%) | 28(16%) | 35 (19%) | 36 (20%) | 33 (18%) | |
| BRAF mutation | 0.04 | |||||
| (−) | 650 (84%) | 166 (88%) | 173 (88%) | 155 (80%) | 156 (81%) | |
| (+) | 122 (16%) | 23 (12%) | 23 (12%) | 38 (20%) | 38 (19%) | |
| KRAS mutation | 0.56 | |||||
| (−) | 459 (60%) | 114 (60%) | 109 (56%) | 121 (63%) | 115 (60%) | |
| (+) | 308 (40%) | 76 (40%) | 85 (44%) | 70 (37%) | 77 (40%) | |
| PIK3CA mutation | 0.52 | |||||
| (−) | 595 (83%) | 155 (86%) | 143 (80%) | 152 (83%) | 145 (82%) | |
| (+) | 123 (17%) | 25 (14%) | 35 (20%) | 31 (17%) | 32 (18%) | |
| LINE-1 methylation level | 0.63 | |||||
| Mean ± SD (%) | 62.2 ± 9.3 | 62.0 ± 8.0 | 61.7 ± 9.6 | 62.1 ± 9.5 | 62.9 ± 10.0 | |
Percentage indicates the proportion of cases with a specific clinical, pathological, or tumor molecular feature in patients with each expression level of let-7a. There were cases that had missing values for any of the characteristics except for age, sex and year of diagnosis.
To assess associations between the ordinal categories (first to fourth quartile) of let-7a expression and categorical data, the chi-square test was performed. To compare mean age and mean LINE-1 methylation levels, the analysis of variance was performed. We adjusted two-sided α level to 0.003 (= 0.05/15) by simple Bonferroni correction.
BMI, body mass index; Q1 to Q4, quartile 1 (lowest) to quartile 4 (highest); SD, standard deviation.
let-7a and let-7b expression and T-cell density in colorectal cancer tissue
We measured the densities of T cells in colorectal cancer tissue by immunohistochemistry and image analysis. Pairwise correlations between the densities of CD3+, CD8+, CD45RO+, and FOXP3+ T cells were significant except between FOXP3+ and CD3+ cells and between FOXP3+ and CD8+ T cells (Supplementary Table S4).
Table 2 shows the distribution of colorectal cancer cases according to the densities of T cells (quartiles) and let-7a or let-7b expression (quartiles) in colorectal cancer tissue. In our primary hypothesis testing, we conducted univariate and multivariate logistic regression analyses to assess the associations of let-7a or let-7b expression as an ordinal quartile predictor variable with the density of CD3+, CD8+, CD45RO+, or FOXP3+ T cells in colorectal cancer tissue as a binary outcome variable (Table 3). let-7a expression was inversely associated with the densities of CD3+ and CD45RO+ T cells in multivariate analyses (multivariate Ptrend = 0.003 and 0.0004, respectively, with adjusted α level at 0.006). Compared with cases in the lowest quartile of let-7a expression, those in the highest quartile were associated with lower density of CD3+ T cells [multivariate OR, 0.40; 95% confidence interval (CI), 0.23 to 0.67] and CD45RO+ T cells (multivariate OR, 0.31; 95% CI, 0.17 to 0.58). In contrast, let-7b expression was not significantly associated with any of the T-cell densities (all multivariate Ptrend ≥ 0.04 with adjusted α level at 0.006).
Table 2.
Distribution of cases according to let-7a and let-7b expression and T-cell densities in colorectal cancer tissue
|
let-7a expression
|
let-7b expression
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T-cell density | Total No. | Q1 | Q2 | Q3 | Q4 | Ptrenda | Q1 | Q2 | Q3 | Q4 | Ptrenda |
| CD3+ cell density | 0.004 | 0.18 | |||||||||
| Q1 | 116 (23%) | 19 (16%) | 33 (24%) | 27 (21%) | 37 (28%) | 33 (26%) | 21 (16%) | 31 (23%) | 30 (25%) | ||
| Q2 | 121 (23%) | 24 (20%) | 32 (24%) | 26 (20%) | 39 (30%) | 37 (30%) | 33 (25%) | 23 (17%) | 28 (23%) | ||
| Q3 | 144 (28%) | 41 (34%) | 33 (24%) | 36 (28%) | 34 (26%) | 29 (23%) | 43 (33%) | 38 (29%) | 34 (28%) | ||
| Q4 | 133 (26%) | 35 (29%) | 37 (27%) | 40 (31%) | 21 (16%) | 26 (21%) | 34 (26%) | 42 (31%) | 30 (24%) | ||
| CD8+ cell density | 0.010 | 0.008 | |||||||||
| Q1 | 130 (26%) | 30 (25%) | 27 (21%) | 32 (25%) | 41 (32%) | 40 (32%) | 35 (27%) | 32 (25%) | 23 (19%) | ||
| Q2 | 129 (25%) | 26 (22%) | 31 (24%) | 34 (27%) | 38 (29%) | 31 (25%) | 38 (30%) | 30 (23%) | 29 (24%) | ||
| Q3 | 127 (25%) | 34 (28%) | 41 (32%) | 27 (21%) | 25 (19%) | 26 (21%) | 29 (23%) | 34 (26%) | 37 (30%) | ||
| Q4 | 120 (24%) | 30 (25%) | 31 (24%) | 34 (27%) | 25 (19%) | 27 (22%) | 26 (20%) | 34 (26%) | 33 (27%) | ||
| CD45RO+ cell density | 0.001 | 0.05 | |||||||||
| Q1 | 133 (26%) | 20 (17%) | 34 (25%) | 29 (22%) | 50 (38%) | 28 (22%) | 31 (23%) | 39 (29%) | 34 (28%) | ||
| Q2 | 131 (25%) | 22 (19%) | 38 (28%) | 33 (25%) | 38 (29%) | 32 (26%) | 36 (27%) | 27 (20%) | 36 (30%) | ||
| Q3 | 126 (24%) | 33 (28%) | 33 (24%) | 37 (28%) | 23 (17%) | 34 (27%) | 27 (20%) | 31 (23%) | 34 (28%) | ||
| Q4 | 128 (25%) | 43 (36%) | 30 (22%) | 34 (26%) | 21 (16%) | 32 (25%) | 39 (30%) | 39 (28%) | 17 (14%) | ||
| FOXP3+ cell density | 0.88 | 0.40 | |||||||||
| Q1 | 126 (26%) | 29 (26%) | 32 (26%) | 30 (24%) | 35 (27%) | 31 (26%) | 32 (26%) | 30 (24%) | 32 (27%) | ||
| Q2 | 130 (26%) | 30 (27%) | 33 (26%) | 36 (29%) | 31 (24%) | 32 (27%) | 30 (24%) | 29 (23%) | 38 (32%) | ||
| Q3 | 121 (25%) | 29 (26%) | 28 (22%) | 31 (25%) | 33 (25%) | 30 (25%) | 29 (23%) | 32 (25%) | 30 (26%) | ||
| Q4 | 114 (23%) | 25 (22%) | 32 (26%) | 26 (21%) | 31 (24%) | 27 (22%) | 34 (27%) | 35 (28%) | 18 (15%) | ||
Ptrend value was calculated by the linear trend test across the ordinal (first to fourth quartile) categories of let-7a or let-7b expression as a continuous variable in the univariate ordinal logistic regression model for the density of CD3+, CD8+, CD45RO+, or FOXP3+ cells (an ordinal quartile outcome variable). We adjusted two-sided α level to 0.006 (= 0.05/8) by simple Bonferroni correction.
Q1 to Q4, quartile 1 (lowest) to quartile 4 (highest).
Table 3.
let-7a and let-7b expression and T-cell densities in colorectal cancer tissue
| let-7a expression | Univariate OR (95% CI) | Multivariate OR (95% CI) | let-7b expression | Univariate OR (95% CI) | Multivariate OR (95% CI) |
|---|---|---|---|---|---|
| Model of CD3+ cell density as a binary outcome (n = 514)a | |||||
| Q1 | 1 (reference) | 1 (reference) | Q1 | 1 (reference) | 1 (reference) |
| Q2 | 0.61 (0.37–1.01) | 0.62 (0.37–1.03) | Q2 | 1.82 (1.11–2.98) | 1.75 (1.06–2.88) |
| Q3 | 0.81 (0.49–1.36) | 0.82 (0.49–1.39) | Q3 | 1.89 (1.15–3.09) | 1.67 (1.01–2.78) |
| Q4 | 0.41 (0.25–0.68) | 0.40 (0.23–0.67) | Q4 | 1.40 (0.85–2.32) | 1.30 (0.78–2.18) |
| Ptrendb | 0.004 | 0.003 | Ptrendb | 0.18 | 0.40 |
| Model of CD8+ cell density as a binary outcome (n = 506)a | |||||
| Q1 | 1 (reference) | 1 (reference) | Q1 | 1 (reference) | 1 (reference) |
| Q2 | 1.08 (0.66–1.79) | 1.05 (0.63–1.76) | Q2 | 1.01 (0.61–1.66) | 1.03 (0.61–1.72) |
| Q3 | 0.81 (0.49–1.33) | 0.77 (0.46–1.29) | Q3 | 1.47 (0.90–2.41) | 1.47 (0.88–2.47) |
| Q4 | 0.55 (0.34–0.92) | 0.54 (0.32–0.91) | Q4 | 1.80 (1.09–2.99) | 1.59 (0.94–2.69) |
| Ptrendb | 0.010 | 0.008 | Ptrendb | 0.008 | 0.04 |
| Model of CD45RO+ cell density as a binary outcome (n = 518)a | |||||
| Q1 | 1 (reference) | 1 (reference) | Q1 | 1 (reference) | 1 (reference) |
| Q2 | 0.50 (0.29–0.87) | 0.50 (0.28–0.88) | Q2 | 1.22 (0.71–2.11) | 1.34 (0.76–2.36) |
| Q3 | 0.60 (0.35–1.03) | 0.52 (0.30–0.92) | Q3 | 1.18 (0.68–2.04) | 1.32 (0.75–2.31) |
| Q4 | 0.33 (0.18–0.60) | 0.31 (0.17–0.58) | Q4 | 0.48 (0.25–0.92) | 0.52 (0.27–1.00) |
| Ptrendb | 0.001 | 0.0004 | Ptrendb | 0.05 | 0.09 |
| Model of FOXP3+ cell density as a binary outcome (n = 491)a | |||||
| Q1 | 1 (reference) | 1 (reference) | Q1 | 1 (reference) | 1 (reference) |
| Q2 | 1.01 (0.61–1.68) | 1.07 (0.64–1.81) | Q2 | 1.12 (0.68–1.85) | 1.09 (0.65–1.83) |
| Q3 | 0.94 (0.57–1.57) | 0.89 (0.53–1.51) | Q3 | 1.26 (0.76–2.07) | 1.17 (0.70–1.96) |
| Q4 | 1.06 (0.64–1.76) | 1.00 (0.60–1.68) | Q4 | 0.76 (0.45–1.27) | 0.77 (0.46–1.32) |
| Ptrendb | 0.88 | 0.84 | Ptrendb | 0.40 | 0.43 |
The multivariate logistic regression model initially included age, sex, year of diagnosis, family history of colorectal cancer in parent or sibling, tumor location, microsatellite instability, CpG island methylator phenotype, KRAS, BRAF, and PIK3CA mutations, and LINE-1 methylation level. A backward stepwise elimination with a threshold of P < 0.05 was used to select variables in the final models.
Ptrend was calculated by the linear trend test across the ordinal categories (first to fourth quartile) of let-7a or let-7b expression as a continuous variable in the logistic regression model for the density of CD3+, CD8+, CD45RO+, or FOXP3+ cells as a binary outcome variable. We adjusted two-sided α level to 0.006 (= 0.05/8) by simple Bonferroni correction.
CI, confidence interval; Q1 to Q4, quartile 1 (lowest) to quartile 4 (highest).
Tumor-associated let-7a and let-7b expression and colorectal cancer mortality
During a median follow-up of 13.5 years for censored cases, 445 deaths including 242 colorectal cancer-specific deaths were documented. In the second primary hypothesis test (Table 4), let-7a expression was positively associated with cancer-specific mortality (multivariate Ptrend = 0.001). Multivariate hazard ratio of the highest vs. lowest quartile of let-7a for cancer-specific mortality was 1.82 (95% CI, 1.42–3.13). We observed similar findings for the 518 cases with density of CD45RO+ cells available (Table S5). In contrast, let-7b expression was not associated with cancer-specific mortality (multivariate Ptrend = 0.44). In Kaplan-Meier analysis (Fig. 1), higher let-7a expression was associated with shorter colorectal cancer-specific survival (P = 0.003).
Table 4.
let-7a and let-7b expression in colorectal cancer tissue and survival
| Cancer-specific mortality
|
Overall mortality
|
||||||
|---|---|---|---|---|---|---|---|
| let-7 expression | No. of cases | No. of events | Univariate HR (95% CI) | Multivariate HRa (95% CI) | No. of events | Univariate HR (95% CI) | Multivariate HRa (95% CI) |
| let-7a (n = 795) | |||||||
| Q1 | 198 | 43 | 1 (reference) | 1 (reference) | 96 | 1 (reference) | 1 (reference) |
| Q2 | 199 | 57 | 1.38 (0.93–2.05) | 1.38 (0.93–2.05) | 120 | 1.28 (0.98–1.68) | 1.34 (1.02–1.75) |
| Q3 | 199 | 66 | 1.63 (1.13–2.45) | 1.66 (1.48–3.25) | 115 | 1.31 (1.00–1.71) | 1.41 (1.08–1.86) |
| Q4 | 199 | 76 | 1.97 (1.25–2.66) | 1.82 (1.42–3.13) | 114 | 1.35 (1.03–1.77) | 1.39 (1.06–1.84) |
| Ptrendb | 0.0002 | 0.001 | 0.04 | 0.02 | |||
| let-7b (n = 792) | |||||||
| Q1 | 198 | 52 | 1 (reference) | 1 (reference) | 109 | 1 (reference) | 1 (reference) |
| Q2 | 198 | 63 | 1.26 (0.87–1.82) | 1.30 (0.90–1.88) | 114 | 1.31 (0.87–1.47) | 1.17 (0.90–1.52) |
| Q3 | 198 | 70 | 1.45 (1.01–2.08) | 1.53 (1.07–2.20) | 114 | 1.08 (0.96–1.63) | 1.26 (0.97–1.64) |
| Q4 | 198 | 56 | 1.08 (0.74–1.58) | 1.11 (0.76–1.62) | 105 | 1.01 (0.77–1.32) | 1.00 (0.77–1.32) |
| Ptrendb | 0.53 | 0.44 | 0.76 | 0.84 | |||
The multivariate Cox regression model initially included sex, age, year of diagnosis, family history of colorectal cancer in parent or sibling, microsatellite instability, CpG island methylator phenotype, KRAS, BRAF, and PIK3CA mutations, and LINE-1 methylation level. A backward stepwise elimination with a threshold of P < 0.05 was used to select variables in the final models.
Ptrend was calculated by the linear trend test across the ordinal categories (first to fourth quartile) of let-7a or let-7b expression as a continuous variable in the Cox regression model for cancer-specific mortality and overall mortality. We adjusted two-sided α level to 0.012 (= 0.05/4) by simple Bonferroni correction.
CI, confidence interval; HR, hazard ratio; Q1 to Q4, quartile 1 (lowest) to quartile 4 (highest).
Figure 1.
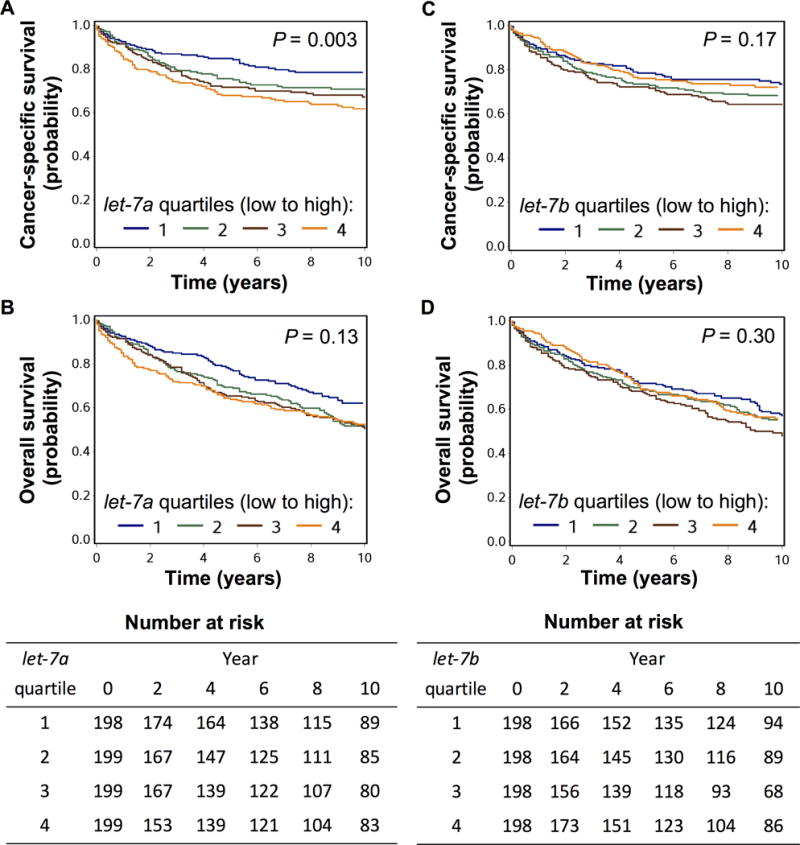
Kaplan-Meier curve for cancer-specific survival and overall survival classified by quartiles of let-7a (A, B) or let-7b expression (C, D) in colorectal cancer tissue. Expression level increases from quartile 1 to quartile 4. Statistical analyses were performed using log rank test.
let-7a and let-7b in cancer, stromal, and adjacent nontumor cells of colonic mucosa
To determine whether let-7 microRNAs might be expressed in colorectal cancer cells and/or cells in tumor stroma, we microdissected tumor cells and tumor stromal tissues in 10 selected cases, using a laser capture microdissection (LCM) technique. Expression let-7a and let-7b in tumor stroma were on average approximately 4 times higher than in paired tumor cells (Supplementary Fig. S2A and B, Wilcoxon signed rank test, P = 0.002 and P = 0.004 for let-7a and let-7b, respectively).
We macrodissected tumor tissue and adjacent nontumor colonic mucosa tissue in 52 colorectal cancer cases. Expression of let-7a and let-7b was generally lower in tumor tissue than in paired adjacent nontumor colonic mucosa (Supplementary Fig. S2C and D, Wilcoxon signed rank test, P < 0.0001 and P = 0.0003 for let-7a and let-7b, respectively).
DISCUSSION
We conducted this study to test the hypothesis that let-7a or let-7b expression might be inversely associated with T-cell densities in colorectal cancer tissue. We found the inverse association between let-7a expression levels and the densities of CD3+ cells and CD45RO+ T cells in colorectal cancer tissue. We also found that tumor-associated let-7a expression was positively associated with colorectal cancer-specific mortality.
The let-7 miRNA family has been reported to regulate immune activation in various cell types. In human epithelial cells, let-7 suppresses immune responses to pathogens by inhibition of Toll-like receptor 4 (TLR4), and downregulation of let-7 restores immune activation (16,33). In mammalian macrophages exposed to pathogen challenge, let-7 controls the immune response via inhibition of the NFKB1 pathway or repression of cytokine expression (17,34,35). Furthermore, the let-7 family has been shown to regulate T-cell functions. In murine CD4+ T cells receiving a T-cell receptor signal, let-7 represses T-cell activation and facilitates T-cell anergy by targeting Mtor mRNA (36). In addition, let-7a has been reported to inhibit Th17 cell differentiation and increase regulatory T cells in a mouse hepatitis model (37), and suppression of let-7 expression has been associated with the differentiation of central memory T cells (18). Our population-based discovery of the inverse association between expression of let-7a and densities of CD3+ and CD45RO+ cells in colorectal cancer tissues suggests that let-7a might inhibit the lymphocytic immune response against colorectal cancer, which is consistent with experimental evidence for immune suppressive effect of the let-7 family.
Tumor molecular analyses are increasingly important in colorectal cancer research (38,39). In addition, ample evidence indicates the relevance of immune characterization together with tumor molecular analyses (40–43). In particular, upregulation of the immune checkpoint ligand CD274 (PD-L1) in colorectal cancer has been associated with lower densities of FOXP3+ cells and tumor microsatellite stability in 823 tumors (44) [in contrast to other much smaller studies (11,45)]. As evidence indicates influences of tumor somatic alterations on immune checkpoint pathways (46), tumor molecular alterations may be targeted to augment effects of immunotherapies. Our findings suggest that let-7a may be a potential therapeutic target to improve the efficacy of immunotherapy.
Previous studies have found a controversial relationship between let-7 expression and cancer survival. Expression of let-7a and let-7f has been associated with longer survival in lung cancer (19), and let-7b expression with longer survival in serous ovarian cancer (20). However, MIRLET7A3 hypomethylation, which increases let-7a-3 expression, has been associated with shorter survival in epithelial ovarian cancers (21). To date, two studies using microarrays have investigated the prognostic value of let-7 in colorectal cancer, but no significant prognostic association has been observed (47,48). Potential reasons for the inconsistent effects of let-7 on cancer survival may include limited sample sizes of those studies, and differences in study populations, cancer types, and measurement methods for let-7 family members. In the current study, we measured let-7a and let-7b that account for the majority of let-7 expressed in human colonic epithelia (22) in 795 patients. As a result, we found a strong positive association of let-7a expression with colorectal cancer-specific mortality.
One limitation of our study is the cross-sectional design, which cannot exclude the possibility of reverse causation. It is possible that CD45RO+ cells might suppress let-7a expression in the tumor microenvironment. Nevertheless, our hypothesis was based on various lines of experimental evidence indicating that let-7 suppresses immune responses (16–18,33–36). Another limitation is the measurement of let-7 expression in colorectal cancer tissues, which contain a mixture of tumor cells and stromal cells, including immune cells. Our measurement in 10 pairs of tumor cells and tumor stroma suggests higher let-7a expression in stromal cells, although further studies are required to validate this finding and to identify the cell types responsible for let-7 production.
Our study has several strengths. First, this study utilizes our molecular pathological epidemiology (49,50) database of 795 colorectal cancer cases in two U.S. nationwide prospective cohorts, which integrates epidemiological exposures, clinicopathological features, tumor molecular markers, and immune reaction status. This enabled us to rigorously test the association of let-7 expression with T-cell density and mortality, controlling for potential confounders. Second, we used robust methods, including validated PCR assays for microRNA expression and objective quantification of T-cell densities by image analysis. Third, our tumor specimens were obtained from many hospitals throughout the U.S., which improves the generalizability of our findings.
In conclusion, higher let-7a expression was associated with lower densities of CD3+ and CD45RO+ cells in colorectal cancer tissue and with higher colorectal cancer-specific mortality. Our findings support a possible role of let-7a in downregulating T cell–mediated immunity in colorectal cancer. Further prospective studies are needed to validate these findings from the current hypothesis-generating study. Upon validation, these population-based data may have implications for expanding the benefit of immune therapies through targeting microRNAs.
Supplementary Material
Acknowledgments
We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY.
Financial support: This work was supported by U.S. National Institutes of Health (NIH) grants (P01 CA87969 and UM1 CA186107 to M.J. Stampfer; P01 CA55075 and UM1 CA167552 to W.C. Willett; P50 CA127003 to C.S.F.; K24 DK098311 and R01 CA137178 to A.T.C.; R01 CA151993 and R35 CA197735 to S.O.; and K07 CA190673 to R.N.); and by grants from the Project P Fund, The Friends of the Dana-Farber Cancer Institute, Bennett Family Fund, and the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance. R.D. is supported by the Program of Introducing Talents of Discipline to Universities B12003 and the International S&T Cooperation Program 2011DFA32570. T.H. was supported by a fellowship grant from the Uehara Memorial Foundation and by a grant from the Mochida Memorial Foundation for Medical and Pharmaceutical Research. Y.M. is supported by a fellowship grant of the Keio Gijuku Fukuzawa Memorial Fund for the Advancement of Education and Research.
Abbreviations
- cDNA
complementary DNA
- CI
confidence interval
- CIMP
CpG island methylator phenotype
- Ct
cycle threshold
- FFPE
formalin-fixed paraffin-embedded
- HPFS
Health Professionals Follow-up Study
- LCM
laser capture microdissection
- LINE-1
long interspersed nucleotide element-1
- MSI
microsatellite instability
- MSS
microsatellite stable
- NHS
Nurses’ Health Study
- OR
odds ratio
- PCR
polymerase chain reaction
- SD
standard deviation
Footnotes
Disclosures: The authors declare that they have no conflicts of interest.
Use of standardized official symbols: We use HUGO (Human Genome Organisation)-approved official symbols for genes and gene products, including BRAF, CACNA1G, CD3, CD8, CD274, CDKN2A, CRABP1, CTLA4, FOXP3, IGF2, KRAS, MIRLET7A1, MIRLET7A2, MIRLET7A3, MIRLET7B, MLH1, MTOR, NEUROG1, NFKB1, PDCD1, PIK3CA, PTPRC, RNU6-2, RUNX3, SOCS1, and TLR4; all of which are described at www.genenames.org. Gene names are italicized, and gene product names are non-italicized.
References
- 1.Ogino S, Galon J, Fuchs CS, Dranoff G. Cancer immunology–analysis of host and tumor factors for personalized medicine. Nature reviews Clinical oncology. 2011;8:711–9. doi: 10.1038/nrclinonc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, et al. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity. 2016;44:698–711. doi: 10.1016/j.immuni.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 3.Di Caro G, Marchesi F, Laghi L, Grizzi F. Immune cells: plastic players along colorectal cancer progression. Journal of cellular and molecular medicine. 2013;17:1088–95. doi: 10.1111/jcmm.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogino S, Nosho K, Irahara N, Meyerhardt JA, Baba Y, Shima K, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:6412–20. doi: 10.1158/1078-0432.CCR-09-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. The Journal of pathology. 2010;222:350–66. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. The New England journal of medicine. 2005;353:2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 7.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–8. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 8.Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nature reviews Clinical oncology. 2016;13:143–58. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 9.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. The New England journal of medicine. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giannakis M, Mu XJ, Shukla SA, Qian ZR, Cohen O, Nishihara R, et al. Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma. Cell Rep. 2016;15:857–65. doi: 10.1016/j.celrep.2016.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer discovery. 2015;5:43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jasinski-Bergner S, Mandelboim O, Seliger B. The role of microRNAs in the control of innate immune response in cancer. Journal of the National Cancer Institute. 2014;106:dju257. doi: 10.1093/jnci/dju257. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Tan S, Kooger R, Zhang C, Zhang Y. MicroRNAs as novel biological targets for detection and regulation. Chem Soc Rev. 2014;43:506–17. doi: 10.1039/c3cs60312a. [DOI] [PubMed] [Google Scholar]
- 14.Cheng CJ, Bahal R, Babar IA, Pincus Z, Barrera F, Liu C, et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature. 2015;518:107–10. doi: 10.1038/nature13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mima K, Nishihara R, Nowak JA, Kim SA, Song M, Inamura K, et al. MicroRNA MIR21 and T Cells in Colorectal Cancer. Cancer Immunol Res. 2016;4:33–40. doi: 10.1158/2326-6066.CIR-15-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen XM, Splinter PL, O’Hara SP, LaRusso NF. A cellular micro-RNA, let-7i, regulates Toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection. The Journal of biological chemistry. 2007;282:28929–38. doi: 10.1074/jbc.M702633200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Androulidaki A, Iliopoulos D, Arranz A, Doxaki C, Schworer S, Zacharioudaki V, et al. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity. 2009;31:220–31. doi: 10.1016/j.immuni.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almanza G, Fernandez A, Volinia S, Cortez-Gonzalez X, Croce CM, Zanetti M. Selected microRNAs define cell fate determination of murine central memory CD8 T cells. PloS one. 2010;5:e11243. doi: 10.1371/journal.pone.0011243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–6. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 20.Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim JH, et al. MicroRNA expression profiles in serous ovarian carcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:2690–5. doi: 10.1158/1078-0432.CCR-07-1731. [DOI] [PubMed] [Google Scholar]
- 21.Lu L, Katsaros D, de la Longrais IA, Sochirca O, Yu H. Hypermethylation of let-7a-3 in epithelial ovarian cancer is associated with low insulin-like growth factor-II expression and favorable prognosis. Cancer Res. 2007;67:10117–22. doi: 10.1158/0008-5472.CAN-07-2544. [DOI] [PubMed] [Google Scholar]
- 22.King CE, Wang L, Winograd R, Madison BB, Mongroo PS, Johnstone CN, et al. LIN28B fosters colon cancer migration, invasion and transformation through let-7-dependent and -independent mechanisms. Oncogene. 2011;30:4185–93. doi: 10.1038/onc.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. The New England journal of medicine. 2012;367:1596–606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishihara R, Lochhead P, Kuchiba A, Jung S, Yamauchi M, Liao X, et al. Aspirin use and risk of colorectal cancer according to BRAF mutation status. JAMA: the journal of the American Medical Association. 2013;309:2563–71. doi: 10.1001/jama.2013.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature protocols. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 26.Ogino S, Kawasaki T, Kirkner GJ, Loda M, Fuchs CS. CpG island methylator phenotype-low (CIMP-low) in colorectal cancer: possible associations with male sex and KRAS mutations. J Mol Diagn. 2006;8:582–8. doi: 10.2353/jmoldx.2006.060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogino S, Kawasaki T, Brahmandam M, Yan L, Cantor M, Namgyal C, et al. Sensitive sequencing method for KRAS mutation detection by Pyrosequencing. J Mol Diagn. 2005;7:413–21. doi: 10.1016/S1525-1578(10)60571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imamura Y, Lochhead P, Yamauchi M, Kuchiba A, Qian ZR, Liao X, et al. Analyses of clinicopathological, molecular, and prognostic associations of KRAS codon 61 and codon 146 mutations in colorectal cancer: cohort study and literature review. Molecular cancer. 2014;13:135. doi: 10.1186/1476-4598-13-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao X, Morikawa T, Lochhead P, Imamura Y, Kuchiba A, Yamauchi M, et al. Prognostic role of PIK3CA mutation in colorectal cancer: cohort study and literature review. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:2257–68. doi: 10.1158/1078-0432.CCR-11-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Meyerhardt JA, Loda M, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90–6. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Chan AT, Schernhammer ES, et al. A Cohort Study of Tumoral LINE-1 Hypomethylation and Prognosis in Colon Cancer. Journal of the National Cancer Institute. 2008;100:1734–8. doi: 10.1093/jnci/djn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irahara N, Nosho K, Baba Y, Shima K, Lindeman NI, Hazra A, et al. Precision of pyrosequencing assay to measure LINE-1 methylation in colon cancer, normal colonic mucosa, and peripheral blood cells. J Mol Diagn. 2010;12:177–83. doi: 10.2353/jmoldx.2010.090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu G, Zhou R, Liu J, Gong AY, Eischeid AN, Dittman JW, et al. MicroRNA-98 and let-7 confer cholangiocyte expression of cytokine-inducible Src homology 2-containing protein in response to microbial challenge. Journal of immunology (Baltimore, Md : 1950) 2009;183:1617–24. doi: 10.4049/jimmunol.0804362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar M, Sahu SK, Kumar R, Subuddhi A, Maji RK, Jana K, et al. MicroRNA let-7 Modulates the Immune Response to Mycobacterium tuberculosis Infection via Control of A20, an Inhibitor of the NF-kappaB Pathway. Cell host & microbe. 2015;17:345–56. doi: 10.1016/j.chom.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Schulte LN, Eulalio A, Mollenkopf HJ, Reinhardt R, Vogel J. Analysis of the host microRNA response to Salmonella uncovers the control of major cytokines by the let-7 family. The EMBO journal. 2011;30:1977–89. doi: 10.1038/emboj.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcais A, Blevins R, Graumann J, Feytout A, Dharmalingam G, Carroll T, et al. microRNA-mediated regulation of mTOR complex components facilitates discrimination between activation and anergy in CD4 T cells. J Exp Med. 2014;211:2281–95. doi: 10.1084/jem.20132059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Wang X, Zhong M, Zhang M, Suo Q, Lv K. MicroRNA let-7a ameliorates con A-induced hepatitis by inhibiting IL-6-dependent Th17 cell differentiation. J Clin Immunol. 2013;33:630–9. doi: 10.1007/s10875-012-9840-7. [DOI] [PubMed] [Google Scholar]
- 38.Colussi D, Brandi G, Bazzoli F, Ricciardiello L. Molecular pathways involved in colorectal cancer: implications for disease behavior and prevention. International journal of molecular sciences. 2013;14:16365–85. doi: 10.3390/ijms140816365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. doi: 10.1038/nrdp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nature reviews Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 42.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. The Journal of pathology. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rozek LS, Schmit SL, Greenson JK, Tomsho LP, Rennert HS, Rennert G, et al. Tumor-Infiltrating Lymphocytes, Crohn’s-Like Lymphoid Reaction, and Survival From Colorectal Cancer. Journal of the National Cancer Institute. 2016;108:djw027. doi: 10.1093/jnci/djw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masugi Y, Nishihara R, Yang J, Mima K, da Silva A, Shi Y, et al. Tumour CD274 (PD-L1) expression and T cells in colorectal cancer. Gut. 2016 doi: 10.1136/gutjnl-2016-311421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenbaum MW, Bledsoe JR, Morales-Oyarvide V, Huynh TG, Mino-Kenudson M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2016;29:1104–12. doi: 10.1038/modpathol.2016.95. [DOI] [PubMed] [Google Scholar]
- 46.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer discovery. 2013;3:1355–63. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schetter AJ, Leung SY, Sohn JJ, Zanetti KA, Bowman ED, Yanaihara N, et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA: the journal of the American Medical Association. 2008;299:425–36. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slattery ML, Herrick JS, Mullany LE, Valeri N, Stevens J, Caan BJ, et al. An evaluation and replication of miRNAs with disease stage and colorectal cancer-specific mortality. International journal of cancer Journal international du cancer. 2015;137:428–38. doi: 10.1002/ijc.29384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397–411. doi: 10.1136/gut.2010.217182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ogino S, Nishihara R, VanderWeele TJ, Wang M, Nishi A, Lochhead P, et al. Review Article: The Role of Molecular Pathological Epidemiology in the Study of Neoplastic and Non-neoplastic Diseases in the Era of Precision Medicine. Epidemiology. 2016;27:602–11. doi: 10.1097/EDE.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


