Case Presentation
A previously healthy 14-year-old girl was transferred to our facility from an outside hospital with a 10-day history of nausea, malaise, intermittent fever (peak 39.5°C), and fatigue. In the 72 hours prior to admission, she had developed progressive yellowing of the skin and scleral icterus. She had not traveled recently and her immunizations were up-to-date. She denied being sexually active or using illicit substances. She was a well-developed Caucasian adolescent who on initial presentation appeared fatigued and jaundiced. Vital signs on admission: temperature, 38.8°C; heart rate, 96 beats per minute; respiratory rate, 18 breaths per minute; and blood pressure, 112/58 mm Hg. Her physical examination revealed scleral icterus, pharyngeal erythema with tonsillar exudates, anterior cervical lymphadenopathy, and right-sided abdominal tenderness. The liver edge was palpable at 1 cm below the right costal margin.
Laboratory evaluation from the outside hospital revealed transaminase elevation and elevated serum bilirubin levels (table 1). Complete blood count did not demonstrate evidence of anemia, thrombocytopenia, leukocytosis, or bandemia. Lipase level was normal at 32 U/L (23–300 U/L), though a C-reactive protein was mildly elevated at 1.20 mg/dL (< 0.99 mg/dL). Acetaminophen level was negative at < 10.0 μg/mL (10–25 μg/mL). Coagulation studies were within the reference range. An initial ultrasound of the abdomen showed diffuse thickening of the gallbladder wall, measuring up to 8.5 mm in thickness, with no evidence of biliary duct dilatation or the presence of stones (figure 1). Splenomegaly was also seen. The patient did report right upper quadrant tenderness during the ultrasound, exhibiting the positive sonographic Murphy’s sign. These findings were consistent with a working diagnosis of acute acalculous cholecystitis and empiric broad-spectrum antibiotic therapy with piperacillin-tazobactam was initiated. All oral feeds were held and aggressive intravenous hydration was initiated.
Table 1.
Clinical Course
| Admission | Day 1 | Day 2 | Day 5 | Day 8 | Day 9 (Discharge) | Day 15 | Day 62 | |
|---|---|---|---|---|---|---|---|---|
| AST (U/L) | 360 | 317 | 270 | 143 | 114 | 90 | 68 | 31 |
| ALT (U/L) | 286 | 232 | 204 | 135 | 122 | 106 | 86 | 29 |
| Total bilirubin (mg/dL) | 10.3 | 8.3 | 7.4 | 4.2 | 1.7 | 1.5 | 1.3 | 0.5 |
| Direct bilirubin (mg/dL) | 7.3 | 5.5 | 4.6 | 1.3 | < 0.1 | < 0.1 | < 0.1 | < 0.1 |
| GGT (U/L) | 408 | 406 | 409 | 249 | 199 | 47 | 10 | |
| EBV RT-PCR (copies/mL) | 24578 | 1005 | ||||||
| White blood cell count (cells/L) | 10.3 × 109 | 9.1 × 109 | 5.4 × 109 | |||||
| Hemoglobin (g/dL) | 12.2 | 10.5 | 12.5 | |||||
| % Atypical lymphocytes | 0 | 13 | 0 |
Figure 1.

Transverse grayscale ultrasound of the gallbladder from the date of admission demonstrates marked circumferential gallbladder wall thickening (calipers) measuring 8.5 mm.
Hospital Course
A repeat complete blood count at our institution on hospital day 2 demonstrated atypical lymphocytosis, raising suspicion for an underlying diagnosis of infectious mononucleosis. Heterophile antibodies were present as detected by the Monospot test. Serologic testing was positive for EBV viral capsid antigen (VCA) IgM, but negative for VCA IgG and EBV nuclear antigen (EBNA) antibodies, most consistent with primary EBV infection. Furthermore, Epstein-Barr viral quantitative real-time polymerase chain reaction (PCR) assay of the patient’s serum drawn on hospital day two revealed 24,578 copies/mL (< 200 copies/mL). Serologic studies for hepatitis A, hepatitis C, and cytomegalovirus were negative. Blood, stool, and urine cultures showed no growth. Anti-hepatitis B core (anti-HBc) IgM was positive, but hepatitis B viral quantitative real-time PCR assay was negative. Our patient had no risk factors for hepatitis B infection and had been immunized. Based on the degree of hepatitis and AAC with mounting evidence of primary EBV infection, a course of intravenous ganciclovir was initiated. By hospital day four, the patient’s abdominal pain had resolved, with improvement in energy level and appetite, but with continued intermittent fevers. Repeat ultrasound examinations on hospital days five (figure 2) and eight (figure 3) showed dramatic improvement from the initial study, with complete subsidence of gallbladder inflammation. The patient was transitioned from intravenous ganciclovir and piperacillin-tazobactam to oral valganciclovir and ciprofloxacin as oral feeds were advanced.
Figure 2.

Transverse grayscale ultrasound of the gallbladder 4 days after admission demonstrates improved circumferential gallbladder wall thickening (calipers), now measuring 4.0 mm.
Figure 3.
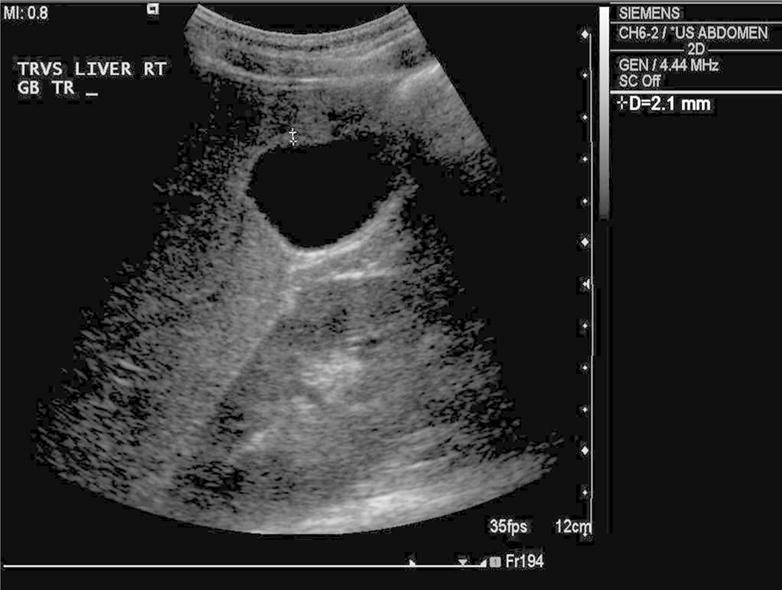
Transverse grayscale ultrasound of the gallbladder 7 days after admission demonstrates resolved gallbladder wall thickening (calipers), now measuring 2.1 mm, within normal range.
The patient was discharged on hospital day nine in good clinical condition with improvement in biochemical markers. After only four days of anti-viral therapy, repeat EBV quantitative PCR assay showed a decrease to 1005 copies/mL (< 200 copies/mL). She was discharged home on a low-fat diet with outpatient antibiotic and anti-viral therapy to complete a total of 14 days of treatment. At her first outpatient follow-up visit, two weeks after hospital discharge, she was found to be in excellent clinical condition with no signs or symptoms. All liver chemistry abnormalities had resolved soon after her first outpatient follow-up.
Final Diagnosis
Acalculous cholecystitis and cholestatic hepatitis secondary to primary Epstein-Barr virus infection.
Discussion
Gallbladder disease, including acute cholecystitis, is uncommon in the general pediatric population. Adult patients with cholecystitis typically have complications related to gallstones and require emergent surgical intervention. In pediatric patients, acute cholecystitis usually occurs secondary to infection or inflammation, rather than gallstones.1 Acute acalculous cholecystitis is an inflammatory process of the gallbladder in the absence of a gallstone, accounting for approximately 30–50% of cases of pediatric cholecystitis.2 AAC may be seen following severe abdominal trauma or in association with systemic diseases, like nephrotic syndrome, Kawasaki disease, and systemic lupus erythematosus.3,4 Furthermore, congenital gallbladder abnormalities, biliary duct anomalies, and other acquired conditions causing biliary stasis can also be associated with AAC. Only sixteen other patients with EBV-associated AAC have been previously reported, with our case being one of the first in North America.5–15 Fortunately, AAC complicating the course of primary EBV infection is associated with a favorable outcome.13
The etiology of AAC is thought to be due to bile stasis, coupled with increased lithogenicity of bile. Critically ill patients are thought to be particularly vulnerable due to increased bile viscosity secondary to fever and dehydration, as well as to decreased cholecystokinin-induced gallbladder contraction from prolonged absence of oral feeding. Decreased cardiac output and poor perfusion can result in gallbladder wall ischemia, which can also contribute to the pathogenesis of AAC in some patients.11 Secondary gallbladder infection in the setting of systemic infection is also thought to result in the development of AAC.
The exact pathogenesis of EBV-associated AAC is unclear. Our patient had cholestatic hepatitis with elevated γ-glutamyltransferase and alkaline phosphatase activities with imaging studies showing no evidence of biliary tract obstruction. EBV-associated hepatitis has recently been recognized as an important cause of cholestasis, suggesting that our patient’s acute infection may have induced the bile stasis and subsequent gallbladder inflammation that led to the development of AAC.16 Dehydration secondary to pharyngitis and loss of appetite may have also increased bile viscosity in this case. Direct invasion of the gallbladder by the Epstein-Barr viral antigen may represent another possible mechanism of pathogenesis. Such a mechanism has been previously described in a case of AAC in the setting of a viral hepatitis A infection, with viral antigen detected in gallbladder epithelial cells.17
Management strategies for AAC vary, ranging from non-operative supportive care to cholecystectomy.2 A recent treatment algorithm for pediatric patients with AAC suggests that emergency surgical intervention should be considered if ultrasonographic abnormalities persist or worsen on follow-up examinations.18 Our patient was monitored with serial laboratory studies and repeat ultrasonography, which demonstrated resolution of gallbladder inflammation. Therefore, surgical intervention was not indicated in her case. Review of the most current literature indicates that that regardless of the underlying etiology, supportive, non-operative management of AAC is safe and effective in most cases. A recent study demonstrates that nonsurgical treatment of AAC is associated with good outcomes when compared to cholecystectomy or cholecystotomy placement, particularly in medically fragile patients with additional co-morbidities.19 Treatment of EBV disease with anti-viral agents in immunocompetent individuals remains a topic of ongoing debate. A meta-analysis performed in the late 1990s of five randomized controlled trials using acyclovir for infectious mononucleosis in immunocompetent hosts showed a trend towards clinical effectiveness but did not reach significance.20 More experience has been gained in recent years in the use of ganciclovir and valganciclovir, two other nucleoside analogs, in immunosuppressed patients for both viral suppression and treatment of EBV-related post-transplant lymphoproliferative disorder.21 Though there is no prospective data on the use of ganciclovir in the setting of EBV-associated acalculous cholecystitis, it has been used to hasten resolution of EBV-associated hepatitis.22,23
To our knowledge, this is one of the first cases of EBV-associated cholestatic hepatitis and acute acalculous cholecystitis reported in an immunocompetent adolescent in North America, and in which anti-viral therapy has been utilized with rapid resolution of sonographic evidence of AAC. We propose that AAC can develop in the setting of a primary EBV infection in otherwise healthy children, particularly in those with cholestatic hepatitis. Infectious diseases consultation is recommended in these cases to discuss the utility of anti-viral therapies. Clinicians should maintain a high index of suspicion for possible gallbladder involvement during EBV infection to avoid overuse of antibiotics and to prevent unnecessary surgical procedures.
Acknowledgments
Funding Source: No funding was secured for this study.
Abbreviations
- AAC
Acute acalculous cholecystitis
- EBV
Epstein-Barr virus
- IM
infectious mononucleosis
- PCR
polymerase chain reaction
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest Disclosure: The authors have no conflicts of interest to disclose. Dr. Say (author guarantor) conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted. Drs. Chaparro and Sivagnanam critically reviewed and revised the manuscript and approved the final manuscript as submitted. Dr. Koning reviewed and interpreted radiographic studies pertaining to this case, reviewed the manuscript, and approved the final manuscript as submitted.
Informed Consent: Informed consent was obtained from the patient and her legal guardian. All identifying patient information has been removed to protect patient privacy.
References
- 1.Suchy FJ. Disease of the gallbladder. In: Kliegman R, editor. Nelson textbook of pediatrics. 19th. Vol. 2011. Philadelphia, PA: Elsevier/Saunders; pp. 1415–1416. [Google Scholar]
- 2.Tsakayannis DE, Kozakewich HP, Lillehei CW. Acalculous cholecystitis in children. Journal of pediatric surgery. 1996;31(1):127–130. doi: 10.1016/s0022-3468(96)90334-6. discussion 130–121. [DOI] [PubMed] [Google Scholar]
- 3.Mendonca JA, Marques-Neto JF, Prando P, Appenzeller S. Acute acalculous cholecystitis in juvenile systemic lupus erythematosus. Lupus. 2009;18(6):561–563. doi: 10.1177/0961203308098587. [DOI] [PubMed] [Google Scholar]
- 4.Chen CJ, Huang FC, Tiao MM, et al. Sonographic gallbladder abnormality is associated with intravenous immunoglobulin resistance in Kawasaki disease. The Scientific World Journal. 2012;2012:485758. doi: 10.1100/2012/485758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lagona E, Sharifi F, Voutsioti A, Mavri A, Markouri M, Attilakos A. Epstein-Barr virus infectious mononucleosis associated with acute acalculous cholecystitis. Infection. 2007;35(2):118–119. doi: 10.1007/s15010-007-6115-y. [DOI] [PubMed] [Google Scholar]
- 6.Prassouli A, Panagiotou J, Vakaki M, et al. Acute acalculous cholecystitis as the initial presentation of primary Epstein-Barr virus infection. Journal of pediatric surgery. 2007;42(1):E11–13. doi: 10.1016/j.jpedsurg.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Iaria C, Arena L, Di Maio G, et al. Acute acalculous cholecystitis during the course of primary Epstein-Barr virus infection: a new case and a review of the literature. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2008;12(4):391–395. doi: 10.1016/j.ijid.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Gora-Gebka M, Liberek A, Bako W, Szarszewski A, Kaminska B, Korzon M. Acute acalculous cholecystitis of viral etiology–a rare condition in children? Journal of pediatric surgery. 2008;43(1):e25–27. doi: 10.1016/j.jpedsurg.2007.10.073. [DOI] [PubMed] [Google Scholar]
- 9.Attilakos A, Prassouli A, Hadjigeorgiou G, et al. Acute acalculous cholecystitis in children with Epstein-Barr virus infection: a role for Gilbert’s syndrome? International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2009;13(4):e161–164. doi: 10.1016/j.ijid.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Poddighe D, Cagnoli G, Mastricci N, Bruni P. Acute acalculous cholecystitis associated with severe EBV hepatitis in an immunocompetent child. BMJ case reports. 2014:2014. doi: 10.1136/bcr-2013-201166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koch AD, van den Bosch HC, Bravenboer B. Epstein-Barr virus-associated cholecystitis. Annals of internal medicine. 2007;146(11):826–827. doi: 10.7326/0003-4819-146-11-200706050-00024. [DOI] [PubMed] [Google Scholar]
- 12.Cholongitas E, Katsogridakis K, Dasenaki M. Acalculous cholecystitis during the course of acute Epstein-Barr virus infection. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2009;13(3):e129–130. doi: 10.1016/j.ijid.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 13.Arya SO, Saini A, El-Baba M, Salimnia H, Abdel-Haq N. Epstein Barr virus-associated acute acalculous cholecystitis: a rare occurrence but favorable outcome. Clinical pediatrics. 2010;49(8):799–804. doi: 10.1177/0009922810363729. [DOI] [PubMed] [Google Scholar]
- 14.Kim A, Yang HR, Moon JS, Chang JY, Ko JS. Epstein-barr virus infection with acute acalculous cholecystitis. Pediatric gastroenterology, hepatology & nutrition. 2014;17(1):57–60. doi: 10.5223/pghn.2014.17.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gagneux-Brunon A, Suy F, Pouvaret A, et al. Acute acalculous cholecystitis, a rare complication of Epstein-Barr virus primary infection: report of two cases and review. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2014;61(1):173–175. doi: 10.1016/j.jcv.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Shaukat A, Tsai HT, Rutherford R, Anania FA. Epstein-Barr virus induced hepatitis: An important cause of cholestasis. Hepatology research: the official journal of the Japan Society of Hepatology. 2005;33(1):24–26. doi: 10.1016/j.hepres.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Mourani S, Dobbs SM, Genta RM, Tandon AK, Yoffe B. Hepatitis A virus-associated cholecystitis. Annals of internal medicine. 1994;120(5):398–400. doi: 10.7326/0003-4819-120-5-199403010-00008. [DOI] [PubMed] [Google Scholar]
- 18.Imamoglu M, Sarihan H, Sari A, Ahmetoglu A. Acute acalculous cholecystitis in children: Diagnosis and treatment. Journal of pediatric surgery. 2002;37(1):36–39. doi: 10.1053/jpsu.2002.29423. [DOI] [PubMed] [Google Scholar]
- 19.Gu MG, Kim TN, Song J, Nam YJ, Lee JY, Park JS. Risk factors and therapeutic outcomes of acute acalculous cholecystitis. Digestion. 2014;90(2):75–80. doi: 10.1159/000362444. [DOI] [PubMed] [Google Scholar]
- 20.Torre D, Tambini R. Acyclovir for treatment of infectious mononucleosis: a meta-analysis. Scandinavian journal of infectious diseases. 1999;31(6):543–547. doi: 10.1080/00365549950164409. [DOI] [PubMed] [Google Scholar]
- 21.Funch DP, Walker AM, Schneider G, Ziyadeh NJ, Pescovitz MD. Ganciclovir and acyclovir reduce the risk of post-transplant lymphoproliferative disorder in renal transplant recipients. Am J Transplant. 2005;5(12):2894–2900. doi: 10.1111/j.1600-6143.2005.01115.x. [DOI] [PubMed] [Google Scholar]
- 22.Adams LA, Deboer B, Jeffrey G, Marley R, Garas G. Ganciclovir and the treatment of Epstein-Barr virus hepatitis. Journal of gastroenterology and hepatology. 2006;21(11):1758–1760. doi: 10.1111/j.1440-1746.2006.03257.x. [DOI] [PubMed] [Google Scholar]
- 23.Pisapia R, Mariano A, Rianda A, Testa A, Oliva A, Vincenzi L. Severe EBV hepatitis treated with valganciclovir. Infection. 2013;41(1):251–254. doi: 10.1007/s15010-012-0303-0. [DOI] [PubMed] [Google Scholar]


